2. 土肥资源高效利用国家工程实验室,泰安 271018;
3. 山东省高校农业环境重点实验室,泰安 271018;
4. 大连民族大学经济管理学院,大连 116650
三氯生(Triclosan, TCS)是一种广谱抗菌剂, 作为一种典型的药品和个人护理品(Pharmaceuticals and personal care products, PPCPs)广泛用于洗发精、肥皂、洗衣液和牙膏等, 应用量巨大[1]。这些日用品使用后经生活污水进入城市污水处理厂, 由于低的溶解度(1.97~4.6 mg L-1, 25 ℃)和高的疏水性(log Kow= 4.76, pKa=8.14), TCS会吸附进入城市污泥, 随着污泥农用不断进入土壤, 因此, 土壤环境可能是该物质重要的汇[2-3]。土壤TCS浓度为0.052~1.0 mg kg-1干重[4], 模拟预测浓度可达4.5 mg kg-1干重[5]。尽管浓度低, 但TCS易在环境中累积, 具有一定持久性[6]。TCS的存在增加了土壤微生物对抗生素的抗性[7]。砂土中5 mg kg-1、黏土中50 mg kg-1的TCS降低了微生物硝化速率[8-9], 低于10 mg kg-1的TCS抑制了土壤呼吸[9-10]。土壤中广泛存在的TCS及其持久性使得其生态效应受到高度关注, 成为近年来土壤环境领域关注的热点[11]。
镉(Cd)是毒性极大的重金属, 已被认定为人类致癌物[12], 通过污水灌溉、化肥和农药使用、畜禽粪便及其堆肥长期施用以及电子废弃物堆弃等渠道进入土壤, Cd的污染在中国是非常严重和普遍的问题[13]。对中国矿区、工业区、公路旁、城市边缘区、城市、污罐区、农田、菜地、茶园、果园、森林等土壤重金属含量调查结果显示, 土壤Cd的浓度为0~578 mg kg-1, 平均值为3.52 mg kg-1, 2 253个采样点有547个点Cd含量超过了1.0 mg kg-1, 93个点超过了10 mg kg-1[14-16]。因此, Cd在土壤中的生态风险一直受到广泛关注[17-18]。
目前, 土壤污染呈现由单一污染到复合污染的态势。TCS的大量广泛使用以及中水灌溉和活性污泥农用导致其源源不断地进入土壤中[2]。活性污泥中还含有许多重金属, 包括Cd[19], 因此, 活性污泥中同时存在着TCS与Cd。Cd还通过上述途径进入土壤导致Cd污染[13]。因此, 土壤环境中存在着TCS与Cd复合污染的可能性。由于TCS和Cd均对土壤环境产生了不利影响, 两者的复合污染可能会降低土壤质量。而目前关于TCS与Cd复合污染土壤的生态效应信息尚缺乏, 因此, 非常有必要探讨TCS与Cd的复合污染对土壤的健康效应。
土壤呼吸是土壤全部代谢过程的总和, 被认为是土壤微生物总活性的指标, 成为土壤污染评价的常用指标之一[20]。土壤蔗糖酶催化水解蔗糖转变为葡萄糖和果糖, 为微生物提供能量, 在土壤碳循环方面非常重要[21]。作为一种氮代谢酶, 蛋白酶在氮素循环和调控植物利用氮方面发挥十分重要的作用。此外, 上述三种指标对Cd的污染均较敏感[22], 如土壤呼吸与Cd含量尤其是Ca(NO3)2提取态Cd含量呈显著负相关[23], 外源Cd含量也可能增强土壤呼吸活性[24]。酸性土壤Cd含量由2.5 mg kg-1增加至5 mg kg-1时, 蔗糖酶活性降低了[25]。前人已经对TCS与Cd单一胁迫的土壤呼吸效应进行了研究, 而TCS与Cd复合污染的生态效应研究还很匮乏。因此, 本文以TCS和Cd为目标污染物, 选择土壤呼吸、蔗糖酶和蛋白酶为指标, 通过室内模拟试验研究两者单一和复合污染对上述3种指标的影响, 试图揭示土壤微生物活性和涉及碳、氮循环的酶活性对TCS和Cd单一及复合污染的响应规律, 为评价此类新型复合污染的环境风险提供重要的科学依据。
1 材料与方法 1.1 供试土壤土壤样品采自泰安市山东农业大学南校区科技试验田, 采样时间为2015年3月24日。除去土壤表面的杂草、枯叶后, 采用五点法采集0~20 cm表层土。取回后, 去除石砾和植物残体等杂物, 完全混合, 室温下风干, 过2 mm筛, 置于4℃冰箱备用。土壤为棕壤, 根据中国土壤系统分类命名为普通简育湿润淋溶土(Typic-Hapli-Udic Argosols)。土壤全氮、全磷和全钾含量分别为0.73、7.38和1.54 g kg-1, 碱解氮、有效磷和速效钾含量分别为132.3、18.4和125.7 mg kg-1, pH为7.6, 有机质为17.6 g kg-1, 黏粒(< 2 μm)、粉粒(50~2 μm)和砂粒(> 50 μm)含量分别为104、577和319 g kg-1。土壤中Cd含量为2.22 mg kg-1。
