2. 河南师范大学旅游学院,河南新乡 453007;
3. 广西大学商学院,南宁 530004
土壤是一种包含多种生境和环境条件的生态系统[1],在全球环境变化过程中具有重要作用[2]。近20年,随着我国产业结构调整以及农户追求经济利益最大化等因素的影响,我国南方地区大面积的水田改为果园等经济林。水田改旱作后土壤的理化性质、土壤肥力、土壤微生物发生改变[3],并导致土壤板结[4-6]、土地退化[7-8]。土壤微生物是土壤功能的重要组成部分[9-10],其在分解土壤动植物残体、促进养分生物地球化学循环、改善生态环境等方面发挥着重要作用[9, 11-15]。土壤微生物多样性是反映土壤健康和质量的一个重要指标[5-6, 16],能够影响生态系统的生产力、稳定性,反映生态系统的环境压力[1]。有研究表明,土地利用方式变化后,随着土壤微生物生境的变化,土壤微生物多样性随之发生改变[17-19]。
一些学者对土地利用方式变化后土壤理化性质和基本微生物性质进行了研究[9, 20-22]。然而,很少有学者关注由厌氧环境占优势的水田转变为好氧环境为主的果园后,土壤微生物学性质特别是微生物遗传多样性和群落结构的变化。本研究着重分析了水田改果园后,不同利用年限土壤微生物群落结构和遗传多样性等微生物学性质的变化,并探讨了土壤微生物学性质对土地利用方式转变的响应规律,希望利用土壤微生物的预警作用来监测土壤生态环境的变化,为土壤生态功能维护和实现土壤可持续利用提供理论指导。
1 材料与方法 1.1 研究区域概况研究区位于浙江省嘉兴市南湖区大桥镇江南村,地理坐标位于30°44′47″ N ~30°45′4″ N和120°51′44″ E~120°52′10″ E之间,地处杭嘉湖平原,隶属于浙北平原区,属于中北亚热带季风气候过渡带,多年平均降水量在1 200~2 000 mm之间,年均气温在15.0~17.7℃。土壤成土母质为湖沼相沉积物,土体深度1 m以上,海拔平均为3.0~3.5 m[23]。
1.2 土壤样品采集水田改果园系列土壤样品采集于2012年10月中旬,共采集18个耕层土壤样品(对应18个田块),采集的果园土壤在利用方式转变前均为水田。根据不同土地利用方式和利用年限农地的分布及数量,确定该类型农地中土壤样品的采集数量(由于水田、种植葡萄树13 a和15 a的田块分布较广,因而在该类型农地中采集的土壤样品较多),共采集水田样本4个,果园土壤样本14个(表 1)。依据第二次土壤普查资料,供试土壤在发生分类中属于水稻土土类,脱潜型水稻土亚类,青紫泥田土属,腐心青紫泥田土种[23]。
|
|
表 1 土壤样品信息 Table 1 General information of the soil samples |
水田耕层土壤样品采集时,水稻接近收获期,田面落干。每个耕层土壤样品(0~15 cm)由5个以上采样点混合而成,混匀分成四份,取一份原状土壤沿自然结构面掰开,自然风干,测定土壤水稳定性团聚体;第二份自然风干后,分别过2 mm、1 mm和0.15 mm孔径筛,测定土壤理化性质。另外两份置于放有冰块塑料袋中带回实验室,一份放入4℃冰箱,一周内完成土壤微生物生物量碳和氮的测定;另一份土样冷冻干燥后,放-80℃冰箱保存,用于提取土壤微生物DNA和测定土壤微生物磷脂脂肪酸。
供试水田与果园位置相邻,而两种土地利用方式在管理上存在一定差异,果园土壤较水田施肥量大,灌溉量小;同一利用方式的农地灌溉、施肥等管理方式相似。土壤样品相关信息详见表 1,具体施肥量信息详见表 2。
|
|
表 2 水田和果园土壤施肥量 Table 2 Fertilization rates in the paddy field and orchard |
自然含水量、土壤颜色、>0.25 mm水稳性团聚体、pH、土壤颗粒组成、有机质、全氮、碱解氮、全磷、有效磷、全钾、速效钾等指标的测定步骤和方法详见文献[24-25]。土壤微生物生物量碳氮采用氯仿熏蒸—K2SO4浸提法。微生物遗传多样性测定过程如下:提取冻干土壤中细菌的总DNA,扩增后,取PCR产物上样做变性梯度凝胶电泳(Denaturing gradient gel electrophoresis,DGGE),从DGGE胶上切下电泳条带,染色后用凝胶成像系统(GelDoc XR+,Bio-Rad,美国)拍照。土壤微生物群落结构多样性测定参照微生物鉴定系统(Version 4.5,MIDI Sherlock,美国),对土壤微生物磷脂脂肪酸(Phospholipid fatty acid,PLFA)各成分脂肪酸进行鉴定,生成磷脂脂肪酸图谱[26]。
1.4 数据处理采用Microsoft Excel 2003软件处理数据,Origin 8.0制图。使用SPSS 17.0软件进行差异显著性分析,采用最小显著差异(Least significant deviation,LSD)法;相关性分析采用皮尔森(Pearson)法。采用香农-威那(Shannon-Wiener,SW)多样性指数表征不同土壤样品中微生物的多样性指数,公式如下:
