2. 环境保护部华南环境科学研究所,广州 510655;
3. 广东工业大学环境科学与工程学院,广州 510006;
4. 中国科学院大学,北京 100049
砷是土壤中的重金属污染物之一,被国际癌症研究机构归类为第一类致癌物[1]。砷的化合物毒性大小依次为:砷(Ⅲ)>一甲基化亚砷酸(MMAs(Ⅲ))>二甲基化亚砷酸(DMAs(Ⅲ))>二甲基化砷(DMAs(Ⅴ))>一甲基化砷(MMAs(Ⅴ))>砷(Ⅴ)[2]。水稻土中自然本底的砷浓度一般较低,而人类工业和农业活动使得大量的外源性砷进入到土壤当中[3]。水稻是世界上最主要的粮食作物之一,易吸收富集砷,因而稻田土壤砷污染会对以水稻为主食的人群身体健康构成严重威胁[4]。
水稻土中砷的迁移转化与砷的生物化学过程紧密相关,砷的氧化还原与甲基化决定水稻土砷的赋存形态与归趋。其中水稻土砷的生物化学行为与界面微环境密切有关,有研究表明间歇式水淹处理比长期水淹处理土壤溶液砷浓度和水稻籽粒砷含量均更低,且有进一步研究指出37种基因型水稻经点喷式管理方式对砷的吸收累积量相比于长期水淹管理方式均显著降低,这证实厌氧水淹过程是导致水稻土砷释放的关键环节。据研究报道,水淹缺氧环境有利于铁/砷还原菌的活动,导致铁矿物的还原溶解和砷释放还原以及砷的甲基化[5-7]。Bennett等[8]研究表明土壤溶液中铁(Ⅱ)浓度和砷(Ⅲ)浓度呈极显著正相关,说明铁矿物还原溶解引起了砷的释放还原。即使在水淹缺氧环境下,砷的氧化也是存在的,有研究证实根表铁膜可作为水稻吸收砷的屏障[9],Hu等[10]进一步研究表明水稻根表铁膜砷氧化微生物丰度与水稻根部、秸秆和籽粒均呈显著负相关性。水淹环境下水稻土中砷的甲基化与砷的氧化还原过程同样重要,Zhang等[11]研究表明,水稻土在水淹厌氧条件下砷还原基因丰度显著高于有氧条件下的丰度,且砷还原基因与砷甲基化基因呈显著正相关性。且有进一步研究表明,一些特定种类的微生物与水稻土中砷的氧化还原和甲基化过程紧密相关[7, 11-15]。水稻土的水淹厌氧环境引起的与铁砷迁移转化有关的微生物在群落水平和代谢、基因表达方面的变化进一步影响着砷的生物化学过程[11, 13-14],并由此改变着水稻土中砷的动力学特性[7, 14, 16-17]。
目前,许多研究从不同的角度深入探讨了水稻土中砷的氧化还原和甲基化过程以及涉及的微生物介导机制,但对这方面研究却鲜有系统性的综合分析。因此,本文综述了水稻土中砷的氧化还原和甲基化的生物化学过程及其影响因素,分析了水淹厌氧条件下水稻土砷的迁移转化特点,并展望了未来的研究方向,以期为水稻土砷污染防治提供科学依据。
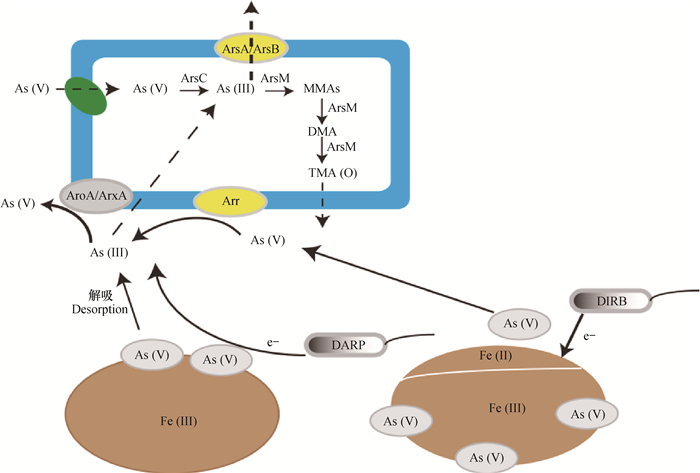
1 水稻土砷的氧化和还原水稻土砷的迁移转化与微生物介导砷的氧化还原等生物化学过程有关,其中铁/砷的还原将有利于砷有效性地提高和进一步的砷甲基化,具体如图 1所示。
|
图 1 水稻土砷的释放、氧化还原与甲基化过程 Fig. 1 Release, oxidation and reduction as well as methylation of arsenic in paddy soil |
砷的还原主要分为异化砷还原与细胞质砷还原。
异化砷还原是指微生物在厌氧条件下利用乳酸盐、乙酸盐、芳香类化合物等有机物作为电子供体将砷(Ⅴ)还原为砷(Ⅲ),同时合成ATP,为微生物生长提供能量;这种厌氧砷呼吸还原机制在原核生物中普遍存在[18-21]。异化砷还原发生在细胞周质上,需要异化砷还原酶异质二聚体蛋白arrAB以及cymA基因编码的细胞色素C的参与。其中,异化砷还原酶由大亚基arrA(大小约为100 kDa)和小亚基arrB(大小约为30 kDa)组成;arrA是砷(Ⅴ)发生还原的场所,arrB负责将经呼吸链传来的基质脱下的电子传递给arrA;细胞色素C则是充当电子穿梭体的角色[18, 21]。
细胞质砷还原是指将进入细胞的砷(Ⅴ)还原为砷(Ⅲ)并通过膜蛋白将砷(Ⅲ)泵出细胞,降低细胞中砷的浓度以达到解毒的目的[22-23]。细胞质砷还原是由细胞质中ars操纵子编码的砷(Ⅴ)还原酶控制的,它与砷(Ⅲ)排出泵共同形成支撑细胞质砷还原的耦合体系。ars操纵子的核心由三个基因组成:arsR,arsB,arsC。其中,arsR为阻遏蛋白基因,与ars启动子结合时会抑制其他ars基因的转录与表达;arsB是载体蛋白基因,与利用膜势能将砷(Ⅲ)排出有关;arsC基因则编码砷(Ⅲ)还原酶,对砷(Ⅲ)还原起着重要作用[18, 23-24]。研究表明,砷(Ⅲ)排出细胞的过程与ATP酶arsA有关;arsA与arsB共同组成排出砷(Ⅲ)的阴离子泵,砷(Ⅲ)与arsA酶结合刺激arsA酶水解ATP产生能量促使阴离子泵将砷(Ⅲ)排出;arsA可通过与arsD金属分子伴侣结合而增强自身与砷(Ⅲ)结合的能力[16]。
异化砷还原与细胞质砷还原分别与砷呼吸还原基因arrA和砷解毒还原基因arsC有关。arsC作为砷解毒基因广泛存在于好氧、厌氧微生物中,而arrA主要存在于厌氧微生物中[7]。Saltikov等[25]研究Shewanella sp. strain ANA-3的砷还原行为发现arrA基因的表达会被氧气与硝态氮抑制,arrA基因的表达在处于对数期和停滞期初期的微生物中受砷(Ⅴ)的刺激作用最显著,且arrA基因对砷(Ⅲ)的敏感度也远超于arsC基因,但arsC基因在有氧和厌氧环境下都能表达。Jia等[7]的研究则表明,水淹水稻根际土中arsC基因的丰度与多样性高于arrA基因,说明了arsC作为砷解毒基因的重要性。
能进行砷还原的微生物种类繁多,如常见的如Geobacter、Shewanella、Anaeromyxobacter和Ohtaekwangia、Desulfuromonas、Desulfocapsa、Desulfobulbus、Lacibacter等[12, 14],以及一些典型的水稻根际微生物,如Rhizobiales和Burkholderials[7],其中部分微生物与在沉积物、咸水湖以及热泉中分离出的砷还原微生物相似[7]。
1.2 砷还原主要影响因素铁矿物还原溶解使其吸持的砷进入液相被普遍认为是促进淹水水稻土中砷还原的关键因子,而铁矿物对砷的固定则被认为有利于抑制砷还原;大量研究表明铁矿物吸持态砷(Ⅴ)较溶液相砷(Ⅴ)更难被微生物还原,拥有更长的还原半衰期[6, 12, 26-27],而Bennett等[8]研究表明土壤溶液中铁(Ⅱ)浓度和砷(Ⅲ)浓度呈极显著正相关(r=0.896,p<0.001),说明铁(Ⅲ)还原溶解有利于砷(Ⅴ)释放还原,也进一步证明了铁矿物固定砷有利于抑制砷还原。