1.2 供试药剂TCS标准品(纯度99.5%)购自Sigma-Aldrich(美国), 溶于丙酮, 配成5 g L-1储备液, 置于4℃冰箱保存; Cd为分析纯氯化镉(CdCl2), 其他试剂均为分析纯。
1.3 试验设计将上述处理好的土样从冰箱中取出, 置于25℃培养箱中避光预培养3 d。称取预培养好的土壤样品200g, 向土样分别加入稀释至不同浓度的TCS和Cd溶液, 充分混匀。为避免溶剂的不利影响, 将土壤样品置于通风橱中2 h至丙酮溶剂挥发完全后继续充分搅拌混匀。参考TCS与Cd的土壤微生态效应文献[12, 24-26], 土样中TCS最终浓度分别为0、1.0、10.0、50.0 mg kg-1干土, Cd的最终浓度为0、10.0 mg kg-1干土, 复合污染处理组浓度(TCS/Cd)为1.0/10.0, 10.0/10.0, 50.0/10.0 mg kg-1干土。调节土壤含水量为最大持水量的60%, 置于培养箱中, 25℃避光培养, 每2~3天补充水分, 使土样含水量在整个实验期间保持恒定。培养后第7、14、28和56天分别取土样测定土壤呼吸强度、蛋白酶和蔗糖酶活性, 每处理设3个重复。
1.4 测定方法土壤呼吸采用室内密闭培养法[27]测定。蔗糖酶活性采用0.1 mol L-1的Na2S2O3滴定法测定, 以1 g土壤37℃下培养24 h后所消耗的0.1 mol L-1 Na2S2O3毫升数表示[28]。蛋白酶活性采用比色法测定, 以24 h后1 g干土中酪氨酸的质量表示, mg g-1 d-1[28]。
1.5 数据分析不同处理对土壤呼吸和酶活性的影响率用如下公式计算:
| $ 影响率(\%)={\rm (A-B)/B×100} $ | (1) |
式中, A为添加污染物时的土壤呼吸或酶活性; B为未添加污染物时的土壤呼吸或酶活性; 正值表示刺激或激活, 负值表示抑制。
计算TCS与Cd复合污染的作用模式是以概率为基础[29], 该方程为:
| $ {\rm P(E) = P_A+ P_B-(P_A×_B)}/100 $ | (2) |
式中, P(E)为抑制率理论预测值; PA为污染物A引起的抑制率; PB为污染物B引起的抑制率; 假设P(T)为抑制率实际测定值。将P(T)与P(E)进行差异显著性分析, 零假设是P(T)高于P(E), 若有显著性差异则联合作用模式是协同作用; 若P(T)低于P(E)且有显著性差异, 则联合作用模式是拮抗作用; 若P(T)与P(E)间无显著性差异, 则联合作用模式是加和效应。
所有测定数据均用Microsoft Excel 2007和SPSS17.0进行计算和统计分析, 以平均值±标准差的形式表示。
2 结果 2.1 三氯生与镉单一及复合污染对土壤呼吸的影响TCS与Cd单一及复合污染对土壤呼吸影响的变化趋势如图 1所示。两者单一和复合胁迫下土壤呼吸活性均呈现先上升接着下降最后又上升的变化趋势(图 1)。Cd(10.0 mg kg-1)单一胁迫时, 培养7d对土壤呼吸有明显的激活作用, 激活率达18.52%, 但14 d和28 d时转为抑制作用, 抑制率达10.12%和10.78%, 56 d时又转为激活作用, 激活率为24.16%(图 1A), 与对照相比有显著差异(p < 0.05), 表明10.0 mg kg-1的Cd仅是短暂抑制了土壤呼吸作用。TCS单一胁迫培养前期(7 d)对土壤呼吸表现为激活作用, 不同浓度TCS的激活率分别为25.26%, 27.98%和18.45%;随着培养时间延长, 14 d和28 d时, 土壤呼吸均被抑制; 但56 d时, 土壤呼吸又被显著激活, 激活率分别为41.84%、31.56%和21.41% (TCS1.0、TCS 10.0和TCS 50 mg kg-1) (图 1B), 与TCS添加浓度显著相关(p < 0.05)。不同浓度的TCS与Cd复合胁迫第7天时刺激了土壤呼吸活性, 随着TCS浓度增加, 激活作用减弱; 14 d和28 d时抑制了土壤呼吸, 随着TCS浓度增加, 抑制作用也随之减弱; 实验最后(56 d)又转为非常明显的刺激作用(图 1C)。
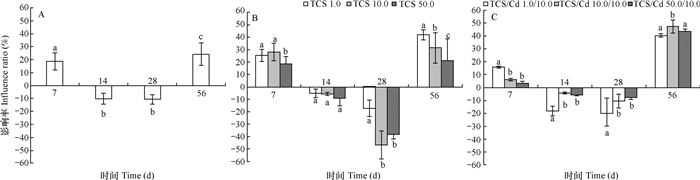
|
