铁是土壤中丰度最高的可变价过渡金属元素。Fe(Ⅲ)氧化物的溶解度远小于Fe(Ⅱ)氧化物, 故其还原过程伴随着吸附于表面或者包裹其中的氧化物结合态重金属的溶解和迁移, 进而增加了重金属As、Co、Mo和Ni的生物有效性和进入食物链的风险[1-2]。此外, 铁还原微生物(Iron reducing bacteria, IRB)可以在还原铁氧化物的同时直接或者间接促进有机污染物的降解[3-4]。
自然界的厌氧环境, 如水体[5]、沉积物[6]、土壤[7]均可发现IRB介导的铁还原过程, 甚至旱地农田的耕层土壤中亦存在铁的还原过程[8]。旱地土壤铁的厌氧还原过程中99.26%的硝酸盐被还原[8], 促使氮素可能以N2O形式损失[9]。此外, 铁的还原不利于形成水稳性结构体, 引起土壤铁解[10], 但可增加磷的生物有效性[11]。豫西黄土区土层深厚, 耕层以下土壤中铁氧化物总量随剖面呈现一定的增加趋势, 但与表层土壤差异不大[12]。虽然土壤剖面中铁氧化物的形态及相对含量存在差异[13], 黏土矿物结构中铁亦可被IRB还原[14-15], 且耕层以下土壤易于保持厌氧环境, 但耕层以下土壤中铁还原、氧化过程特征如何鲜见报道。
近中性条件下, 还原产生Fe(Ⅱ)在厌氧光照条件下可被光合型Fe(Ⅱ)氧化(Phototrophic ferrous oxidation, PFO)微生物[16]、硝酸盐依赖性Fe(Ⅱ)氧化(Nitrate dependent ferrous oxidation, NDFO)微生物氧化[17], 或者被嗜中性微好氧亚铁氧化菌氧化[18], 其氧化过程中伴随着生物成矿和与重金属离子的共沉淀[19], 从而降低重金属的生物有效性。研究发现水稻土、旱作褐土耕层和表层土壤均存在光合型亚铁氧化过程, Fe(Ⅱ)氧化量与土壤无定形铁、水溶性硫酸盐含量、阳离子交换量显著负相关, 与土壤总氮、总磷显著正相关[20-21]。研究发现微生物可以通过气、水迁移等途径实现不同区域的水平迁移[22], 且土壤耕层以下土壤与表层土壤存在气体交换和水分的淋溶入渗, Fe(Ⅱ)氧化微生物可能随气、水或者植物根系而向下迁移。但耕层以下土壤长期不见光。此外, 硝酸盐在旱地农田土壤剖面中出现富集现象, 但含量低于研究硝酸盐依赖型Fe(Ⅱ)氧化过程采用的硝酸盐浓度[23-25], Fe(Ⅱ)氧化过程是否存在、其特征如何尚不明晰。
鉴于此, 本文采用恒温厌氧泥浆培养的方法研究旱地淋溶褐土的剖面土壤样品在光照和避光条件下铁的氧化还原过程, 以期探索旱地土壤耕层以下土壤中是否存在铁还原过程和光合型Fe(Ⅱ)氧化过程, 为拓展对铁氧化还原微生物生境的认识、深入理解铁氧化还原过程提供依据。
1 材料与方法 1.1 供试材料供试土壤剖面样品采自河南省洛阳市洛宁县王村乡聂坟村旱作农田(111°39′19.30″E, 34°27′14.76″N), 土壤类型为褐土。采样点位于豫西丘陵山区, 暖温带大陆性季风气候, 海拔561 m, 年均降水量570 mm, 主要作物为冬小麦/夏玉米。样品用带刻度的不锈钢管型土钻采集, 每20 cm深度取土钻中心土样, 避光自然风干, 粉碎过20目筛备用。供试土壤基本性质见表 1。