| $ {\rm{SW = - }}\sum {\mathit{Pi}{\rm{ln}}} \mathit{Pi} $ |
式中,Pi为某群落中第i个类型的物种占总物种的百分比。
2 结果与讨论 2.1 水田和果园土壤理化性质本研究中水田改果园系列耕层土壤为壤黏土(国际制土壤质地分类),耕层土壤中黏粒含量在299~325 g kg-1之间,表明供试土壤质地非常相似。供试水田中自然含水量、pH、>0.25 mm水稳性团聚体、有机质、全氮、碱解氮含量整体上高于果园土壤,而全磷、有效磷、全钾、速效钾含量整体上低于果园土壤(表 3)。这与水田改果园后人为滞水水分状况消失、土壤透气性改善、土壤有机质矿化速度加快、氯化钾和过磷酸钙等酸性化肥施用量增加等因素有关。
|
|
表 3 水田和果园耕层土壤理化性质 Table 3 Physiochemical properties of the soils in the cultivated layers of the paddy fields and orchards |
水田耕层土壤微生物生物量碳(Microbial biomass carbon,MBC)平均含量为815.2 mg kg-1,改果园后土壤MBC平均含量降低至455.3 mg kg-1,降幅达44.1%,且其含量与果园年龄呈极显著负相关(图 1)。水田耕层土壤微生物生物量氮(Microbial biomass nitrogen,MBN)平均含量为35.65 mg kg-1,改果园后土壤MBN平含均量降低至14.30 mg kg-1,降幅达59.9%,且其含量与果园年龄呈极显著负相关(图 1)。有研究[27]表明水田改为菜地后土壤MBC、MBN下降40%以上,这与本研究的结论相似。另有研究[28]表明洞庭湖区水田MBC、MBN明显高于油菜地等旱地土壤。
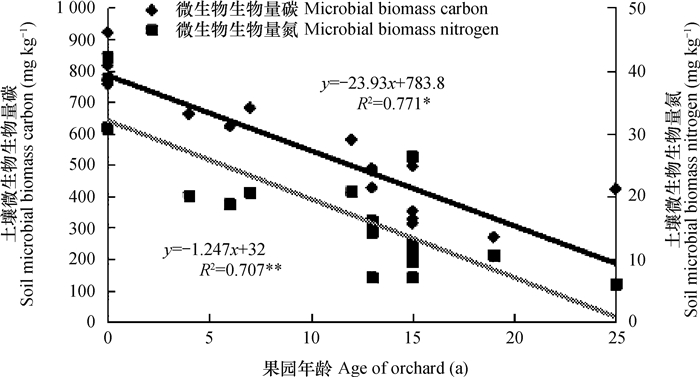
|
图 1 土壤微生物生物量碳氮与果园年龄之间的相关性 Fig. 1 Correlation coefficient of soil microbial biomass carbon and nitrogen with age of orchard |
相关分析表明,耕层土壤MBC与土壤pH、自然含水量呈显著(p<0.05)或极显著(p<0.01)正相关(表 4),但与土壤有机质、全氮等的相关性未达到显著水平(p>0.05)(表 4),这与研究区水田改果园后,施入大量蓖麻饼等有机物料而较难为土壤生物所利用有关,这也表明施用有机肥时应考虑生物有效性问题。耕层土壤MBN与土壤pH、有机质、碱解氮、自然含水量和全氮含量呈显著(p<0.05)或极显著(p<0.01)正相关(表 4)。耕层土壤MBC和MBN与土壤全磷、有效磷、全钾、速效钾均呈极显著负相关(p<0.01)(表 4)。由于养分失衡等原因,虽然改果园后土壤磷素和钾素含量增加,而MBC和MBN依然下降。此外,水田改果园后土壤微生物生物量下降还有以下原因:一方面,稻田土壤为微生物提供了相对充足的有机碳源、氮源和水分等主要营养物质,使得稻田土壤微生物生长旺盛,土壤微生物生物量相对较高;另一方面,水田改果园后土壤酸化,抑制了土壤微生物活性,特别是占土壤微生物多数的细菌活性。
|
|
表 4 土壤理化性质与土壤微生物生物量相关性(n=18) Table 4 Correlation coefficient of soil microbial biomass with soil physicochemical properties(n=18) |
Sparling[29]研究表明,如果土壤被过度使用,土壤微生物生物量碳库将会以较快的速率下降,最终造成微生物商(微生物生物量碳与总有机碳的比值,Microbial biomass carbon/ total organic carbon,MBC/TOC)降低。水田耕层土壤微生物商随着改果园年限的延长而下降,并与改果园年限呈极显著负相关(p<0.01)(图 2),表明本研究中水田改果园后土壤存在过度使用、土壤退化等问题,影响了土壤的可持续发展,同时说明,水田在积累有机碳的同时,也有利于土壤微生物生物量的提高,这与沈冰洁等[30]的结论一致。
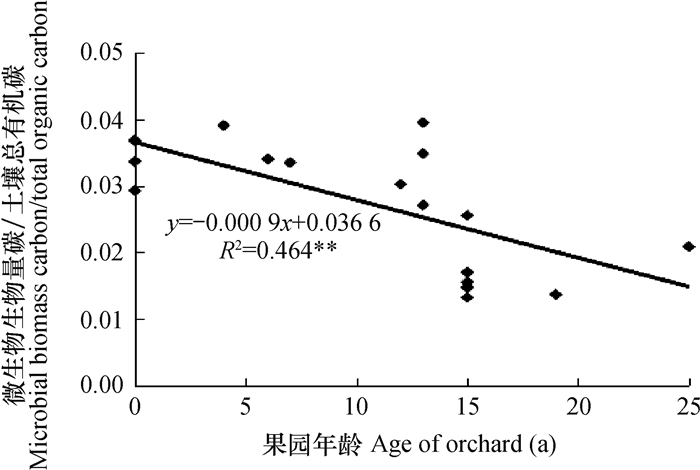
|
图 2 土壤微生物商与果园年龄之间的相关性 Fig. 2 Correlation coefficient of ratios of microbial biomass carbon to total organic carbon with age of orchar |