图 1 三氯生与镉单一及复合污染对土壤呼吸的影响率 Fig. 1 Influence ratio of single-factor and combined pollution of triclosan and cadmium on soil respiration 注: A:镉单一污染; B:三氯生单一污染; C:三氯生与镉复合污染。浓度单位: mg kg-1。不同字母表示同一时间不同处理间差异显著(p<0.05)。下同 Note: A: Cd; B: Triclosan; C: Triclosan+Cd. Units: mg kg-1. Different letters in the same sampling time represent significant difference between treatments at 0.05 level. The same below |
TCS与Cd单一及复合污染对土壤蛋白酶活性的影响如图 2所示。Cd单一胁迫在整个实验期间对土壤蛋白酶活性均有明显的刺激作用, 激活率在前14 d无明显变化, 28 d时显著降低, 但56 d时显著增加, 达到最大值(94.5%)(图 2A)。不同浓度的TCS单一胁迫均刺激了蛋白酶活性, 激活程度随培养时间及浓度水平的变化而变化; 56 d时激活效应最强, 1.0、10.0和50.0 mg kg-1浓度下分别达到31.8%、42.1%和68.2%, 且与TCS添加浓度呈显著正相关(p < 0.05)(图 2B), 表明高浓度TCS强烈刺激了土壤蛋白酶活性。TCS与Cd复合胁迫仍对蛋白酶活性表现强烈的刺激作用, 随着培养时间延长, 激活率先降低后升高, 56 d时达到最大, TCS/Cd 1.0/10.0、TCS/Cd 10.0/10.0和TCS/Cd 50.0/10.0 mg kg-1时, 激活率分别为98.0%、110.3%和102.7%(图 2C), 但同一时间不同浓度的激活程度无显著性差异(p > 0.05)。Cd的激活作用大于不同浓度(1.0、10.0和50.0 mg kg-1)TCS的效果, 表明Cd在两者的复合污染中起主要作用。
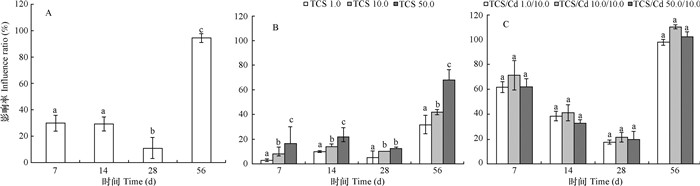
|
图 2 三氯生与镉单一及复合污染对土壤蛋白酶活性的影响率 Fig. 2 Influence ratio of single-factor and combined pollution of triclosan and cadmium on soil protease |
TCS与Cd单一及复合污染对蔗糖酶活性的影响如图 3所示。10.0 mg kg-1Cd单一胁迫下, 蔗糖酶活性均被显著抑制, 14 d时抑制率达到最大值(81%), 但不同培养时间的抑制率之间无显著差异(p > 0.05)(图 3A)。TCS单一胁迫也显著降低了蔗糖酶活性, 抑制率随培养时间和浓度的变化而变化(图 3B):同一污染浓度下, 随培养时间的变化, 56 d时各浓度的抑制率最小; 相同培养时间时, TCS浓度越高抑制效应越强, 50.0 mg kg-1TCS在7 d、14 d、28 d和56 d的抑制率分别为58.34%、43.81%、62.86%和20.01%。TCS与Cd的复合污染胁迫在整个实验期间同样显著抑制了蔗糖酶活性, 但抑制程度随培养时间的延长和浓度水平的不同变化不明显(图 3C): TCS/Cd 1.0/10.0 mg kg-1时, 抑制率为82.9%~89.6%, TCS/Cd 10.0/10.0 mg kg-1和TCS/Cd 50.0/10.0 mg kg-1时抑制率稍有降低, 分别为70.7%~82.1%和71.6%~83.9%。整个实验期间Cd和TCS的复合污染对蔗糖酶的联合效应与Cd单一污染相比无显著差异(p > 0.05), 表明TCS和Cd复合作用下Cd的毒性效应起决定作用。
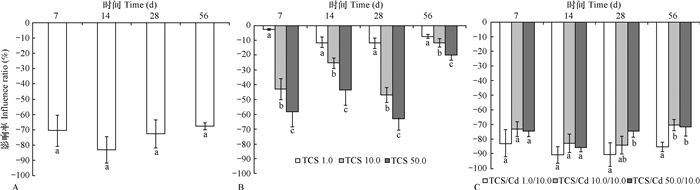
|
图 3 三氯生与镉单一及复合污染对土壤蔗糖酶活性的影响率 Fig. 3 Influence ratio of single-factor and combined pollution of triclosan and cadmium on soil inverase |