水铁矿、针铁矿、赤铁矿对固定水稻土砷有重要作用,其中比表面积和反应活性最大的无定型水铁矿的砷吸持量最大,结晶度较高的针铁矿与赤铁矿的砷吸持量低于水铁矿[28-31]。Zobrist等[32]研究发现Sulfurospirillum barnesii还原液相砷(Ⅴ)远高效于还原水铁矿吸持态砷(Ⅴ),而砷(Ⅴ)与水铁矿共沉淀时还原速率更低。Jones等[33]研究发现Clostridium sp. strain CN-0还原液相砷(Ⅴ)的速率是还原针铁矿悬液(砷/铁=5.3×10-4 mol mol-1)中砷(Ⅴ)的速率的1200倍以上,其中液相砷(Ⅴ)还原速率超过12 mmol d-1,并指出砷(Ⅴ)解吸控制着液相砷(Ⅲ)的生成速率。近来,Huang等[12]进一步研究发现,液相砷(Ⅴ)、0.2 g L-1针铁矿和水铁矿悬液中的砷(Ⅴ)在Shewanella putrefaciens strain CN-32介导下的还原半衰期分别为3 h、32 h以及227 h,同样表明铁矿物固定砷能抑制砷还原,且水铁矿的抑制效果最显著。尽管铁还原菌偏爱集聚在结晶度低、反应活性高的铁矿物上[34],但Zhang等[35]指出一般的水稻田耕作环境中被还原的草酸铵提取铁(即无定型铁矿物)不超过其总量的1%,因此水铁矿固定砷对抑制砷还原起关键作用。与水铁矿、针铁矿不同,赤铁矿主要通过结构嵌入固定砷,Bolanz等[36]研究发现比表面积低至1 m2 g-1的赤铁矿可固定化1.8 wt%的砷(Ⅴ),远超过其表面所能承载的量,表明赤铁矿通过结构内嵌吸持了大量砷;由于赤铁矿结晶度高,不易被微生物还原,因此其对砷的吸持有利于避免砷(Ⅴ)释放到溶液中[36-37],但现有研究鲜有涉及赤铁矿抑制砷还原的具体效果。进一步研究表明,水稻土无定型铁与可交换态和专属吸附态砷总和呈负相关(r=-0.694,p<0.001)[38],且在外源添加时水铁矿相比于针铁矿与赤铁矿对有效态砷截留效果最好,其中无定型铁与无定型铁结合态砷呈显著正相关关系(r=0.879,p=0.009)[29]。以往的研究表明,水淹缺氧条件下水稻土溶液中的有效态砷主要以与铁矿物亲和力较低、移动性较强的砷(Ⅲ)形式存在[4],这与水淹缺氧条件铁/砷还原微生物驱动土壤微界面铁砷还原释放有关。相应地,水淹缺氧条件下水稻土有较高的pH,Honma等[39]进一步发现水稻土pH与总砷呈函数关系[As]=3.56×10-12 exp(4.72pH),溶解亚铁与总砷呈函数关系[As]=0.0024[Fe(Ⅱ)]2+0.3125[Fe(Ⅱ)]+3.5886,而厌氧条件下砷主要以砷(Ⅲ)存在,因此较高的pH或有利于水稻土砷还原。
水稻土中铁矿物发生相态转变或者还原生成次生矿物的过程以及氮铁循环、碳铁循环均可能影响砷固定并进一步影响砷还原,具体见于表 1和表 2。
|
|
表 1 铁矿物相态转变生成次生矿物对砷还原的影响 Table 1 Effects of secondary minerals formed during changes of iron minerals in phase on arsenic reduction |
|
|
表 2 对砷固定化、砷还原可能产生影响的氮铁、碳铁循环过程 Table 2 Nitrogen-iron and carbon-iron recycling processes that may have potential influence on arsenic immobilization and reduction |
除铁矿物之外,土壤有机质也是影响砷还原的关键因素。这与土壤有机质是铁代谢微生物和矿物质相互作用过程的重要参与者有关,一方面低分子有机质可以提供电子耦合铁的还原过程从而影响砷的界面环境化学行为,另一方面,有机质的氧化能够直接耦合沉积物、土壤矿物和溶液中砷的微生物还原[55]。稳定性低的有机质促进微生物砷还原的作用最为显著,如碳含量低、含氧基团和活性基团多的有机质、生物质有机质、溶解有机质等[56-58]。Sahoo和Kim[59]研究表明有机质刺激微生物活动使土壤氧化还原电位下降,有利于铁矿物还原溶解促进砷释放还原[12]。Chen等[60]研究发现生物炭促进了砷污染沉积物中的铁还原和砷还原,且总铁还原量与总砷还原量中的87%~90%与83%~88%与微生物降解生物炭相关,同时Geobacter、Anaeromyxobacter、Desulfosporosinus和Pedobacter等铁/砷还原菌的丰度显著上升。Wang等[54]同样发现加入生物炭的砷污染水稻土中铁还原菌Geobacter、Anaeromyxobacter、Desulfosporosinus和Pedobacter的丰度显著上升,且总砷释放量、砷(Ⅲ)释放量的提高与亚铁生成量呈显著相关性,砷还原基因arrA、arsC的丰度比与未加入生物炭时分别提高了20倍与1.4倍。
同样,有机质还可以通过其他方式和机制影响砷还原,具体见表 3。
|
|
表 3 有机质促进砷还原的方式与机理或实例 Table 3 Modes and mechanisms or examples of organic matter stimulating arsenic reduction |
砷的氧化由化能自养型砷氧化微生物与异养型砷氧化微生物介导[16]。化能自养型砷氧化微生物以氧气、硝酸盐、氯酸盐等为电子受体氧化砷氧化,或在厌氧条件下以铁矿物,硝酸根等为电子受体氧化砷[21, 23, 52, 70],进行产能并同化CO2以合成细胞物质,支持细胞生长。异养型砷氧化微生物介导的砷氧化过程常被视为一种解毒反应,即将砷(Ⅲ)转化为砷(Ⅴ)从而降低砷对细胞的毒性[16]。
砷(Ⅲ)的氧化是由aio基因介导的,一般采用aroA基因表示;aio砷(Ⅲ)氧化酶由两个一大一小的、分别由aioA与aioB基因编码的亚单位组成,这两个亚基均与砷(Ⅴ)还原酶的两个亚基有亲缘关系[16, 21, 71-72],广泛存在于细菌与古菌的砷(Ⅲ)氧化酶中,其功能是将从砷(Ⅲ)脱下来的电子传递到可溶于细胞质中的电子载体中[71-72]。Kumari和Jagadevan[16]指出砷氧化时,含有钼蝶呤的砷氧化酶中心与砷(Ⅲ)相连,钼(Ⅵ)被砷(Ⅲ)脱下的两个电子还原为钼(Ⅳ),随后两个电子先后经过大、小两个亚单位被传递至内膜的呼吸链上并由天青蛋白或细胞色素C传递至电子受体(氧气)。砷(Ⅲ)浓度较高的环境下微生物砷氧化基因丰度明显变高,砷氧化活动明显更活跃,表明砷氧化是一种微生物砷解毒机制。近来arxA基因(编码arxA砷氧化酶)对砷氧化的作用也得以确认,带有arxA基因的砷氧化微生物以硝态氮为电子受体,通过砷氧化固定二氧化碳[7, 16, 19]。另有研究表明,较高的磷浓度或有利于砷氧化微生物(如Synechocystis)更高效地氧化砷(Ⅲ),同时抑制其微生物细胞内砷(Ⅴ)还原[71-72]。
水稻土中与aroA基因有关的砷氧化菌主要隶属于以下的门:α-Proteobacteria与β-Proteobacteria。Zhang等[11]也在水稻土中检测到了α-Proteobacteria与β-Proteobacteria两个门的微生物,并且发现水稻根际菌Rhizobiales与Burkholderiales在砷氧化中起重要作用。进一步的研究报道水稻土同样存在Sinorhizobium sp. DAO10(aio)[73]、Alkalilimnicola ehrlichii strain MLHE-1(arxA)和Paracoccus strain SY(aio)[74]等砷氧化菌。水稻根膜方面,Hu等[10]研究发现水稻根际铁膜上的砷氧化微生物主要隶属于Acidovorax sp.与Hydrogenophaga sp.。