|
|
表 1 供试土壤基本性质 Table 1 Basic soil properties of the sample profiles |
称取每个剖面深度的土壤样品3.000 g若干份于容积为10 mL的血清瓶中, 加入3 mL去离子水后盖上橡胶塞, 充氮5 min以排出顶空中氧气, 在氮气保护下加铝盖密封, 1/2置于光照培养箱(宁波莱福, FPG3)中30±1℃[25]培养作为光照处理(2 000 lux), 1/2置于恒温培养箱(上海博讯, GSP-9270MBE)中30±1℃避光培养(避光处理)。在40 d的持续恒温培养过程中, 定期采样分析Fe(Ⅱ)含量。每次采样时各处理取出3瓶, 打开密封铝盖和橡胶塞, 充分摇匀后用移液器吸取0.4 mL泥浆, 溶于预先加入4.6 mL0.5 mol·L-1盐酸并称重的塑料离心管中, 在30±1 ℃恒温培养箱中浸提24 h。浸提液过0.22 μm滤膜后测定滤液中Fe(Ⅱ)浓度。
培养结束后各处理取3瓶, 用27 mL去离子水将瓶内泥浆全部洗入100 mL三角瓶, 摇匀过滤后分析其水溶性硝酸盐、硫酸盐、无机碳和有机碳的含量。
土壤pH、有机碳、碱解氮、有效磷、缓效钾、速效钾、阳离子交换量、物理性黏粒采用常规分析方法测试[26]。
0.5 mol·L-1 HCl提取Fe(Ⅱ)、水溶性硫酸盐、硝酸盐和水溶性碳含量利用文献[8]中方法测试。
1.3 数据处理参照文献[8]中提到的Logistic方程式拟合避光培养过程中0.5 mol·L-1可提取Fe(Ⅱ)含量随时间的变化, 求出体系中Fe(Ⅱ)的最大累积量(还原潜势)、速率常数、最大速率(Vmax)及其出现的时间。
光照条件下Fe(Ⅱ)最大累积量为培养过程中Fe(Ⅱ)的最大值; 氧化开始时间为培养过程Fe(Ⅱ)开始降低的时间; Fe(Ⅱ)氧化量为Fe(Ⅱ)的最大值与培养结束时体系Fe(Ⅱ)含量的差值; Fe(Ⅱ)氧化速率为平均氧化速率, 数值上等于Fe(Ⅱ)氧化量除以培养过程中氧化持续时间。
数据采用OriginPro 8.5和SPSS 13.0分析处理。
2 结果 2.1 避光时剖面土壤铁的还原特征豫西旱地土壤剖面0~100 cm范围内均存在铁还原过程。避光培养过程中不同土层0.5 mol·L-1 HCl可提取Fe(Ⅱ)随培养时间均呈现“S”型增长趋势(图 1a), 剖面深度并不影响Fe(Ⅱ)累积趋势, 但剖面不同深度土壤中Fe(Ⅱ)的累积量之间存在明显差异(图 1a)。由图 2可知, 旱地土壤剖面铁氧化物的还原潜势在0~60 cm的深度随剖面深度的增加显著降低, 60~80 cm和80~100 cm之间无显著差异。耕层(0~20 cm)和20~40 cm土层中铁氧化物的还原速率常数、最大速率及其出现时间均无显著差异, 剖面深度超过40 cm后呈现铁氧化物的还原速率常数、最大速率均呈现显著降低趋势, 最大速率出现时间则显著后延。
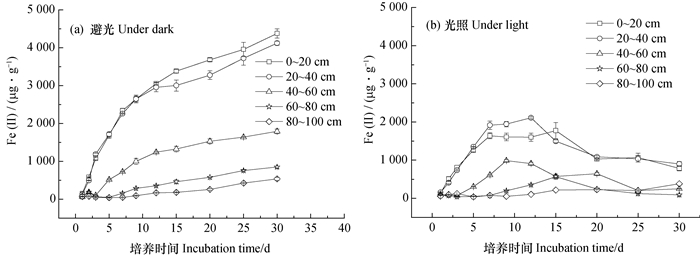
|