如图 4所示, 不同浓度TCS和Cd复合污染对土壤呼吸的联合效应中, 7 d时P (E)大于P (T), 且差异均显著, 表明两者的复合污染表现为拮抗作用, 其联合效应不随浓度的改变而改变。14 d和28 d时, 复合污染联合效应中P (E)小于P (T), 除了TCS/Cd1.0/10.0时差异不显著表现为加和作用外, 其余结果差异均显著, 因此表现为协同作用。56 d时仅有TCS/Cd1.0/10.0的联合效应表现为明显的拮抗作用, 其他两种剂量下均为加和作用。因此, TCS与Cd复合污染对土壤呼吸的联合效应作用模式随着培养时间的变化而变化(图 4A)。
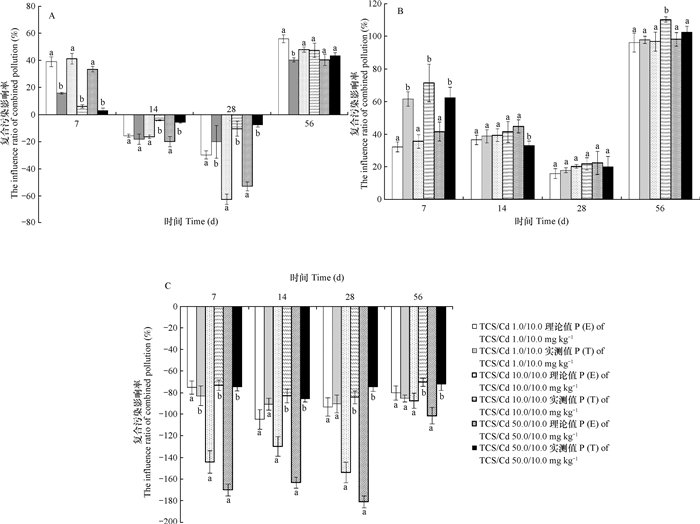
|
图 4 三氯生与镉复合污染对土壤呼吸(A)、蛋白酶(B)和蔗糖酶(C)影响率的理论值与实测值比较 Fig. 4 Comparison of theoretically predicted value with observed value of combined effects of TCS and Cd on soil respiration (A), protease (B) and invertase (C) 注:不同字母代表同一时间同一浓度下的理论值与实测值的差异显著(p<0.05)。P(E):影响率理论预测值, P(T):影响率实际测定值 Note: Different letters in the same sampling time represent significant difference between theoretically expected and observed values in the same concentration treatments at 0.05 level. P(E): Theoretically predicated affecting rate, P(T): Observed affecting rate |
TCS与Cd复合污染对蛋白酶的联合效应中, 培养7 d时P(E)小于P(T), 且差异性显著, 均表现为协同作用。随着培养时间延长, 仅有50.0 mg kg-1 TCS和Cd复合污染在14 d时以及10.0 mg kg-1 TCS和Cd复合污染在56 d时P(E)与P(T)差异显著, 分别表现为拮抗和协同作用, 其他浓度和时间的复合污染P(E)与P(T)差异不显著, 表现为加和效应。因此, TCS与Cd复合污染对土壤蛋白酶的联合毒性效应作用模式随污染物浓度和培养时间的变化而变化(图 4B)。
TCS与Cd复合污染对土壤蔗糖酶的联合毒性效应中, 仅有1.0 mg kg-1TCS和Cd复合污染在14 d、28 d和56 d时P(E)与P(T)差异不显著, 表现为加和作用, 其他浓度和培养时间均表现为协同作用。因此, TCS与Cd复合污染对蔗糖酶的联合效应以协同作用为主(图 4C)。
3 讨论本研究中Cd单一胁迫7 d土壤呼吸增加, 这与Lu等[24]的结果相同, 这是因为实验培养7 d时土壤可利用碳较多, Cd胁迫下微生物需要更多的能量生存, 因此, 仅有少部分碳被吸收而大部分碳被消耗为CO2导致土壤呼吸增加。随后土壤呼吸下降, 这与Usman[30]的结果一致, 随着培养时间延长, 土壤可利用碳量下降, 同时, 一部分微生物在Cd的毒性下被抑制或杀灭, 因此, 培养至中间时土壤呼吸持续下降; 而培养结束时土壤呼吸再被激活, 可能是因为微生物在Cd胁迫下通过适应和变异产生耐性菌种, 该菌种对土壤呼吸促进作用大于Cd对其他微生物呼吸的抑制作用, 导致土壤整体呼吸强度升高; 另一方面, 被杀灭微生物的分解也会增加土壤呼吸[31]。TCS对土壤呼吸的影响已有部分研究, Waller和Kookana[9]研究发现, 低浓度(1.0 mg kg-1)TCS对土壤呼吸无显著影响, 中浓度(10.0 mg kg-1)TCS仅在砂土中激活了土壤呼吸, 高浓度(50.0 mg kg-1)TCS仅在黏土中抑制了土壤呼吸。Butler等[10]的研究同样得到不同类型土壤(壤砂土、黏土和砂壤土)中TCS对土壤呼吸的影响差异较大:施用之初土壤呼吸在壤砂土中被显著抑制, 随着浓度增加, 抑制作用增强, 但在黏土和砂壤土中10.0 mg kg-1TCS对土壤呼吸无显著抑制作用, 其他浓度的TCS明显抑制了土壤呼吸。因此, 土壤类型显著影响TCS对土壤呼吸的胁迫效应, 如pH、黏粒含量和有机质在土壤呼吸变化中分别起到1%、16%和15%的贡献[10]。本研究所用的土壤与上述土壤性质有明显差异, 导致TCS的生物有效性明显不同, 因此, 研究结果有较大差异。