对土壤总细菌16S rDNA基因片段的DGGE图谱(图 3)分析表明,不同含量的细菌在图谱上形成明暗不同的条带,条带类型和相对亮度表明土壤细菌的种类和相对丰度,各泳道差异表征各样品细菌多样性差异及样品之间亲缘关系。如图 3所示,代表水田样本的泳道(S1~S4)中条带相对于果园土壤的泳道(S5~S18)复杂,且条带较深,这表明水田土壤中微生物种类更加丰富,且含量较高;这与水田土壤中含有较高的水分、有机碳源和氮源等营养物质且氧化还原环境较复杂有关。图 3中,代表水田土壤样本的泳道(S1~S4)中条带比较相似,而代表果园土壤的泳道(S5~S18)中条带较相似,表明同一土地利用方式土壤微生物基因多样性相似度较高。
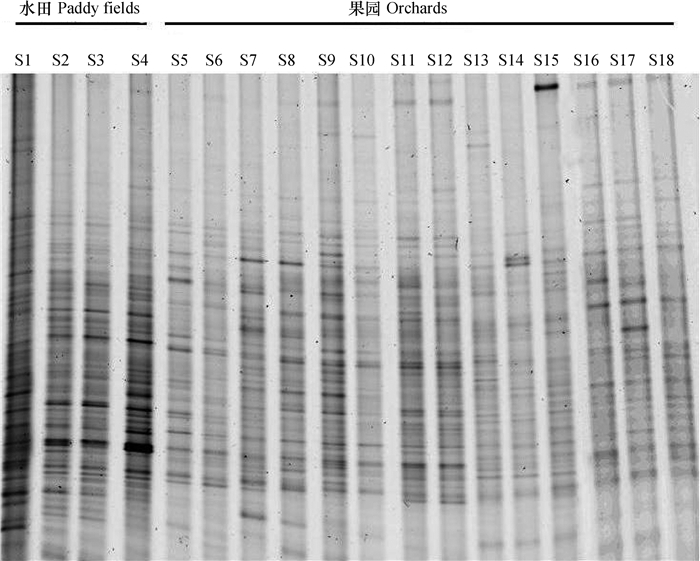
|
图 3 水田和果园土壤总细菌变性梯度凝胶电泳指纹图谱 Fig. 3 Denaturing gradient gel electrophoresis (DGGE) banding patterns of 16S rDNA gene fragments of soil bacteria in the paddy and orchard soils |
对耕层土壤细菌DGGE指纹图谱(图 3)进行聚类分析,结果显示,耕层土壤样品被分为两簇,一簇属于水田土壤,另一簇为果园土壤;而果园土壤又可以分为两簇,分别为短期果园土壤和长期果园土壤(图 4)。水田改果园后,水田和果园土壤之间细菌的基因多样性存在较大差异,水田和果园土壤的相似度为0.46,而短期果园和长期果园土壤中细菌基因条带之间的相似度为0.56(图 4)。以上结果表明,土壤细菌的基因多样性发生了阶段性变化,水田和果园之间的差异性,要大于短期果园土壤与长期果园土壤之间的差异性(图 4),土地利用方式变化(水田改果园)要大于利用年限对土壤细菌基因多样性的影响,这与Xue等[19]关于利用方式和利用年限对微生物群落结构影响的结论一致。
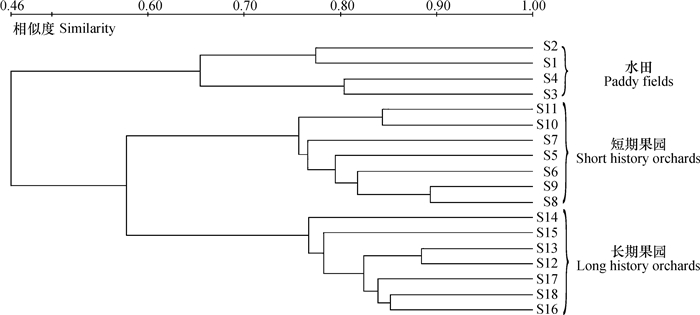
|
图 4 水田和果园土壤总细菌变性梯度凝胶电泳指纹图谱的聚类分析 Fig. 4 Cluster analysis of the figureprints in the DGGE profiles of the soil bacteria in the paddy and orchard soils |
通过对图 3进行多样性分析,发现不同样品的香农-威那多样性指数存在一定的差异性。整体表现为,水田土壤(S1~S4)中细菌的香农-威那多样性指数高于果园土壤(S5~S18)(表 5)。表明水田改果园后,由于土壤水分、有机质和氮素等营养物质减少,氧化还原状况趋于稳定,导致耕层土壤中细菌多样性降低。
|
|
表 5 水田和果园耕层土壤总细菌的香农-威那多样性指数 Table 5 Shannon-Wiener indexes of soil total bacteria in the cultivated soil layer of the paddy fields and orchards |
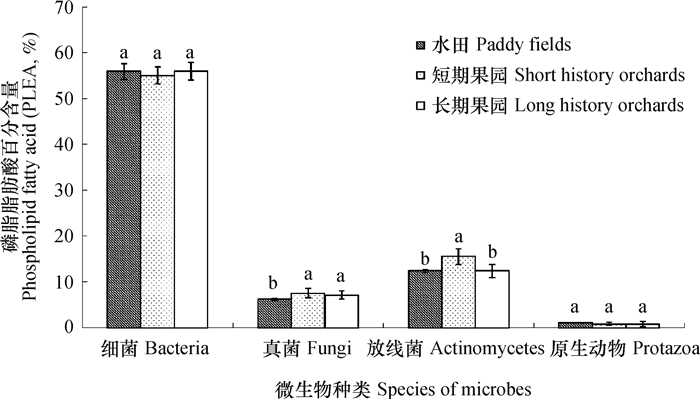
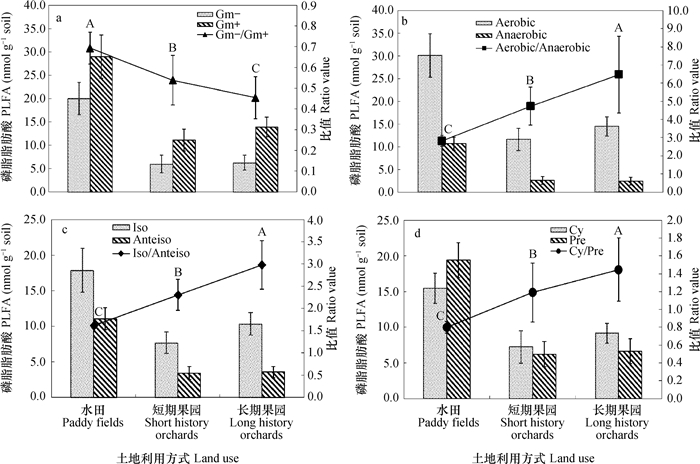
磷脂脂肪酸(PLFA)存在于所有活体细胞膜中,常被作为土壤微生物群落的生物标记[31-32]。本研究中,随着水田改果园年限的延长,耕层土壤微生物主要营养物质(水分、碳源和氮源)下降,在水田中土壤微生物总PLFAs平均含量为145.0 nmol,在果园土壤中其平均含量降低至60.79 nmol,降幅达58.1%;土壤微生物总PLFAs含量与微生物生物量碳呈极显著正相关,相关系数r = 0.74**(n = 18)。在水田土壤样品中平均检测到54种PLFAs,果园土壤样品中平均检测到46种PLFAs,表明水田改果园后土壤微生物PLFAs种类减少。水田改果园后,受土壤通气性增强等因素的影响,耕层土壤中真菌和放线菌在土壤微生物中所占比例增加,细菌和原生动物在土壤微生物中所占比例变化不明显(图 5)。改果园后,革兰氏阴性细菌/革兰氏阳性细菌(Gram negative bacteria/Gram positive bacteria,Gm-/Gm+)显著降低(图 6a);由于土壤通气性增强等因素的影响,耕层土壤中好氧细菌/厌氧细菌(Aerobic bacteria/ Anaerobic bacteria,Aerobic/Anaerobic)显著增加(图 6b),并与改果园年限呈显著正相关,相关系数r = 0.53*(n = 18)。
|
图 5 水田和果园土壤中主要微生物类群相对含量 Fig. 5 Relative abundance of microbial specific groups in paddy and orchard soils 注:同一微生物类群不同英文字母表示差异性显著(p<0.05) Note:Different letters in the same group of microbes mean significant difference at 0.05 level |