图 1 剖面土壤避光(a)与光照(b)厌氧培养过程中0.5 mol·L-1HCl可浸提Fe(Ⅱ)含量变化 Fig. 1 Content of 0.5 mol·L-1 HCl extractable Fe(Ⅱ)in the profile soils under anaerobic incubation in darkness(a)and in light(b) |
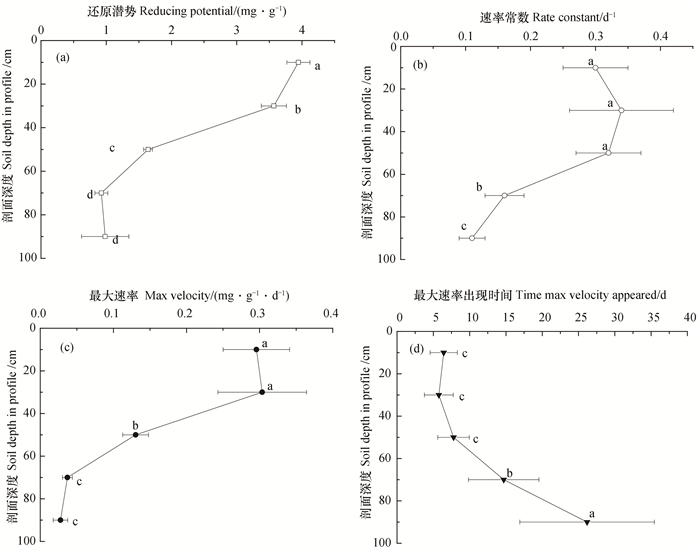
|
图 2 避光培养时土壤铁还原过程的关键参数随剖面深度的变化 Fig. 2 Variation of key parameters of the reducing processes of iron in the soil incubated in darkness with soil depth in profile 注:字母不同表示差异显著(P < 0.05) Note:Different letters mean significant difference at 0.05 level |
光照条件下, 0~80 cm土层中Fe(Ⅱ)随培养时间的累积趋势与避光明显不同(图 1), 培养前期表现为Fe(Ⅲ)还原, 后期Fe(Ⅱ)累积量明显降低, 表现为Fe(Ⅱ)氧化, 说明旱地土壤0~80 cm土层中均存在光合型Fe(Ⅱ)氧化微生物。
光照条件下20~40 cm土层中Fe(Ⅱ)的累积量、氧化量和氧化速率均为最大。除了20~40 cm土层外, Fe(Ⅱ)累积量、氧化量和氧化速率均随剖面深度呈现降低趋势(图 3)。
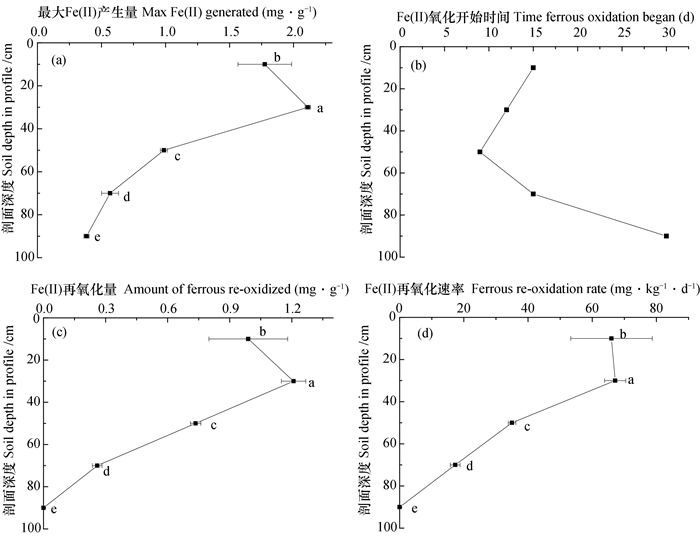
|
图 3 光照培养时铁还原氧化过程的关键参数随剖面深度的变化 Fig. 3 Variation of key parameters of iron redox processes in the soil anaerobically incubated in light with soil depth in profile |
虽80~100 cm土层在培养过程中未出现Fe(Ⅱ)累积量降低所表现出的氧化现象, 但该土层在30 d培养结束时Fe(Ⅱ)的累积量为383.3 μg·g-1, 较之避光时降低了28.74%。前期Fe(Ⅱ)最大累积量以20~40 cm土层最大, 与剖面深度的关系与避光基本一致。对于耕层(0~20 cm)和20~40 cm土层而言, 虽然Fe(Ⅱ)分别在培养12 d和15 d时开始明显降低, 但均自第7天开始不再明显上升, 显著区别于避光处理(图 1 a)。
2.3 培养结束后的水溶性碳在避光或光照条件, 培养结束后剖面土壤中水溶性有机碳均呈现降低趋势, 减少量随剖面深度增加而降低(图 4)。避光处理水溶性有机碳平均降低19.38 mg·kg-1, 平均降幅14.98%;不同土层减少量随剖面深度减小, 降幅在0~60 cm土层之间未见显著变化; 光照处理水溶性有机碳平均降低34.62 mg·kg-1, 平均降幅32.09%。
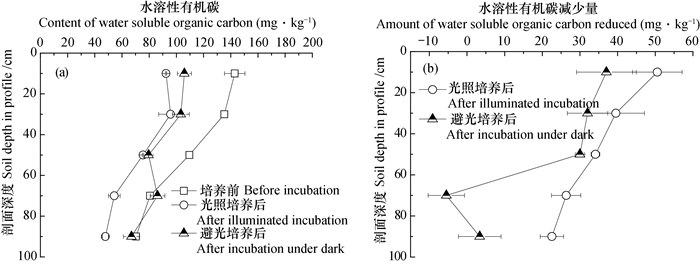
|