1.4 砷氧化主要影响因素大量研究表明水稻土氧化还原电位是影响砷氧化的重要因素。Rhine等[75]研究有氧/厌氧交替环境下砷(Ⅴ)呼吸还原菌strain Y5和自养型砷(Ⅲ)氧化菌strain OL1共同介导的砷(Ⅴ)/砷(Ⅲ)动力学,发现从有氧切换到厌氧环境时砷的主要存在价态从砷(Ⅴ)变为砷(Ⅲ)。Jia等[7]和Williams等[76]发现水淹水稻土中氧气量更高的水淹根际土与非根际土相比具有更高的砷氧化菌丰度和砷(Ⅴ)浓度。通常,水稻的通气组织越发达(泌氧量越大),根际周边区域氧化还原电位越高,砷(Ⅲ)的氧化现象就越显著[77]。Hu等[10]发现根表铁膜砷氧化微生物对抑制水稻富集砷起重要作用,而这与砷氧化微生物将砷(Ⅲ)转化为能被水稻根表铁膜强烈吸附的砷(Ⅴ)有关。
厌氧沉积物或水淹土壤等厌氧还原性环境中硝态氮可以作为电子受体促进微生物砷氧化[78-79]。Zhang等[19]发现水淹厌氧砷污染水稻土中砷(Ⅲ)的氧化耦合了硝态氮的还原,且该过程是由Paracoccus属的自养菌strain SY介导的。同样,一些活性很高的有机质可以直接氧化砷(Ⅲ)。Jiang等[69]研究发现氧化态AQDS能将砷(Ⅲ)直接氧化为砷(Ⅴ),而Chen等[60]也发现灭菌水淹厌氧环境中存在砷氧化现象,并推测可能与有机质直接氧化砷有关。
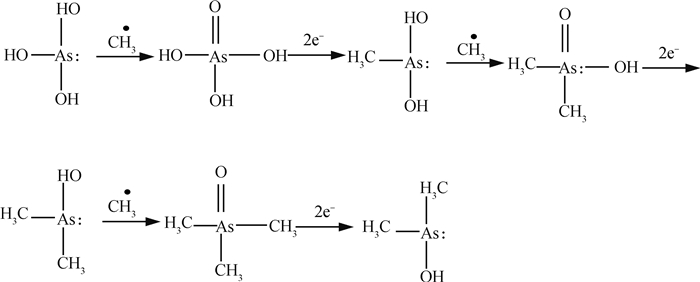
2 水稻土砷的甲基化过程 2.1 砷甲基化的主要机理砷甲基化是水稻土中存在的普遍现象,水稻土、水稻籽粒以及水稻土上方的大气均曾被检测到甲基化砷的存在[80-82],研究表明每年约有419~1 252 t的砷从水稻土中释放到大气中[83]。砷甲基化是水稻土中微生物自带的一种减毒脱毒机制,由砷甲基化基因(arsM)控制[84]。砷还原酶(arsC)与砷甲基化酶(arsM)是这些微生物细胞内砷甲基化的主控因子,砷还原酶将细胞质中的砷(Ⅴ)还原为毒性更大的砷(Ⅲ),而砷(Ⅲ)在arsM的催化甲基化作用下,依次生成一甲基化砷(MMA)、二甲基化砷(DMA)、三甲基化砷(TMA),并通过扩散作用被排出细胞体外[85]。目前,最合理并为接受的砷甲基化反应模式可以用图 2表示。
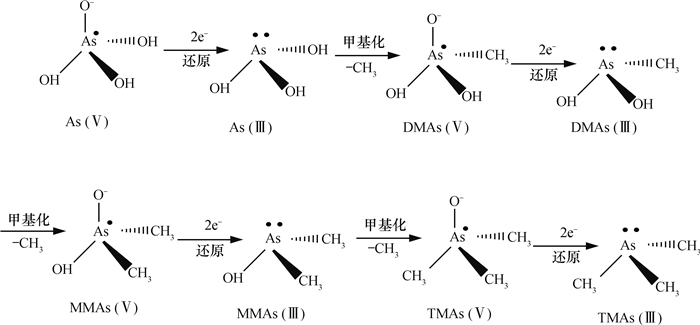
该过程实际上是一个还原-氧化-甲基化交替的过程,砷(Ⅲ)化合物H3AsO3中的羟基不断被甲基取代,最终形成挥发性的MMA、DMA、TMA,这个循环过程可以用图 3更清晰地表达出来。
|
图 3 砷还原-氧化-甲基化交替反应式[80, 87] Fig. 3 Chemical equation of alternation of reduction-oxidation-methylation of arsenic[80, 87] |
甲基供体是使无机砷转化为甲基化砷的重要物料,而不同微生物对应的甲基供体有所差异。大多数真菌细胞内发生的砷甲基化反应以S-腺苷甲硫氨酸(SAM)为甲基供体,而在细菌的细胞中,甲钴胺是主要的甲基供体[88]。迄今为止,研究中涉及较多的甲基供体为SAM。甲基供体SAM在砷甲基化过程中经历的过程为:SAM以及砷结合在微生物体内arsM酶上相应的位点上,随后SAM将甲基传递给砷(Ⅲ)[86],使原本同时与三个含硫半胱氨酰的氨基酸残基相连的砷(Ⅲ)转化为甲基化砷-酶的二元中间体。随后,该中间体水解,甲基化砷解离出来,还原性的谷胱甘肽(GSH)作为电子供体通过还原作用使arsM酶的活性恢复[84]。
水稻土中的砷甲基化微生物多种多样并隶属于不同的门,包括Proteobacteria、Firmicutes、Bacteroidetes、Gemmatimonadales等,其中也包括一些硫酸盐还原菌和产甲烷菌[11, 13]。Jia等[15]发现水稻土与根际土中Actinobacteria、Gemmat-imonadales、α-Proteobacterales、δ-Proteobacterales、β-Proteobacterales、Sphaerobacterales、Firmicates、CFB group bacteria、Halobacteriales、Archaea等门的菌种都与砷甲基化有关,且根际土或根表上出现的主要为Proteobacteria、Gemmatimonadales、Sphaerobacterales、Firmicutes。
2.2 砷甲基化的主要影响因素水稻土中的砷甲基化过程受多种因素影响,且有机质是关键因素。加入有机质使微生物获得充分养料,提高砷甲基化菌丰度,从而促进水稻土中还原性环境的形成以及砷甲基化过程,研究表明,有机质形成时间越短、性质越不稳定时,越易被利用,对砷甲基化的促进效果越显著[89-90]。不同种类的微生物能产生不同的砷甲基化产物,而施加不同的有机质促进砷甲基化的效果也有所差异;Huang等[91]往砷污染水稻土中加入干酒糟与三叶草,发现加入干酒糟后砷挥发量较未加入时高出100倍以上;加入干酒糟的处理组砷挥发量较加入三叶草的处理组高出两倍以上,两个处理组的砷甲基化产物也有所差异。Jia等[15]研究表明,往水稻土中加入稻草后arsM基因的丰度平均上升了139.4%,证实了添加有机质对微生物砷甲基化的促进作用,并指出不同微生物对砷甲基化的具体影响有所差异。水分也是影响砷甲基化的重要因素,适当湿度有利于创造厌氧或兼性厌氧的还原性环境,进而促进微生物进行砷甲基化,且研究表明土壤含水量为H2O 250~350 g kg-1土时砷甲基化的强化效果最显著[92-94]。此外,偏酸性的环境也有利于增强砷甲基化微生物的活动,研究表明pH从8.5降至8.0便可显著提高TMA的生成量[91, 95-96];Huang等[91]的研究中往水稻土添加三叶草粉末及干酒糟后砷挥发量均显著增加,而其pH则由8.7分别降至8.3与8.1。
3 水淹厌氧条件对水稻土砷的生物化学作用机制水稻种植需要经历的淹水阶段是影响水稻土砷迁移转化的关键因素,大量研究证实水淹厌氧条件能显著影响水稻土中砷的氧化、还原与甲基化,而这与水稻土中铁砷代谢相关微生物的群落结构的变化是密切相关的。水淹厌氧条件有利于水稻土砷的释放还原与甲基化,而不利于砷氧化[97]。砷在水淹缺氧条件下的具体环境化学行为可参考图 4。

|
图 4 水淹还原性条件下水稻土砷的环境化学行为 Fig. 4 Environmental chemical behavior of arsenic in flooded reducing paddy soil |