|
图 6 水田和果园土壤中不同类型微生物脂肪酸绝对含量和其相对比例 Fig. 6 Absolute abundance and ratio of microbial fatty acid in paddy and orchard soils relative to type of the acid 注:Gm-:革兰氏阴性细菌;Gm+:革兰氏阳性细菌;Aerobic:好氧细菌;Anaerobic:厌氧细菌;Iso:异式脂肪酸;Anteiso:反异支链脂肪酸;Cy:环丙基脂肪酸;Pre:环丙基脂肪酸前体物。同一指标不同英文字母表示差异性显著(p<0.01)。下同 Note:Gm-:Gram negative bacteria; Gm+:Gram positive bacteria; Aerobic:Aerobic bacteria; Anaerobic:Anaerobic bacteria; Iso:Iso fatty acids; Anteiso:Anteiso fatty acids; Cy:Cyclo fatty acids; Pre:Precurso fatty acids. Different letters affixed to the same index mean for significant difference at 0.01 level. The same below |
有研究表明,用异式脂肪酸(Iso fatty acids,Iso)与反异支链脂肪酸(Anteiso fatty acids,Anteiso)的比值可以表征养分胁迫[32]。本研究表明,水田改果园后,耕层土壤表征养分胁迫的Iso /Anteiso显著增加(图 6c),并与改果园年限呈极显著正相关,相关系数r = 0.63**(n = 18)。在饥饿压力下,革兰氏阴性菌会将某些单烯PLFA转化为环丙基脂肪酸,因此,环丙基脂肪酸和前体物的比值(cyclo fatty acids/ precurso fatty acids,Cy/Pre)也可用来指示微生物所处的压力状态[33-34]。改果园后,表征养分胁迫的Cyc/Pre显著增强(图 6d),并与改果园年限呈极显著正相关,相关系数r= 0.61**(n = 18)。以上结果表明,水田改果园后,耕层土壤微生物的养分胁迫增强,引起土壤微生物数量减少,这主要与改果园后自然含水量及碳氮元素含量的降低及氮、磷、钾等营养元素的不均衡有关。
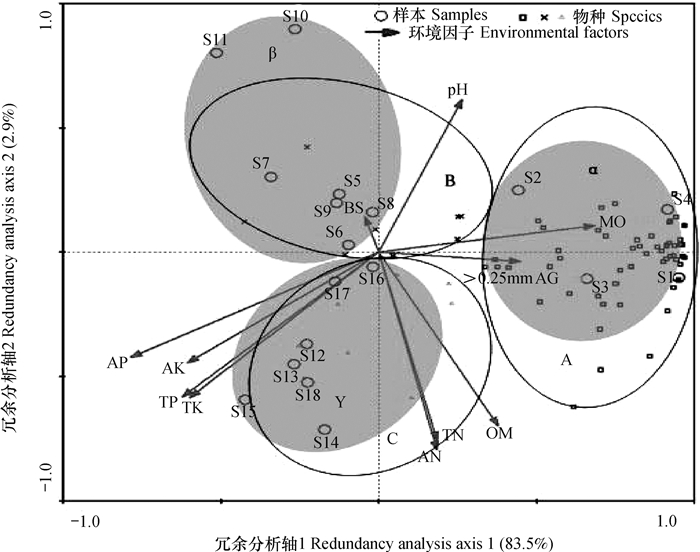
如图 7所示,由于土壤微生物群落结构的差异,水田样本及其最适物种分别集中在α(阴影区)和A区域内,短期果园土壤样本及其最适物种分别集中在β(阴影区)和B区域内,长期果园土壤样本及其最适物种分别集中在γ(阴影区)和C区域内。依据土壤样本在冗余分析排序图中聚集的结果可将果园土壤分为短期果园土壤(S5~S11,≤13 a)和长期果园土壤(S12~S18,>13 a)两个阶段,该分组结果与DGGE指纹条带的聚类分析结果一致(图 4)。图 7所示,>0.25 mm水稳性团聚体和水分含量两种环境因子沿水田样本聚集方向(α区域)增加最明显,说明它们与水田微生物群落关系最密切,即在A区域出现的土壤微生物对水分依赖性较强。有机质和氮素延伸方向位于α和γ区域之间,表明A和C区域土壤微生物对有机质和氮素依赖性较强,这与水田中有机质和氮素含量较高及长期果园土壤施用大量蓖麻饼有一定关系。pH和盐基饱和度在短期果园土壤(β区域)方向延伸,说明这两种环境因子对短期果园土壤微生物影响较大,这与水田改果园后土壤酸化有一定关系。钾素和磷素在长期果园土壤(γ区域)方向延伸,说明磷素和钾素对C区域内土壤微生物影响更明显,这与长期果园土壤中大量施用化肥关系密切。
|
图 7 耕层土壤微生物磷脂脂肪酸冗余分析排序图 Fig. 7 Sequencing graph of soil microorganism phospholipid fatty acids in the plow layer soils in redundancy analysis 注:图中S1~S18为样品号;不同种类脂肪酸在A、B、C三个区域中分别用方形、叉形和三角形表示;土壤样本用圆圈表示;环境影响因子用带有箭头的线段表示。