图 4 厌氧培养结束后剖面土层中水溶性有机碳含量(a)及减少量(b) Fig. 4 Water soluble organic carbon in the soil profile after anaerobic incubation, (a)content and(b)amount reduced |
避光培养显著增加了剖面中水溶性无机碳的含量, 平均增量55. 50 mg·kg-1, 增量在0~60 cm深度随剖面深度增加而显著减少, 40~100 cm深度土层之间无显著差异(图 5)。光照培养结束后剖面土壤水溶性无机碳含量平均为21.65 mg·kg-1, 不同深度土层之间无显著差异。光照培养降低了0~80 cm土层中水溶性无机碳的含量, 减少量介于16.45~39.80 mg·kg-1之间(图 5)。
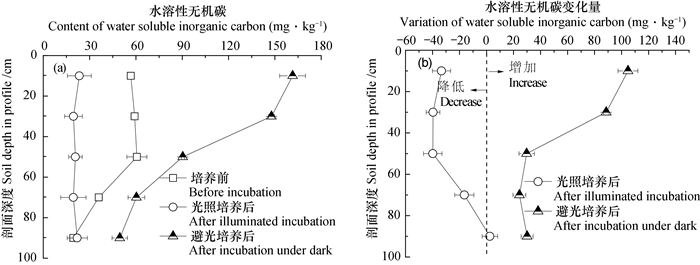
|
图 5 厌氧培养结束后剖面土层中水溶性无机碳含量(a)及变化量(b) Fig. 5 Water soluble inorganic carbon in the soil profile after anaerobic incubation, (a)content and(b)variation |
进一步分析培养过程水溶性碳变化量与铁氧化还原关键参数的关系发现(表 2), 避光时铁还原潜势与水溶性无机碳增量之间存在正相关关系, 铁还原速率常数、最大速率与水溶性有机碳减少量呈显著正相关关系, 显示铁的避光还原过程促进了有机碳的矿化。光照时, Fe(Ⅱ)氧化量与水溶性无机碳减少量显著正相关, 氧化速率水溶性有机碳变化量正相关(表 2), 其原因可能与水溶性无机碳被光合产氧微生物用以产氧, 促使Fe(Ⅱ)的再氧化有关。
|
|
表 2 铁氧化还原关键参数与培养过程水溶性碳、硝酸盐、硫酸盐变化量的相关系数 Table 2 Correlation coefficient between key parameters of iron redox and variations of water soluble carbon, nitrate and sulphate in the process of anaerobic incubation |
培养结束后, 体系中水溶性硝酸盐含量如表 3所示, 可以看出无论避光还是光照条件, 培养结束后水溶性硝酸盐含量均呈现降低趋势, 降幅介于83.41%~98.60%, 且随剖面深度增加而减少。光照处理平均降幅93.12%, 与避光处理的降幅之间无显著差异。
|
|
表 3 培养结束剖面土层中水溶性硝酸盐含量 Table 3 Water soluble nitrate in the soil profile after the incubation |
避光培养结束后水溶性硫酸盐含量降低, 降幅介于22.19%~92.36%, 降幅随剖面深度增加而减少(表 4)。光照培养结束后0~40 cm土层水溶性硫酸盐含量略有增加, 增幅介于7.30%~8.63%;40~100 cm土层水溶性硫酸盐含量降低, 降幅15.21%~26.44%。
|
|