水稻土中铁矿物是砷的主要吸附载体,水淹厌氧条件的形成以及低氧化还原电位环境有利于促进铁还原菌和砷还原菌的活动,不利于砷氧化菌的活动,导致铁矿物还原溶解使砷释放至溶液中并被微生物还原,从而提高了水淹水稻土溶液中总砷和砷(Ⅲ)的浓度。Yamaguchi等[5]发现,水淹条件下当水稻土的氧化还原电位由+100 mV下降至-68 mV与-75 mV时,溶液中砷的释放量每千克土分别提高了6.9 μmol和19 μmol。Syu等[98]研究发现砷含量为343.3 mg kg-1的砷污染水稻土经历淹水后土壤溶液砷含量大幅度上升至3 000 μg L-1以上,且伴随着氧化还原电位的急剧下降与铁(Ⅱ)含量的快速上升,而Wu等[99]研究四种不同基因型的水稻在有氧和无氧条件下对砷的累积发现无氧条件下根部的总砷量(147~243 mg kg-1)显著高于有氧条件下根部总砷量(88.8~218 mg kg-1)。Somenahally等[100]研究表明相比厌氧水淹处理,间歇式水淹处理中水稻根际土孔隙水总砷约降低86%,而这与地杆菌、希瓦氏菌和厌氧黏细菌等异化铁还原菌在厌氧水淹条件下丰度更高有关。Zecchin等[101]研究指出水稻根际土在长期持续水淹后Geobacteraceae族的铁还原菌的丰度显著上升并伴随着砷(Ⅴ)从铁矿物中溶出。而Das等[14]进一步研究表明,与不淹水和干湿交替处理相比,水淹厌氧处理下水稻土的铁还原菌丰度可分别高出56.4和6.5个数量级,砷还原菌丰度可分别高出31.8和15.9个数量级,溶解亚铁、砷含量显著更高,且水稻土孔隙水中砷(Ⅲ)占总砷的87.3%~93.6%。这说明水淹厌氧条件不仅驱动铁还原促进砷释放,还可以促进砷的直接还原。其中,携带砷(Ⅴ)还原基因的微生物多为厌氧微生物[7]。大量研究证实水淹缺氧条件下土壤溶液砷以砷(Ⅲ)为主,且砷(Ⅲ)浓度可能随着土壤氧化还原电位的降低与砷浓度的提高而提高[14]。Ohtsuka等[102]研究了水稻土中与异化砷呼吸还原酶(arrA)有关的砷还原菌的活动,结合X射线吸收近边结构(X-ray absorption near edge structure,XANES)分析表征矿物相,发现地杆菌Geobacter OR-1能还原土壤溶液中的砷与铁矿物表面的砷使土壤固相砷(Ⅲ)浓度显著增大,且往灭菌实验土中接种该菌种时土浆上清液中的砷(Ⅲ)浓度(904 nmol L-1)远大于未接种该菌种时的浓度(78 nmol L-1)。Tian等[103]研究指出了厌氧环境对促进微生物砷还原基因arrC与arrA的表达的重要性。Huang等[12]研究了Shewanella putrefaciens strain CN-32在一系列铁矿物表面的砷还原活动并指出,水淹厌氧条件下矿物表面的砷直接还原与溶液相中的砷还原均是砷还原的重要途径,而矿物吸附相砷的还原对其解吸和溶液相砷浓度的提高有重要意义。相反,水稻土落干-复氧有利于氧化铁矿物的形成与抑制砷释放还原。Zecchin等[101]的研究同样表明有氧种植或在水稻开花两周前排水与淹水种植相比土壤溶液中溶解亚铁和砷(Ⅲ)的浓度显著更低。Das等[14]研究发现落干管理相比于干湿交替管理与水淹管理能使水稻土中具有更高比例的无定型铁矿物结合态砷。进一步研究表明,水淹厌氧水稻土受根尖泌氧的影响,水稻根部铁圈内部复氧更快,主要形成一些无定型铁矿物以及水铁矿、针铁矿,吸附砷(Ⅴ)为主,而铁圈外部复氧速率较慢,主要形成纤铁矿,且主要吸附砷(Ⅲ);一般而言,复氧较快时铁(Ⅱ)氧化易生成水铁矿、针铁矿,较慢时则易生成纤铁矿[104]。尽管次生矿物的生成对固定砷、抑制砷还原的具体影响尚需进一步评估,这些研究也说明了水淹厌氧条件对驱动砷释放还原起关键作用。
能同时参与铁(Ⅲ)还原与砷(Ⅴ)还原的主要微生物及其特点见表 4。
|
|
表 4 水淹厌氧条件下与水稻土铁砷还原有关的主要微生物[17,58,68,105] Table 4 Main microbes involved in iron and arsenic reduction in flooded anaerobic paddy soil[17,58,68,105] |
水淹厌氧条件也有利于水稻土中砷的甲基化,这与较低的Eh有利于砷甲基化微生物进行砷甲基化有关[92, 95-97],且砷(Ⅲ)可能作为砷甲基化的“基质”,Huang等[91]往水淹水稻土中添加三叶草与酒糟等有机质,发现厌氧环境的形成伴随着水稻土中arsC基因丰度、砷(Ⅲ)浓度的上升,甲基化砷的量的增大,其中投入酒糟时甲基化砷挥发量可高达每月9.8 μg kg-1土,较不投加时高出100倍以上。Zhang等[11]进一步研究表明水淹厌氧条件下砷还原基因arsC和砷甲基化基因arsM的丰度均不断提高,且两者呈显著正相关(R2=0.90,p<0.05);这与微生物中砷(Ⅴ)的还原产物砷(Ⅲ)可能作为砷甲基化反应所需的“基质”,arsM基因常与抗砷基因arsC基因相邻,arsM基因控制的砷甲基化活动有助于微生物减轻砷对自身毒性是相对应的[105]。Huang等[105]研究一株从砷污染水稻土中分离出来的SM-1菌株,发现其在水淹厌氧条件下能进行高效甲基化作用的原因在于其细胞排出砷(Ⅲ)的能力较弱,并且它的砷甲基化基因ArarsM与砷(Ⅲ)具有高度响应性,这进一步证明了水淹厌氧条件有利于砷的甲基化。水稻种植经历的水淹阶段强化了砷的甲基化,因而水稻能吸收更多甲基化砷且大米中常能检测到甲基化砷,尤其是DMA与MMA[106-107]。Jia等[108]研究则进一步指出,水稻土中的甲基化砷的挥发量远大于水稻吸收并挥发出来的量。
4 展望砷污染水稻土中砷的氧化、还原和甲基化过程十分常见,砷的归趋与砷代谢微生物所介导的砷生物化学作用密切相关。水淹厌氧环境可以显著改变水稻土中功能微生物群落而进一步影响砷的生物化学行为和砷的归趋。当前大量的研究集中于探索水淹厌氧条件下水稻土中铁的氧化还原对砷的环境化学行为影响,不同水分管理方式对水稻土砷代谢微生物的影响以及功能微生物对砷的生物化学行为的作用机制和砷的生物化学行为与水稻砷累积的响应关系等方面,基于本文对水稻土砷生物化学行为的综述,认为未来的研究应着眼于:
1)水淹厌氧条件下水稻土中铁还原微生物、砷还原微生物和砷甲基化微生物共同影响着砷的生物化学过程,有机质能活化砷代谢微生物。目前关于不同类型的有机质对砷代谢微生物多样性的调节作用尚不清楚。理清有机质和砷生物化学作用需要回答以下问题:①不同类型有机质对异化铁还原、砷还原与甲基化的生物化学作用机制;②不同类型有机质与砷的物理化学交互作用;③有机质-铁矿物-砷三者之间存在螯合、竞争和耦合作用,三者之间在不同铁矿物、不同有机质类型和不同环境条件下的相互作用关系。
2)目前已有大量研究探讨了与砷的氧化、还原及甲基化有关的酶的作用,深入研究这些酶在砷污染土壤环境中的动力学行为,有助于更深入地理解不同砷代谢微生物及其氧化还原和甲基化等生物化学过程对不同环境条件的响应,一定程度上有利于降低水稻对砷的富集累积而取到减毒脱毒的目的。这方面研究需要进一步明确以下问题:①水稻土砷代谢微生物相关酶在不同微环境下的响应;②水稻土砷代谢微生物相关酶与砷的迁移转化和归趋的关联性;③有机质与砷甲基化微生物相关酶两者的关系。
3)施加氮肥和有机肥等对砷生物化学行为的进一步影响和砷的归趋问题有待研究:①水稻土碳循环对铁循环与砷生物化学行为的影响;②水稻土氮循环对铁循环和砷生物化学过程的影响,尤其是Feammox过程对砷的环境化学行为影响;③氮循环或者碳循环耦合铁或者砷氧化还原以及竞争关系尚需探究,如异化铁还原与铁轮反应之间的竞争关系。
4)水淹条件是放大砷的生物化学作用的关键环节。相比于长期水淹管理,点喷式和间歇式水淹处理的水管理模式是被证实能显著地降低水稻对水稻土砷吸收累积的有效办法。目前基于水淹-落干与长期水淹、水淹-落干与点喷式管理、水稻根际土微氧环境与非根际土等三个截然相反的条件下,界面微环境中砷功能微生物群落变化以及相应地砷的生物化学行为的改变对土壤界面与土壤溶液砷的动态影响的研究尚不系统。
| [1] |
Martinez V D, Vucic E A, Becker-Santos D D, et al. Arsenic exposure and the induction of human cancers . Journal of Toxicology, 2011(5): 1-13.