此外, TCS既可作为微生物的碳源, 同时又对微生物具有毒性作用[10]。本研究土壤呼吸在前期被激活, 中期被抑制, 56 d时又被激活, 原因可能是培养7 d时TCS被微生物作为碳源加以利用, 刺激了微生物活性, 而未利用的TCS对微生物产生毒性作用从而导致了随后的抑制效应, 56 d后TCS被部分降解, 毒性降低, 大部分微生物适应了TCS胁迫之后能够利用其作为碳源和能源生长, 从而增强呼吸作用。本研究复合污染对土壤呼吸的影响趋势与TCS和Cd单一污染时的作用均遵循“激活-抑制-激活”规律, 这与芘、Cd单一及复合污染胁迫下土壤呼吸先下降后上升的污染扩散模式[32]相似。
蛋白酶参与土壤氨基酸、蛋白质以及其他含蛋白质氮有机化合物的转化, 是与氮循环密切相关的重要胞外酶, 因此, 探讨污染物对土壤蛋白酶活性的生态效应非常必要。本实验Cd胁迫促进了蛋白酶活性增加, 表明对Cd产生耐性的微生物生长较快, 其对蛋白酶的促进作用大于Cd对蛋白酶的抑制作用, 整体提高了酶活性。因此, Cd污染土壤中可以富集培养并筛选分离出耐性菌属, 驯化培养后用于污染土壤的微生物修复[32]。不同浓度TCS单一胁迫均刺激了蛋白酶活性, 可能是因为尽管TCS具有广谱的杀菌活性, 但在0~50 mg kg-1时显示出抑菌活性, 在高浓度下才表现出杀菌活性。抑菌活性下的TCS仍可被耐性的微生物作为碳源和能源, 促进了蛋白酶活性增加[11]。TCS与Cd复合污染作用下, 重金属的存在抑制了TCS的降解[33], TCS与重金属的复合污染表现出协同作用, 即增强了TCS或Cd单一污染下的激活效应; 且TCS与铜的复合污染增强了微生物的代谢活性, 尤其是氮代谢作用, 因此可能在一定程度上增强了蛋白酶的活性[11]。
蔗糖酶参与土壤碳素循环, 是土壤中研究最多的酶之一。Cd单一胁迫下, 蔗糖酶活性均被显著抑制。原因可能是Cd胁迫引起酶分子中的活性部位--巯基和含咪唑的配位结合, 形成较稳定的络合物, 产生了与底物的竞争性抑制, 最终导致酶活性下降[34]。TCS单一胁迫时, 蔗糖酶活性也被显著抑制, 是由于TCS具有广谱抗菌活性, 通过模拟烯酰还原酶的天然基质影响微生物脂肪酸合成路径, 导致对酶的不可逆抑制[35]。TCS与Cd复合污染抑制了土壤蔗糖酶活性, 这与TCS与铜或锌复合污染降低了硫酸酯酶和磷酸酶活性结果相同, 原因可能是TCS与Cd的复合污染起协同作用, 削弱了土壤碳素能量循环和营养周转率, 影响了蔗糖酶活性。本研究表明, 蔗糖酶活性对TCS与Cd单一及复合污染的敏感性最高, 可作为两者复合污染的生态评价指标之一。
尽管TCS单一污染的生态效应研究较多, 但对TCS与重金属复合污染的土壤生态效应信息了解较少。复合污染的作用类型有协同、加和和拮抗三种, 基于不同的作用方式预测复合胁迫类型的模型有多个[36], 本研究利用的模型基于这样的假设:污染物对生物体是同时起作用的。TCS和Cd复合污染总体上以协同效应为主, 可能是因为重金属的存在抑制了TCS降解, 使得两者的共存时间增加, 两者经过6个月的复合作用对土壤酶活性仍具有协同效应[33]。复合污染的联合毒性效应随着TCS浓度变化而发生显著变化, 这是因为TCS作为抗菌剂在低浓度时具有抑菌活性, 高浓度时才具有杀菌活性, 因此, 不同浓度下与Cd的复合胁迫效应不同。此外, TCS易吸附在土壤有机质中使其生物有效性下降[37], 因此, TCS和Cd复合污染的联合效应随着培养时间的变化也发生显著变化。
目前, 土壤环境面临的一个重要问题是抗菌剂或抗生素的过量使用导致耐药细菌的产生[2]。研究表明, 暴露在TCS中的土壤微生物会通过各种机制发展对TCS或其他药物的抗性[38]。TCS单一或与Cd复合胁迫下土壤呼吸先被抑制后被激活了, 表明部分存活的微生物群落发展了抗性。但在对土壤蛋白酶和蔗糖酶的生态效应中, 整个实验期间均被激活或抑制了, 表明仍有部分微生物未表现对TCS的抗性, 即50 mg kg-1的TCS并未对微生物产生明显的选择性压力而导致敏感微生物被耐受微生物替代, 且Cd的胁迫也未增加微生物对TCS的抗性。但是, 不同土壤中TCS与铜/锌的复合胁迫下微生物对TCS的抗性表现差异很大[11], 因此, 不同浓度TCS单一或与Cd复合污染下微生物的抗性需要深入研究。