不同区域物名称如下:A:12:0,14:0,15:0,16:0,17:0,18:0,i14:0,i15:0,a15:0,i16:0,i17:0,i19:0,10Me 16:0,10Me 17:0,10Me 18:0,cyc 17:0,cyc 19:0ω8c,a16:0,i16:0 3OH,18:1ω5c,18:1ω7c,17:1ω8c,15:0 3OH,16:1ω5c,16:1ω7c,10:0 3OH,i18:1,i20:0,14:1ω5c,10:0,11:0,11:0 2OH,i11:0 3OH,i12:0,12:1 3OH,12:0 3OH,12:0 2OH,i13:0,a13:0,13:0,i13:0 3OH,a14:0,15:1ω6c,i17:0 3OH,20:0,20:1ω7c,19:1ω11c,11Me 18:1ω7c,i19:1,19:1ω6c,i15:0 3OH,16:1ω9c,a17:1,i14:0 3OH,i12:0 3OH,i15:0 2OH,16:1 2OH,16:0 2OH,13:0 2OH,18:0 3OH,i16:1,i14:1;B:18:1 2OH,a18:0,18:3ω6c,i15:1,20:1ω9c,20:4ω6c,i18:0,a17:0;C:11:0 3OH,i11:0,10:0 2OH,14:0 3OH,15:0 2OH,18:2ω6c,18:1ω9c,a17:0 ω9c Note:S1~S18 are sample numbers; The squares, triangles and forks represent different types of PLFAs in A, B and C areas; Circles represent soil samples; the lines with an arrow head represent environmental factors; The names of the species in different regions are listed as follows:A:12:0, 14:0, 15:0, 16:0, 17:0, 18:0, i14:0, i15:0, a15:0, i16:0, i17:0, i19:0, 10Me 16:0, 10Me 17:0, 10Me 18:0, cyc 17:0, cyc 19:0ω8c, a16:0, i16:0 3OH, 18:1ω5c, 18:1ω7c, 17:1ω8c, 15:0 3OH, 16:1ω5c, 16:1ω7c, 10:0 3OH, i18:1, i20:0, 14:1ω5c, 10:0, 11:0, 11:0 2OH, i11:0 3OH, i12:0, 12:1 3OH, 12:0 3OH, 12:0 2OH, i13:0, a13:0, 13:0, i13:0 3OH, a14:0, 15:1ω6c, i17:0 3OH, 20:0, 20:1ω7c, 19:1ω11c, 11Me 18:1ω7c, i19:1, 19:1ω6c, i15:0 3OH, 16:1ω9c, a17:1, i14:0 3OH, i12:0 3OH, i15:0 2OH, 16:1 2OH, 16:0 2OH, 13:0 2OH, 18:0 3OH, i16:1, i14:1;B:18:1 2OH, a18:0, 18:3ω6c, i15:1, 20:1ω9c, 20:4ω6c, i18:0, a17:0;C:11:0 3OH, i11:0, 10:0 2OH, 14:0 3OH, 15:0 2OH, 18:2ω6c, 18:1ω9c, a17:0 ω9c |
耕层土壤冗余分析结果显示,第一排序轴解释了样本中83.5%的变异,第二排序轴解释了样本中2.9%的变异,前4个排序轴共同解释了土壤微生物群落结构演替中89.4%的变异。前2个排序轴的物种—环境相关系数均达到0.92,说明土壤微生物区系与土壤环境因子之间关系密切,这11种土壤环境因子的改变是影响土壤微生物群落结构的主要原因。土壤环境因子中土壤含水量、有机质、全氮、碱解氮和有效磷与前2个排序轴相关性最强,呈极显著正相关(p<0.01),因而这5种因子是决定水田和果园微生物群落结构差异的最重要因子,与沈冰洁等[30]关于水田和旱地中土壤有机碳和全氮对土壤微生物群落结构影响最为显著的结论相比,本结论中影响因子更全面。前四个排序轴的物种—环境累积百分比变化率达到97.9%。第一排序轴与自然含水量、全磷、有效磷、全钾和速效钾均呈显著(p<0.05)或极显著(p<0.01)相关,第二排序轴与pH、有机质、全氮和碱解氮呈显著相关(p<0.05),第三排序轴与盐基饱和度呈极显著负相关。
3 结论土地利用方式及其转变时间能够对土壤理化性质和微生物多样性带来较大影响。水田改果园后,土壤微生物生物量和微生物商显著下降,土壤微生物群落结构发生显著变化,并且随果园年龄的增加而日趋明显。聚类分析和冗余分析表明,在水田和果园生态系统中,土壤水分、有机质、氮素和有效磷是影响土壤微生物群落结构的最主要环境因素(p<0.01)。土地利用方式转变对土壤微生物的影响要大于利用年限的影响。研究表明,水田是地球生态系统中重要的碳汇,其微生物多样性较丰富,水田种植是一种可以促进土壤可持续利用的有效方式。水田改为果园不利于土壤可持续利用,此外,长期种植果树可能会引起土壤养分失衡等一系列问题。因而,应尽量避免将大面积水田改为果园,并应对已改制的果园实行轮作等种植方式以维护土壤生态功能,促进土壤可持续发展。