(  0) 0) |
| [2] |
Vega L, Styblo M, Patterson R, et al. Differential effects of trivalent and pentavalent arsenicals on cell proliferation and cytokine secretion in normal human epidermal keratinocytes . Toxicology and Applied Pharmacology, 2001, 172: 225-232. DOI:10.1006/taap.2001.9152
(  0) 0) |
| [3] |
Liu C P, Luo C L, Gao Y, et al. Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern China . Environmental Pollution, 2010, 158(3): 820-826. DOI:10.1016/j.envpol.2009.09.029
(  0) 0) |
| [4] |
Stroud J L, Norton G J, Islam M R, et al. The dynamics of arsenic in four paddy fields in the Bengal Delta . Environmental Pollution, 2011, 159(4): 947-953. DOI:10.1016/j.envpol.2010.12.016
(  0) 0) |
| [5] |
Yamaguchi N, Nakamura T, Dong D, et al. Arsenic release from flooded paddy soils is influenced by speciation, Eh, pH, and iron dissolution . Chemosphere, 2011, 83(7): 925-932. DOI:10.1016/j.chemosphere.2011.02.044
(  0) 0) |
| [6] |
Weber F A, Hofacker A F, Voegelin A, et al. Temperature dependence and coupling of iron and arsenic reduction and release during flooding of a contaminated soil . Environmental Science & Technology, 2010, 44(1): 116-122.
(  0) 0) |
| [7] |
Jia Y, Huang H, Chen Z, et al. Arsenic uptake by rice is influenced by microbe-mediated arsenic redox changes in the rhizosphere . Environmental Science & Technology, 2014, 48(2): 1001-1007.
(  0) 0) |
| [8] |
Bennett W W, Teasdale P R, Panther J G, et al. Investigating arsenic speciation and mobilization in sediments with DGT and DET:A mesocosm evaluation of oxic-anoxic transitions . Environmental Science & Technology, 2012, 46(7): 3981-3989.
(  0) 0) |
| [9] |
Amaral D C, Lopes G, Guilherme L R G, et al. A new approach to sampling intact Fe plaque reveals Si-induced changes in Fe mineral composition and shoot as in rice . Environmental Science & Technology, 2017, 51(1): 38-45.
(  0) 0) |
| [10] |
Hu M, Li F B, Liu C P, et al. The diversity and abundance of As (Ⅲ) oxidizers on root iron plaque is critical for arsenic bioavailability to rice . Scientific Reports, 2015, 5: 13611. DOI:10.1038/srep13611
(  0) 0) |
| [11] |
Zhang S Y, Zhao F J, Sun G X, et al. Diversity and abundance of arsenic biotransformation genes in paddy soils from southern China . Environmental Science & Technology, 2015, 49(7): 4138-4146.
(  0) 0) |
| [12] |
Huang J H, Voegelin A, Pombo S A, et al. Influence of arsenate adsorption to ferrihydrite, goethite, and boehmite on the kinetics of arsenate reduction by Shewanella putrefaciens strain CN-32 . Environmental Science & Technology, 2011, 45(18): 7701-7709.
(  0) 0) |
| [13] |
Xiao K Q, Li L G, Ma L P, et al. Metagenomic analysis revealed highly diverse microbial arsenic metabolism genes in paddy soils with low-arsenic contents . Environmental Pollution, 2015, 211: 1-8.
(  0) 0) |
| [14] |
Das S, Chou M L, Jean J S, et al. Water management impacts on arsenic behavior and rhizosphere bacterial communities and activities in a rice agro-ecosystem . Science of the Total Environment, 2015, 542: 642-652.
(  0) 0) |
| [15] |
Jia Y, Huang H, Zhong M, et al. Microbial arsenic methylation in soil and rice rhizosphere . Environmental Science & Technology, 2013, 47(7): 3141-3148.
(  0) 0) |
| [16] |
Kumari N, Jagadevan S. Genetic identification of arsenate reductase and arsenite oxidase in redox transformations carried out by arsenic metabolising prokaryotes:A comprehensive review . Chemosphere, 2016, 163: 400-412. DOI:10.1016/j.chemosphere.2016.08.044
(  0) 0) |
| [17] |
朱超, StefanRatering, 曲东, 等. 短期淹水培养对水稻土中地杆菌和厌氧粘细菌丰度的影响. 生态学报, 2011, 31(15): 4251-4260. Zhu C, Ratering S, Qu D, et al. Effects of short-term flooding on Geobacteraceae spp. and Anaeromyxobacter spp. abundance in paddy soil (In Chinese). Acta Ecologica Sinica, 2011, 31(15): 4251-4260. (  0) 0) |
| [18] |
陈倩, 苏建强, 叶军, 等. 微生物砷还原机制的研究进展. 生态毒理学报, 2011, 6(3): 225-233. Chen Q, Su J Q, Ye J, et al. Advances in mechanisms of microbial arsenate reduction (In Chinese). Asian Journal of Ecotoxicology, 2011, 6(3): 225-233. (  0) 0) |
| [19] |
Zhang J, Zhou W X, Liu B B, et al. Anaerobic arsenite oxidation by an autotrophic arsenite-oxidizing bacterium from an arsenic-contaminated paddy soil . Environmental Science & Technology, 2015, 49(10): 5956-5964.
(  0) 0) |
| [20] |
Drewniak L, Sklodowska A. Arsenic-transforming microbes and their role in biomining processes . Environmental Science and Pollution Research, 2013, 20: 7728-7739. DOI:10.1007/s11356-012-1449-0
(  0) 0) |
| [21] |
Slyemi D, Bonnefoy V. How prokaryotes deal with arsenic . Environmental Microbiology Reports, 2012, 4(6): 571-586.