4 结论三氯生与Cd单一及复合污染对土壤呼吸呈现激活-抑制-激活的生态效应; 刺激了蛋白酶活性, 激活率先降低后升高, 56 d时达到最大; 整个实验期间均抑制了蔗糖酶活性, Cd单一污染培养14 d抑制率达到最大值(81%), TCS单一胁迫呈现负的剂量效应关系, 两者复合污染无显著的剂量效应关系。联合效应评价模型预测表明, 相比TCS或Cd单一污染, 两者的复合胁迫对土壤呼吸呈现随时间变化的拮抗-协同-加和效应, 对土壤蛋白酶呈现协同-加和-协同的联合效应, 而对土壤蔗糖酶活性则主要为协同效应。联合效应随着TCS浓度的增加和培养时间的延长而加剧或降低。
致谢 感谢殷梦菲、李茜、刘继文、刘晓斐、葛成飞、宋开付在实验过程中给予的大力帮助。
| [1] |
Guo J H, Iwata H. Risk assessment of triclosan in the global environment using a probabilistic approach . Ecotoxicology and Environmental Safety, 2017, 143: 111-119. DOI:10.1016/j.ecoenv.2017.05.020
(  0) 0) |
| [2] |
Sherburne J J, Anaya A M, Fernie K J, et al. Occurrence of triclocarban and triclosan in an agro-ecosystem following application of biosolids . Environmental Science & Technology, 2016, 50(24): 13206-13214.
(  0) 0) |
| [3] |
Anderson C G, Joshi G, Bair D A, et al. Use of nuclear receptor luciferase-based bioassays to detect endocrine active chemicals in a biosolids-biochar amended soil . Chemosphere, 2017, 181: 160-167. DOI:10.1016/j.chemosphere.2017.04.035
(  0) 0) |
| [4] |
Butler E, Whelan M J, Sakrabani R, et al. Fate of triclosan in field soils receiving sewage sludge . Environmental Pollution, 2012, 167: 101-109. DOI:10.1016/j.envpol.2012.03.036
(  0) 0) |
| [5] |
Fuchsman P, Lyndall J, Bock M, et al. Terrestrial ecological risk evaluation for triclosan in land-applied biosolids . Integrated Environmental Assessment and Management, 2010, 6(3): 405-418. DOI:10.1897/IEAM_2009-071.1
(  0) 0) |
| [6] |
Zarate F M, Schulwitz S E, Stevens K J, et al. Bioconcentration of triclosan, methyl-triclosan, and triclocarban in the plants and sediments of a constructed wetland . Chemosphere, 2012, 88(3): 323-329. DOI:10.1016/j.chemosphere.2012.03.005
(  0) 0) |
| [7] |
Tkachenko O, Shepard J, Aris V M, et al. A triclosan-ciprofloxacin cross-resistant mutant strain of Staphylococcus aureus displays an alteration in the expression of several cell membrane structural and functional genes . Research in Microbiology, 2007, 158(8): 651-658.