| [1] |
Torsvik V, Øvreås L. Microbial diversity and function in soil:From genes to ecosystems . Current Opinion in Microbiology, 2002, 5(3): 240-245. DOI:10.1016/S1369-5274(02)00324-7
(  0) 0) |
| [2] |
Schimel D S. Terrestrial ecosystems and the carbon cycle . Global Change Biology, 1995, 1(1): 77-91. DOI:10.1111/gcb.1995.1.issue-1
(  0) 0) |
| [3] |
王敬, 张金波, 蔡祖聪. 太湖地区稻麦轮作农田改葡萄园对土壤氮转化过程的影响. 土壤学报, 2016, 53(1): 166-176. Wang J, Zhang J B, Cai Z C. Effects of conversion of paddy field into vineyard on soil nitrogen transformation in the Taihu Lake region of China (In Chinese). Acta Pedologica Sinica, 2016, 53(1): 166-176. DOI:10.11766/trxb201508020194 (  0) 0) |
| [4] |
Neill C, Piccolo M C, Cerri C C, et al. Net nitrogen mineralization and net nitrification rates in soils following deforestation for pasture across the southwestern Brazilian Amazon Basin landscape . Oecologia, 1997, 110(2): 243-252. DOI:10.1007/s004420050157
(  0) 0) |
| [5] |
Geissen V, Peña-Peña K, Huerta E. Effects of different land use on soil chemical properties, decomposition rate and earthworm communities in tropical Mexico . Pedobiologia, 2009, 53(1): 75-86. DOI:10.1016/j.pedobi.2009.03.004
(  0) 0) |
| [6] |
阳显斌, 李廷轩, 张锡洲, 等. 烟蒜轮作与套作对土壤微生物类群数量的影响. 土壤, 2016, 48(4): 698-704. Yang X B, Li T X, Zhang X Z, et al. Effects of tobacco garlic crop rotation and tobacco garlic crop intercropping on soil microbial groups in tobacco fields (In Chinese). Soils, 2016, 48(4): 698-704. (  0) 0) |
| [7] |
Celik I. Land-use effects on organic matter and physical properties of soil in a southern Mediterranean highland of Turkey . Soil and Tillage Research, 2005, 83(2): 270-277. DOI:10.1016/j.still.2004.08.001
(  0) 0) |
| [8] |
Li X G, Li F M, Zed R, et al. Soil physical properties and their relations to organic carbon pools as affected by land use in an alpine pastureland . Geoderma, 2007, 139(1/2): 98-105.
(  0) 0) |
| [9] |