(  0) 0) |
| [22] |
Silver S, Phung L T. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic . Applied and Environmental Microbiology, 2005, 71(2): 599-608. DOI:10.1128/AEM.71.2.599-608.2005
(  0) 0) |
| [23] |
Chang J S, Ren X H, Kyoung-Woong K. Biogeoc-hemical cyclic activity of bacterial arsB in arsenic-contaminated mines . Journal of Environmental Sciences, 2008, 20(11): 1348-1355. DOI:10.1016/S1001-0742(08)62232-9
(  0) 0) |
| [24] |
韩永和, 王珊珊. 微生物耐砷机理及其在砷地球化学循环中的作用. 微生物学报, 2016, 56(6): 901-910. Han Y H, Wang S S. Arsenic resistance mechanisms in microbes and their roles in arsenic geochemical cycling-A review (In Chinese). Acta Microbiologica Sinica, 2016, 56(6): 901-910. (  0) 0) |
| [25] |
Saltikov C W, Wildman R A Jr, Newman D K. Expression dynamics of arsenic respiration and detoxification in Shewanella sp. Strain ANA-3 . Journal of Bacteriology, 2005, 187: 7390-7396. DOI:10.1128/JB.187.21.7390-7396.2005
(  0) 0) |
| [26] |
Matsumoto S, Kasuga J, Makino T, et al. Evaluation of the effects of application of iron materials on the accumulation and speciation of arsenic in rice grain grown on uncontaminated soil with relatively high levels of arsenic . Environmental and Experimental Botany, 2016, 125: 42-51. DOI:10.1016/j.envexpbot.2016.02.002
(  0) 0) |
| [27] |
Zhang Z N, Yin N Y, Du H L, et al. The fate of arsenic adsorbed on iron oxides in the presence of arsenite oxidizing bacteria . Chemosphere, 2016, 151: 108-115. DOI:10.1016/j.chemosphere.2016.02.065
(  0) 0) |
| [28] |
Hartley W, Lepp N W. Remediation of arsenic contaminated soils by iron-oxide application, evaluated in terms of plant productivity, arsenic and phytotoxic metal uptake . Science of the Total Environment, 2008, 390: 35-44. DOI:10.1016/j.scitotenv.2007.09.021
(  0) 0) |
| [29] |
钟松雄, 尹光彩, 何宏飞, 等. 不同铁矿物对水稻土砷的稳定化效果及机制. 环境科学学报, 2017, 37(5): 1931-1938. Zhong S X, Yin G C, He H F, et al. Stabilization effect of arsenic by different iron minerals in paddy soils and the related mechanism (In Chinese). Acta Scientiae Circumstantiae, 2017, 37(5): 1931-1938. (  0) 0) |
| [30] |
Mamindy-Pajany Y, Hurel C, Marmier N, et al. Arsenic (Ⅴ) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron:Effects of pH, concentration and reversibility . Desalination, 2011, 281(20): 93-99.
(  0) 0) |
| [31] |
王强, 卜锦春, 魏世强, 等. 赤铁矿对砷的吸附解吸及氧化特征. 环境科学学报, 2008, 28(8): 1612-1617. Wang Q, Bu J C, Wei S Q, et al. Characteristics of isothermal adsorption and desorption, and oxidation of As (Ⅲ) ion on a hematite surface (In Chinese). Acta Scientise Circumstantise, 2008, 28(8): 1612-1617. (  0) 0) |
| [32] |
Zobrist J, Dowdle P R, Davis J A, et al. Mobilization of arsenite by dissimilatory reduction of adsorbed arsenate . Environmental Science & Technology, 2000, 34(22): 4747-4753.
(  0) 0) |
| [33] |
Jones C A, Langner H W, Anderson K, et al. Rates of microbially mediated arsenate reduction and solubilization . Soil Science Society of America Journal, 2000, 64: 600-608. DOI:10.2136/sssaj2000.642600x
(  0) 0) |
| [34] |
Daniel S, Stephan S, Ralf C. Identification of rice root associated nitrate, sulfate and ferric iron reducing bacteria during root decomposition . FEMS Microbiology Ecology, 2004, 50(2): 101-110. DOI:10.1016/j.femsec.2004.06.001
(  0) 0) |
| [35] |
Zhang Z N, Yin N Y, Du H L, et al. The fate of arsenic adsorbed on iron oxides in the presence of arsenite-oxidizing bacteria . Chemosphere, 2016, 151: 108-115. DOI:10.1016/j.chemosphere.2016.02.065
(  0) 0) |
| [36] |
Bolanz R M, Wierzbicka-Wieczorek M, Čaplovičová M, et al. Structural incorporation of As5+ into hematite . Environmental Science & Technology, 2013, 47: 9140-9147.
(  0) 0) |
| [37] |
Palumbo-Roe B, Wragg J, Cave M. Linking selective chemical extraction of iron oxyhydroxides to arsenic bioaccessibility in soil . Environmental Pollution, 2015, 207: 256-265. DOI:10.1016/j.envpol.2015.09.026
(  0) 0) |
| [38] |
Liu C P, Yu H Y, Liu C S, et al. Arsenic availability in rice from a mining area:Is amorphous iron oxide-bound arsenic a source or sink? . Environmental Pollution, 2015, 199: 95-101. DOI:10.1016/j.envpol.2015.01.025
(  0) 0) |
| [39] |
Honma T, Ohba H, Kaneko-Kadokura A, et al. Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains . Environmental Science & Technology, 2016, 50(8): 4178-4185.
(  0) 0) |
| [40] |
Cutting R S, Coker V S, Telling N D, et al. Microbial reduction of arsenic-doped schwertmannite by Geobacter sulfurreducens . Environmental Science & Technology, 2012, 46(22): 12591-12599.
(  0) 0) |
| [41] |
Burton E D, Johnston S G, Kraal P, et al. Sulfate availability drives divergent evolution of arsenic speciation during microbially mediated reductive transformation of schwertmannite . Environmental Science & Technology, 2013, 47(5): 2221-2229.
(  0) 0) |
| [42] |
Rawson J, Prommer H, Siade A, et al. Numerical modeling of arsenic mobility during reductive iron mineral transformations . Environmental Science & Technology, 2016, 50: 2459-2467.
(  0) 0) |
| [43] |
Jiang S H, Lee J H, Kim D, et al. Differential arsenic mobilization from As-bearing ferrihydrite by iron-respiring Shewanella strains with different arsenic-reducing activities . Environmental Science & Technology, 2013, 47(15): 8616-8623.
(  0) 0) |
| [44] |
Muehe E M, Morin G, Scheer L, et al. Arsenic (Ⅴ) incorporation in vivianite during microbial reduction of arsenic (Ⅴ) -bearing biogenic Fe (Ⅲ) (oxyhydr) oxides . Environmental Science & Technology, 2016, 50(5): 2281-2291.
(  0) 0) |
| [45] |
Zhang J, Zhao S C, Xu Y, et al. Nitrate stimulates anaerobic microbial arsenite oxidation in paddy soils . Environmental Science & Technology, 2017, 51(8): 4377-4386.
(  0) 0) |
| [46] |
Zhu Y G, Xue X M, Kappler A, et al. Linking genes to microbial biogeochemical cycling:Lessons from arsenic . Environmental Science & Technology, 2017, 51(3): 7326-7339.
(  0) 0) |
| [47] |
Li X M, Zhang W, Liu T X, et al. Changes in the composition and diversity of microbial communities during anaerobic nitrate reduction and Fe (Ⅱ) oxidation at circumneutral pH in paddy soil . Soil Biology & Biochemistry, 2016, 94: 70-79.
(  0) 0) |
| [48] |
Yu H Y, Li F B, Liu C S. Chapter Five-Iron redox cycling coupled to transformation and immobilization of heavy metals:Implications for paddy rice safety in the red soil of south China . Advances in Agronomy, 2016, 137: 279-317. DOI:10.1016/bs.agron.2015.12.006
(  0) 0) |
| [49] |
Wang M L, Hu R G, Zhao J S, et al. Iron oxidation affects nitrous oxide emissions via donating electrons to denitrification in paddy soils . Geoderma, 2016, 271: 173-180. DOI:10.1016/j.geoderma.2016.02.022
(  0) 0) |
| [50] |
Clement J C, Shrestha J, Ehrenfeld J G, et al. Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils . Soil Biology & Biochemistry, 2005, 37(12): 2323-2328.