(  0) 0) |
| [8] |
StasinakisA S, Petalas A V, Mamais D, et al. Investigation of triclosan fate and toxicity in continuous-flow activated sludge systems . Chemosphere, 2007, 68(2): 375-381. DOI:10.1016/j.chemosphere.2007.01.047
(  0) 0) |
| [9] |
Waller N J, Kookana R S. Effect of triclosan on microbial activity in Australian soils . Environmental Toxicology and Chemistry, 2009, 28(1): 65-70. DOI:10.1897/08-224.1
(  0) 0) |
| [10] |
Butler E, Whelan M J, Ritz K, et al. Effects of triclosan on soil microbial respiration . Environmental Toxicology and Chemistry, 2011, 30(2): 360-366. DOI:10.1002/etc.v30.2
(  0) 0) |
| [11] |
Gielen G J H P, Schaik A P, Northcott G, et al. Effect of copper and zinc on microbial tolerance to triclosan in two soil types . Journal of Soils and Sediments, 2016, 16(7): 1944-1959. DOI:10.1007/s11368-016-1389-2
(  0) 0) |
| [12] |
International Agency for Research on Cancer(IARC). Berrylium, cadmium, mercury, and exposures in the glass manufacturing industry. Lyon: IARC Scientific Publications, 1993
(  0) 0) |
| [13] |
樊霆, 叶文玲, 陈海燕, 等. 农田土壤重金属污染状况及修复技术研究. 生态环境学报, 2013, 22(10): 1727-1736. Fan T, Ye W L, Chen H Y, et al. Review on contamination and remediation technology of heavy metal in agricultural soil (In Chinese). Ecology and Environmental Sciences, 2013, 22(10): 1727-1736. DOI:10.3969/j.issn.1674-5906.2013.10.015 (  0) 0) |
| [14] |
Duan Q N, Lee J C, Liu Y S, et al. Distribution of heavy metal pollution in surface soil samples in China: A graphical review . Bulletin of Environmental Contamination and Toxicology, 2016, 97(3): 303-309. DOI:10.1007/s00128-016-1857-9
(  0) 0) |
| [15] |
王玉军, 欧名豪. 徐州农田土壤养分和重金属含量与分布研究. 土壤学报, 2017, 54(6): 1438-1450. Wang Y J, Ou M H. Contents and distribution of soil nutrients and heavy metal elements in farmlands of Xuzhou (In Chinese). Acta Pedologica Sinica, 2017, 54(6): 1438-1450. (  0) 0) |
| [16] |
张晗, 靳青文, 黄仁龙, 等. 大宝山矿区农田土壤重金属污染及其植物累积特征. 土壤, 2017, 49(1): 141-149. Zhang H, Jin Q W, Huang R L, et al. Characteristics of heavy metal pollution in agricultural soils and bioaccumulation in plants of Dabaoshan mine (In Chinese). Soils, 2017, 49(1): 141-149. (  0) 0) |
| [17] |
蒋逸骏, 胡雪峰, 舒颖, 等. 湘北某镇农田土壤-水稻系统重金属累积和稻米食用安全研究. 土壤学报, 2017, 54(2): 410-420. Jiang Y J, Hu X F, Shu Y, et al. Accumulation of heavy metals in the soil-rice system and assessment of dietary safety of the rice produced in the paddy fields: A case study of a town in the northern part of Hunan Province, China (In Chinese). Acta Pedologica Sinica, 2017, 54(2): 410-420. (  0) 0) |
| [18] |
张奥博, 褚先尧, 殷汉琴, 等. 龙游硫铁矿区农田土壤重金属污染的空间变异及在水稻中的积累. 土壤, 2017, 49(4): 760-769. Zhang A B, Chu X Y, Yin H Q, et al. Spatial variation of eight heavy metals in farmland soils and their accumulation in rice grains in Longyou pyrite mine, Zhejiang Province (In Chinese). Soils, 2017, 49(4): 760-769. (  0) 0) |
| [19] |
Kumpiene J, Brännvall E, Wolters M, et al. Phosphorus and cadmium availability in soil fertilized with biosolids and ashes . Chemosphere, 2016, 151: 124-132. DOI:10.1016/j.chemosphere.2016.02.069
(  0) 0) |
| [20] |
Hollender J, Althoff K, Mundt M, et al. Assessing the microbial activity of soil samples, its nutrient limitation and toxic effects of contaminants using a simple respiration test . Chemosphere, 2003, 53(3): 269-275. DOI:10.1016/S0045-6535(03)00556-3
(  0) 0) |
| [21] |
Chen X, Chen H Y H, Chen X, et al. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations . Applied Soil Ecology, 2016, 107: 162-169. DOI:10.1016/j.apsoil.2016.05.016
(  0) 0) |
| [22] |
Ye X, Kang S, Wang H, et al. Modified natural diatomite and its enhanced immobilization of lead, copper and cadmium in simulated contaminated soils . Journal of Hazardous Materials, 2015, 289: 210-218. DOI:10.1016/j.jhazmat.2015.02.052
(  0) 0) |
| [23] |
Lee S H, Lee J S, Choi Y J, et al. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments . Chemosphere, 2009, 77(8): 1069-1075. DOI:10.1016/j.chemosphere.2009.08.056