Jangid K, Williams M A, Franzluebbers A J, et al. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties . Soil Biology and Biochemistry, 2011, 43(10): 2184-2193. DOI:10.1016/j.soilbio.2011.06.022
(  0) 0) |
| [10] |
Zhang Q, Sun J, Liu S, et al. Manure refinement affects apple rhizosphere bacterial community structure:A study in sandy soil . PLoS One, 2013, 8(10): 1-8.
(  0) 0) |
| [11] |
何振立. 土壤微生物量及其在养分循环和环境质量评价中的意义. 土壤, 1997, 29(2): 61-69. He Z L. The significance of soil microorganisms and their nutrient cycling in environmental quality evaluation (In Chinese). Soils, 1997, 29(2): 61-69. (  0) 0) |
| [12] |
Lauber C L, Strickland M S, Bradford M A, et al. The influence of soil properties on the structure of bacterial and fungal communities across land-use types . Soil Biology and Biochemistry, 2008, 40(9): 2407-2415. DOI:10.1016/j.soilbio.2008.05.021
(  0) 0) |
| [13] |
Wakelin S A, Macdonald L M, Rogers S L, et al. Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils . Soil Biology and Biochemistry, 2008, 40(3): 803-813. DOI:10.1016/j.soilbio.2007.10.015
(  0) 0) |
| [14] |
周赛, 梁玉婷, 张厚喜, 等. 我国中亚热带毛竹林土壤微生物群落的空间分布特征及其影响因素. 土壤, 2015, 47(2): 369-377. Zhou S, Liang Y T, Zhang H X, et al. Characteristics of spatial pattern and influence factors for soil microbial community structure in moso bamboo forests in middle subtropical China (In Chinese). Soils, 2015, 47(2): 369-377. (  0) 0) |
| [15] |
雷海迪, 尹云锋, 刘岩, 等. 杉木凋落物及其生物炭对土壤微生物群落结构的影响. 土壤学报, 2016, 53(3): 790-799. Lei H D, Yin Y F, Liu Y, et al. Effects of fir (Cunninghamia lanceolata) litter and its biochar on soil microbial community structuren (In Chinese). Acta Pedologica Sinica, 2016, 53(3): 790-799. (  0) 0) |
| [16] |
Balota E L, Yada I F, Amaral H, et al. Long-term land use influences soil microbial biomass p and s, phosphatase and arylsulfatase activities, and s mineralization in a brazilian oxiso . Land Degradation and Development, 2014, 25(4): 397-406. DOI:10.1002/ldr.v25.4
(  0) 0) |
| [17] |
Yao H, He Z, Wilson M J, et al. Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use . Microbial Ecology, 2000, 40(3): 223-237.