(  0) 0) |
| [51] |
Ding L J, An X L, Li S, et al. Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from paddy soils in a chronosequence . Environmental Science & Technology, 2014, 48(18): 10641-10647.
(  0) 0) |
| [52] |
Li X F, Hou L J, Liu M, et al. Evidence of nitrogen loss from anaerobic ammonium oxidation coupled with ferric iron reduction in an intertidal wetland . Environmental Science & Technology, 2015, 49(19): 11560-11568.
(  0) 0) |
| [53] |
Zhou G W, Yang X R, Li H, et al. Electron shuttles enhance anaerobic ammonium oxidation coupled to iron (Ⅲ) reduction . Environmental Science & Technology, 2016, 50(17): 9298-9307.
(  0) 0) |
| [54] |
Wang N, Xue X M, Juhasz A L, et al. Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic . Environmental Pollution, 2017, 220: 514-522. DOI:10.1016/j.envpol.2016.09.095
(  0) 0) |
| [55] |
Vaxevanidou K, Giannikou S, Papassiopi N. Microbial arsenic reduction in polluted and unpolluted soils from Attica, Greece . Journal of Hazardous Materials, 2012, 241(1): 307-315.
(  0) 0) |
| [56] |
Ma J, Guo H M, Lei M, et al. Arsenic adsorption and its fractions on aquifer sediment:Effect of pH, arsenic species, and iron/manganese minerals . Water, Air, & Soil Pollution, 2015, 226(8): 1-15.
(  0) 0) |
| [57] |
Haynes R J. Labile organic matter as an indicator of organic matter quality in arable and pastoral soils in New Zealand . Soil Biology & Biochemistry, 2000, 32(2): 211-219.
(  0) 0) |
| [58] |
Katsumi N, Yonebayashi K, Okazaki M, et al. Characterization of soil organic matter with different degrees of humification using evolved gas analysis-mass spectrometry . Talanta, 2016, 155: 28-37. DOI:10.1016/j.talanta.2016.04.007
(  0) 0) |
| [59] |
Sahoo P K, Kim K. A review of the arsenic concentration in paddy rice from the perspective of geoscience . Geosciences Journal, 2013, 17(1): 107-122. DOI:10.1007/s12303-013-0004-4
(  0) 0) |
| [60] |
Chen Z, Wang Y P, Xia D, et al. Enhanced bioreduction of iron and arsenic in sediment by biochar amendment influencing microbial community composition and dissolved organic matter content and composition . Journal of Hazardous Materials, 2016, 311: 20-29. DOI:10.1016/j.jhazmat.2016.02.069
(  0) 0) |
| [61] |
Mikutta C, Kretzschmar R. Spectroscopic evidence for ternary complex formation between arsenate and ferric iron complexes of humic substances . Environmental Science & Technology, 2011, 45(22): 9550-9557.
(  0) 0) |
| [62] |
Catrouillet C, Davranche M, Dia A, et al. Does As (Ⅲ) interact with Fe (Ⅱ) , Fe (Ⅲ) and organic matter through ternary complexes? . Journal of Colloid and Interface Science, 2016, 470: 153-161. DOI:10.1016/j.jcis.2016.02.047
(  0) 0) |
| [63] |
Anawar H M, Tareq S M, Ahmed G.. Is organic matter a source or redox driver or both for arsenic release in groundwater? . Physics and Chemistry of the Earth, 2013, 58/60(6): 49-56.
(  0) 0) |
| [64] |
Chen L X. The research on adsorption and desorption of natural organic matter on hematite. Beijing: China University of Geosciences, 2013
(  0) 0) |
| [65] |
Lü J T, Zhang S Z, Wang S S, et al. Molecular-scale investigation with ESI-FT-ICR-MS on fractionation of dissolved organic matter induced by adsorption on iron oxyhydroxides . Environmental Science & Technology, 2016, 50(5): 2328-2336.
(  0) 0) |
| [66] |
Neumann R B, Pracht L E, Polizzotto M L, et al. Biodegradable organic carbon in sediments of an arsenic contaminated aquifer in Bangladesh . Environmental Science & Technology Letter, 2014, 1(4): 221-225.
(  0) 0) |
| [67] |
Redman A, Macalady D L, Ahmann D. Natural organic matter affects arsenic speciation and sorption onto hematite . Environmental Science & Technology, 2002, 36(13): 2889-2896.
(  0) 0) |
| [68] |
黎慧娟, 彭静静. 异化Fe (Ⅲ) 还原微生物研究进展. 生态学报, 2012, 32(5): 1633-1642. Li H J, Peng J J. Recent advances in studies on dissimilatory Fe (Ⅲ) -reducing microorganisms (In Chinese). Acta Ecologica Sinica, 2012, 32(5): 1633-1642. (  0) 0) |
| [69] |
Jiang J, Bauer I, Paul A, et al. Arsenic redox changes by microbially and chemically formed semiquinone radicals and hydroquinones in a humic substance model quinone . Environmental Science & Technology, 2009, 43(10): 3639-3645.
(  0) 0) |
| [70] |
陈倩, 苏建强, 叶军. 土壤中耐砷细菌的筛选和砷还原基因多样性分析. 生态环境学报, 2011, 20(12): 1919-1926. Chen Q, Su J Q, Ye J. Isolation of arsenic-resistant bacterias from soil and the diversity of arsenate reducing genes (In Chinese). Ecology and Environmental Sciences, 2011, 20(12): 1919-1926. DOI:10.3969/j.issn.1674-5906.2011.12.024 (  0) 0) |
| [71] |
Zhao F J, Harris E, Yan J, et al. Arsenic methylation in soils and its relationship with microbial arsM abundance and diversity, and As speciation in rice . Environmental Science & Technology, 2013, 47(13): 7147-7154.
(  0) 0) |
| [72] |
Zhang S Y, Rensing C, Zhu Y G. Cyanobacteria-mediated arsenic redox dynamics is regulated by phosphate in aquatic environments . Environmental Science & Technology, 2014, 48(2): 994-1000.
(  0) 0) |
| [73] |
Rhine E D, Ní Chadhain S M, Zylstra G J, et al. The arsenite oxidase genes (aroAB) in novel chemoautotrophic arsenite oxidizers . Biochemical and Biophysical Research Communications, 2007, 354(3): 662-667. DOI:10.1016/j.bbrc.2007.01.004
(  0) 0) |
| [74] |
Zargar K, Hoeft S, Oremland R, et al. Identification of a novel arsenite oxidase gene, arxA, in the haloalka-liphilic, arsenite-oxidizing bacterium Alkalilimnicola ehrlichii strain MLHE-1 . Journal of Bacteriology, 2010, 192(14): 3755-3762. DOI:10.1128/JB.00244-10
(  0) 0) |
| [75] |
Rhine E D, Garcia-Dominguez E, Phelps C D, et al. Environmental microbes can speciate and cycle arsenic . Environmental Science & Technology, 2005, 39(24): 9569-9573.
(  0) 0) |
| [76] |
Williams P N, Santner J, Larsen M, et al. Localized flux maxima of arsenic, lead, and iron around root apices in flooded lowland rice . Environmental Science & Technology, 2014, 48(15): 8498-8506.
(  0) 0) |
| [77] |
Colmer T D. Long-distance transport of gases in plants:A perspective on internal aeration and radial oxygen loss from roots . Plant, Cell and Environment, 2003, 26(1): 17-36. DOI:10.1046/j.1365-3040.2003.00846.x
(  0) 0) |
| [78] |
Sun W, Sierraalvarez R, Milner L, et al. Arsenite and ferrous iron oxidation linked to chemolithotrophic denitrification for the immobilization of arsenic in anoxic environments . Environmental Science & Technology, 2009, 43(17): 6585-6591.