(  0) 0) |
| [24] |
Lu M, Xu K, Chen J. Effect of pyrene and cadmium on microbial activity and community structure in soil . Chemosphere, 2013, 91(4): 491-497. DOI:10.1016/j.chemosphere.2012.12.009
(  0) 0) |
| [25] |
Sun Y, Sun G, Xu Y, et al. Assessment of natural sepiolite on cadmium stabilization, microbial communities, and enzyme activities in acidic soil . Environmental Science and Pollution Research, 2013, 20(5): 3290-3299. DOI:10.1007/s11356-012-1261-x
(  0) 0) |
| [26] |
Lin D, Xie X, Zhou Q, et al. Biochemical and genotoxic effect of triclosan on earthworms(Eisenia fetida)using contact and soil tests . Environmental Toxicology, 2012, 27(7): 385-392. DOI:10.1002/tox.v27.7
(  0) 0) |
| [27] |
鲁如坤. 土壤农业化学分析方法. 北京: 中国农业科学技术出版社, 2000. Lu R K. Analytical methods for soil and agro-chemistry(In Chinese) (In Chinese). Beijing: China Agricultural Science and Technology Press, 2000. (  0) 0) |
| [28] |
关松荫. 土壤酶及其研究法. 北京: 农业出版社, 1986. Guan S Y. Soil enzymes and its research methods(In Chinese) (In Chinese). Beijing: Agriculture Press, 1986. (  0) 0) |
| [29] |
Vellinger C, Gismondi E, Felten V, et al. Single and combined effects of cadmium and arsenate in Gammarus pulex(Crustacea, Amphipoda): Understanding the links between physiological and behavioural responses . Aquatic Toxicology, 2013, 140/141: 106-116. DOI:10.1016/j.aquatox.2013.05.010
(  0) 0) |
| [30] |
Usman A R A. Influence of NaCl-induced salinity and Cd toxicity on respiration activity and Cd availability to barley plants in farmyard manure-amended soil. Applied and Environmental Soil Science, 2015, 2015: Article ID: 483836 http://agris.fao.org/agris-search/search.do?recordID=US201700061197
(  0) 0) |
| [31] |
Mushtaq N. Effect of heavy metals contamination of soil by industrial/domestic effluents on size and activity of soil microbial biomass. Rawalpindi: Arid Agricultural University, 2010 http://agris.fao.org/openagris/search.do?recordID=AV2012064232
(  0) 0) |
| [32] |
陈欣瑶, 杨惠子, 李敏, 等. 芘、镉单一及复合污染胁迫下土壤生态功能稳定性的影响机制. 环境科学, 2017, 38(1): 352-360. Chen X Y, Yang H Z, Li M, et al. Mechanism of soil eco-functional stability under pyrene/cadmium simplex and combined pollution stress (In Chinese). Environmental Science, 2017, 38(1): 352-360. (  0) 0) |
| [33] |
Horswell J, Prosser J A, Siggins A, et al. Assessing the impacts of chemical cocktails on the soil ecosystem . Soil Biology and Biochemistry, 2014, 75: 64-72. DOI:10.1016/j.soilbio.2014.03.013
(  0) 0) |
| [34] |
周小梅, 赵运林, 董萌, 等. 镉胁迫对洞庭湖湿地土壤微生物数量与活性的影响. 土壤通报, 2016, 47(5): 1148-1153. Zhou X M, Zhao Y L, Dong M, et al. Effects of cadmium stress on soil microbial quantityand activity in Dongting Lake wetland (In Chinese). Chinese Journal of Soil Science, 2016, 47(5): 1148-1153. (  0) 0) |
| [35] |
McMurry L M, Oethinger M, Levy S B. Triclosan targets lipid synthesis . Nature, 1998, 394(6693): 531. DOI:10.1038/28970
(  0) 0) |
| [36] |
Li X, Zhou Q, Luo Y, et al. Joint action and lethal levels of toluene, ethylbenzene, and xylene on midge(Chironomus plumosus)larvae . Environmental Science and Pollution Research, 2013, 20(2): 957-966. DOI:10.1007/s11356-012-1264-7
(  0) 0) |
| [37] |
Huang X, Wu C, Hu H, et al. Sorption and degradation of triclosan in sediments and its effect on microbes . Ecotoxicology and Environmental Safety, 2015, 116: 76-83. DOI:10.1016/j.ecoenv.2015.03.002
(  0) 0) |
| [38] |
Carey D E, McNamara P J. The impact of triclosan on the spread of antibiotic resistance in the environment. Frontiers in Microbiology, 2015, 5: Article 780 http://www.ncbi.nlm.nih.gov/pubmed/25642217
(  0) 0) |
2. National Engineering Laboratory for Efficient Utilization of Soiland Fertilizer Resources, Taian, Shandong 271018, China;
3. Key Laboratory of AgriculturalEnvironment in Universities of Shandong, Taian, Shandong 271018, China;
4. School of Economics and Management, Dalian Minzu University, Dalian, Liaoning 116650, China
 2018, Vol. 55
2018, Vol. 55