(  0) 0) |
| [18] |
Bossio D A, Girvan M S, Verchot L, et al. Soil microbial community response to land use change in an agricultural landscape of western Kenya . Microbial Ecology, 2005, 49(1): 50-62. DOI:10.1007/s00248-003-0209-6
(  0) 0) |
| [19] |
Xue D, Yao H Y, Ge D Y, et al. Soil microbial community structure in diverse land use systems:A comparative study using Biolog, DGGE, and PLFA analyses . Pedosphere, 2008, 18(5): 653-663. DOI:10.1016/S1002-0160(08)60060-0
(  0) 0) |
| [20] |
Lauber C L, Hamady M, Knight R, et al. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale . Applied and Environmental Microbiology, 2009, 75(15): 5111-5120. DOI:10.1128/AEM.00335-09
(  0) 0) |
| [21] |
Rajaniemi T K, Allison V J. Abiotic conditions and plant cover differentially affect microbial biomass and community composition on dune gradients . Soil Biology and Biochemistry, 2009, 41(1): 102-109. DOI:10.1016/j.soilbio.2008.10.001
(  0) 0) |
| [22] |
Wang B Z, Zhang C X, Liu J L, et al. Microbial community changes along a land-use gradient of desert soil origin . Pedosphere, 2012, 22(5): 593-603. DOI:10.1016/S1002-0160(12)60044-7
(  0) 0) |
| [23] |
嘉兴市土壤志编辑委员会. 嘉兴土壤. 杭州: 浙江科学技术出版社, 1991, 218-223. Jiaxing Soil Log Editorial Committee. Jiaxing soil (In Chinese). Hangzhou: Zhejiang Science and Technology Press, 1991, 218-223. (  0) 0) |
| [24] |
鲁如坤. 土壤农业化学分析方法. 北京: 中国农业科学技术出版社, 2000. Lu R K. Analytical methods for soil and agro-chemistry (In Chinese). Beijing: China Agricultural Science and Technology Press, 2000. (  0) 0) |
| [25] |
张甘霖, 龚子同. 土壤调查实验室分析方法. 北京: 科学出版社, 2012, 1-243. Zhang G L, Gong Z T. Analytical methods of soil survey laboratory (In Chinese). Beijing: Science Press, 2012, 1-243. (  0) 0) |
| [26] |
Wu Y P, Ding N, Wang G, et al. Effects of different soil weights, storage times and extraction methods on soil phospholipid fatty acid analyses . Geoderma, 2009, 150(1): 171-178.
(  0) 0) |
| [27] |
李忠佩, 吴晓晨, 陈碧云. 不同利用方式下土壤有机碳转化及微生物群落功能多样性变化. 中国农业科学, 2007, 40(8): 1712-1721. Li Z P, Wu X C, Chen B Y. Changes in transformation of soil organic carbon and functional diversity of soil microbial community under different land use patterns (In Chinese). Scientia Agricultura Sinica, 2007, 40(8): 1712-1721. (  0) 0) |
| [28] |
彭佩钦, 吴金水, 黄道友, 等. 洞庭湖区不同利用方式对土壤微生物生物量碳氮磷的影响. 生态学报, 2006, 26(7): 2261-2267. Peng P Q, Wu J S, Huang D Y, et al. Microbial biomass C, N, P of farmland soils in different land uses and cropping systems in Dongting Lake region (In Chinese). Acta Ecologica Sinica, 2006, 26(7): 2261-2267. (  0) 0) |
| [29] |
Sparling G P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter . Australia Journal of Soil Research, 1992, 30(2): 195-207. DOI:10.1071/SR9920195
(  0) 0) |
| [30] |
沈冰洁, 祝贞科, 袁红朝, 等. 不同种植方式对亚热带红壤微生物多样性的影响. 环境科学, 2015, 36(10): 3839-3844. Shen B J, Zhu Z K, Yuan H Z, et al. Effects of different plantation type on the abundance and diversity of soil microbes in subtropical red soils (In Chinese). Environmental Science, 2015, 36(10): 3839-3844. (  0) 0) |
| [31] |
White D C, Davis W M, Nickels J S, et al. Determination of the sedimentary microbial biomass by extractible lipid phosphate . Oecologia, 1979, 40(1): 51-62. DOI:10.1007/BF00388810
(  0) 0) |
| [32] |
钟文辉, 蔡祖聪. 土壤微生物多样性研究方法. 应用生态学报, 2004, 15(5): 899-904. Zhong W H, Cai Z C. Methods for studying soil microbial diversity (In Chinese). Chinese Journal of Applied Ecology, 2004, 15(5): 899-904. (  0) 0) |
| [33] |
陈振翔, 于鑫, 夏明芳, 等. 磷脂脂肪酸分析方法在微生物生态学中的应用. 生态学杂志, 2005, 24(7): 828-832. Chen Z X, Yu X, Xia M F, et al. Application of phospholipid fatty acid (PLFA) analysis in microbial ecology (In Chinese). Chinese Journal of Ecology, 2005, 24(7): 828-832. (  0) 0) |
| [34] |
McKinley V L, Peacockb A D, White D C. Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils . Soil Biology and Biochemistry, 2005, 37(10): 1946-1958. DOI:10.1016/j.soilbio.2005.02.033
(  0) 0) |
2. College of Tourism, Henan Normal University, Xinxiang, Henan 453007, China;
3. Business College, Guangxi University, Nanning 530004, China
 2018, Vol. 55
2018, Vol. 55