(  0) 0) |
| [79] |
Rhine E D, Phelps C D, Young L Y. Anaerobic arsenite oxidation by novel denitrifying isolates . Environmental Microbiology, 2006, 8(5): 899-908. DOI:10.1111/emi.2006.8.issue-5
(  0) 0) |
| [80] |
Wang P P, Sun G X, Jia Y, et al. A review on completing arsenic biogeochemical cycle:Microbial volatilization of arsines in environment . Journal of Environmental Sciences, 2014, 26: 371-381. DOI:10.1016/S1001-0742(13)60432-5
(  0) 0) |
| [81] |
Bae M J, Li F Q, Kwon Y S, et al. Concordance of diatom, macroinvertebrate and fish assemblages in streams at nested spatial scales:Implications for ecological integrity . Ecological Indicators, 2014, 47: 89-101. DOI:10.1016/j.ecolind.2014.07.030
(  0) 0) |
| [82] |
Schwarz M V J, Frenzel P. Population dynamics and ecology of ciliates (Protozoa, Ciliophora) in an anoxic rice field soil . Biology and Fertility of Soils, 2003, 38(4): 245-252. DOI:10.1007/s00374-003-0644-z
(  0) 0) |
| [83] |
Zheng R L, Sun G X, Zhu Y G. Effects of microbial processes on the fate of arsenic in paddy soil . Science Bulletin, 2013, 58(2): 186-193. DOI:10.1007/s11434-012-5489-0
(  0) 0) |
| [84] |
Cullen W R. Chemical mechanism of arsenic biomethylation . Chemical Research in Toxicology, 2014, 27(4): 457-461. DOI:10.1021/tx400441h
(  0) 0) |
| [85] |
Kruger M C, Bertin P N, Heipieper H J, et al. Bacterial metabolism of environmental arsenic-mechanisms and biotechnological applications . Applied Microbiology and Biotechnology, 2013, 97: 3827-3841. DOI:10.1007/s00253-013-4838-5
(  0) 0) |
| [86] |
Meng X Y, Qin J, Wang L H, et al. Arsenic biotransformation and volatilization in transgenic rice . New Phytologist, 2011, 191(1): 49-56. DOI:10.1111/j.1469-8137.2011.03743.x
(  0) 0) |
| [87] |
Song X L, Geng Z R, Zhu J S, et al. Structure-function roles of four cysteine residues in the human arsenic (+3 oxidation state) methyltransferase (hAs3MT) by site-directed mutagenesis . Chemico-Biological Interactions, 2009, 179(2/3): 321-328.
(  0) 0) |
| [88] |
Stolz J F, Basu P, Santini J M, et al. Arsenic and selenium in microbial metabolism . Annual Review of Microbiology, 2006, 60(1): 107-130. DOI:10.1146/annurev.micro.60.080805.142053
(  0) 0) |
| [89] |
Zhao F J, Zhu Y G, Meharg A A. Methylated arsenic species in rice:Geographical variation, origin, and uptake mechanisms . Environmental Science & Technology, 2013, 47(9): 3957-3966.
(  0) 0) |
| [90] |
Li H J, Peng J J, Weber K A, et al. Phylogenetic diversity of Fe (Ⅲ) -reducing microorganisms in rice paddy soil:Enrichment cultures with different short-chain fatty acids as electron donors . Soils Sediments, 2011, 11(7): 1234-1242. DOI:10.1007/s11368-011-0371-2
(  0) 0) |
| [91] |
Huang H, Jia Y, Sun G X, et al. Arsenic speciation and volatilization from flooded paddy soils amended with different organic matters . Environmental Science & Technology, 2012, 46(4): 2163-2168.
(  0) 0) |
| [92] |
Bentley R, Chasteen T G. Microbial methylation of metalloids:arsenic, antimony, and bismuth . Microbiology and Molecular Biology Reviews, 2002, 66(2): 250-271. DOI:10.1128/MMBR.66.2.250-271.2002
(  0) 0) |
| [93] |
吴剑, 杨柳燕, 肖琳, 等. 砷污染土壤生物挥发研究进展. 土壤, 2007, 39(4): 522-527. Wu J, Yang L Y, Xiao L, et al. Bio-volatilization of arsenic from polluted soil (In Chinese). Soils, 2007, 39(4): 522-527. (  0) 0) |
| [94] |
Gao S, Burau R G. Environmental factors affecting rates of arsine evolution from and mineralization of arsenicals in soil . Journal of Environmental Quality, 1997, 26(3): 753-763.
(  0) 0) |
| [95] |
高伟, 郑国砥, 高定, 等. 堆肥处理过程中猪粪有机物的动态变化特征. 环境科学, 2006, 27(5): 986-990. Gao W, Zheng G D, Gao D, et al. Transformation of organic matter during thermophilic composting of pig manure (In Chinese). Environmental Science, 2006, 27(5): 986-990. (  0) 0) |
| [96] |
Wang J, Han T, Si Y B, et al. Biotransformation and methylation of different valence state arsenic by Shewanella oneidensis MR-1 . China Environmental Science, 2015, 35(11): 3396-3402.
(  0) 0) |
| [97] |
Huang J. Impact of microorganisms on arsenic biogeochemistry:A review . Water, Air & Soil Pollution, 2014, 225. DOI:10.1007/s11270-0013-1848-y
(  0) 0) |
| [98] |
Syu C H, Huang C C, Jiang P Y, et al. Arsenic accumulation and speciation in rice grains influenced by arsenic phytotoxicity and rice genotypes grown in arsenic-elevated paddy soils . Journal of Hazardous Materials, 2015, 286: 179-186. DOI:10.1016/j.jhazmat.2014.12.052
(  0) 0) |
| [99] |
Wu C, Huang L, Xue S G, et al. Oxic and anoxic conditions affect arsenic (As) accumulation and arsenite transporter expression in rice . Chemosphere, 2017, 168: 969-975. DOI:10.1016/j.chemosphere.2016.10.114
(  0) 0) |
| [100] |
Somenahally A C, Hollister E B, Yan W G, et al. Water management impacts on arsenic speciation and iron-reducing bacteria in contrasting rice-rhizosphere compartments . Environmental Science & Technology, 2011, 45(19): 8328-8335.
(  0) 0) |
| [101] |
Zecchin S, Corsini A, Martin M, et al. Rhizospheric iron and arsenic bacteria affected by water regime:Implications for metalloid uptake by rice . Soil Biology & Biochemistry, 2017, 106: 129-137.
(  0) 0) |
| [102] |
Ohtsuka T, Yamaguchi N, Makino T. Arsenic dissolution from Japanese paddy soil by a dissimilatory arsenate-reducing bacterium Geobacter sp. OR-1 . Environmental Science & Technology, 2013, 47(12): 6263-6271.
(  0) 0) |
| [103] |
Tian H X, Shi Q T, Jing C Y. Arsenic biotransformation in solid waste residue:Comparison of contributions from bacteria with arsenate and iron reducing pathways . Environmental Science & Technology, 2015, 49(4): 2140-2146.
(  0) 0) |
| [104] |
Yamaguchi N, Ohkura T, Takahashi Y, et al. Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix . Environmental Science & Technology, 2014, 48(3): 1549-1556.
(  0) 0) |
| [105] |
Huang K, Chen C, Zhang J, et al. Efficient arsenic methylation and volatilization mediated by a novel bacterium from an arsenic-contaminated paddy soil . Environmental Science & Technology, 2016, 50(12): 6389-6396.
(  0) 0) |
| [106] |
Zavala Y J, Gerads R, Gorleyok H, et al. Arsenic in rice:Ⅱ. Arsenic speciation in USA grain and implications for human health . Environmental Science & Technology, 2008, 42(10): 3861-3866.
(  0) 0) |
| [107] |
Ma L, Wang L, Jia Y Y, et al. Arsenic speciation in locally grown rice grains from Hunan Province, China:Spatial distribution and potential health risk . Science of the Total Environment, 2016, , 557/558: 438-444. DOI:10.1016/j.scitotenv.2016.03.051
(  0) 0) |
| [108] |
Jia Y, Huang H, Sun G X, et al. Pathways and relative contributions to arsenic volatilization from rice plants and paddy soil . Environmental Science & Technology, 2012, 46(15): 8090-8096.
(  0) 0) |
2. South China Institute of Environmental Science, Ministry of Environmental Protection of the People's Republic of China, Guangzhou 510655, China;
3. School of Environmental Science and Technology, Guangdong University of Technology, Guangzhou 510006, China;
4. University of Chinese Academy of Sciences, Beijing 100049, China
 2018, Vol. 55
2018, Vol. 55