表 4 培养结束剖面土层中水溶性硫酸盐含量 Table 4 Water soluble sulfate in the soil profile after the incubation |
相关分析显示, 水溶性硝酸盐减少量与避光时铁还原潜势、最大还原速率之间存在极显著正相关关系, 与光照时铁的还原量、再氧化量及再氧化速率呈显著正相关关系(表 2)。避光时水溶性硫酸盐减少量与铁还原潜势、最大还原速率呈极显著正相关关系; 光照时与最大Fe(Ⅱ)产生量、再氧化量、再氧化速率之间存在显著负相关关系。
2.5 铁还原氧化关键参数与培养前土壤性质的关系本试验在特定土壤的垂直方向上探索了土壤性质与铁氧化还原的关系(表 5)。避光时铁还原潜势与培养前有机碳、水溶性有机碳含量显著正相关, 这与以前在不同地区耕层土壤水平方向得到的结果一致[8];同时也与水溶性硝酸盐含量、有效磷含量、速效钾含量显著正相关, 与物理性黏粒含量显著负相关。由此可见耕层以下土壤有效态N、P、K含量较低可能是制约铁还原的原因之一。铁还原的最大速率除与上述指标正相关外, 还与水溶性硝酸盐显著正相关。
|
|
表 5 铁氧化还原关键参数与培养前土壤性质的相关系数 Table 5 Correlation coefficient between key parameters of iron redox and soil properties before the incubation |
光照时影响最大Fe(Ⅱ)产生量的因素与避光时的因素基本相同。Fe(Ⅱ)的氧化量除与培养前水溶性有机碳、缓效钾、速效钾、水溶性硝酸盐含量正相关外, 也与水溶性无机碳含量显著正相关, 说明光照时Fe(Ⅱ)氧化的过程需要消耗土壤的水溶性无机碳。Fe(Ⅱ)氧化速率则与有机碳、水溶性有机碳、缓效钾、速效钾、水溶性硝酸盐正相关。
3 讨论褐土是我国小麦与玉米产区的重要土壤类型之一, 分布总面积达2.52×107 hm2, 具有低有机碳、高碳酸盐等特征[12]。土壤有机质和全氮含量是褐土区农业生产和可持续发展的重要影响因素[27]。土壤铁氧化物的厌氧还原与有机碳的矿化、氮的损失密切相关, 且受土壤pH[28]、有机碳[29]、含水量和光照[30]等因素影响。
本试验显示避光时豫西旱地褐土0~100 cm的剖面土壤中的铁氧化物均可被还原, 铁还原潜势介于0.92~3.95 mg·g-1之间, 表明铁还原微生物可在旱地土壤剖面0~100 cm深度存在, 拓展了对铁还原微生物生境的认识。不同区域褐土中铁还原潜势与有机碳含量之间存在显著正相关关系[8], 本试验结果显示铁还原潜势与有机碳含量之间的正相关关系在土壤剖面中依然存在(表 2)。耕层以下土壤尤其是40 cm以下, 长期少人为耕作扰动, 处于厌氧状态, 而且铁氧化物含量有增加趋势[12], 但厌氧培养后铁还原量较之表层显著降低, 可能原因之一就是40 cm以下土壤有机碳含量平均含量3.20 mg·g-1, 不足0~40 cm土层的1/2。此外, 下层土壤底物供给不适宜微生物繁育, 研究也显示土壤微生物量碳随剖面深度显著降低[31];下层土壤铁氧化物活化度较低[32]也可能是下层土壤铁还原潜势显著降低的原因。
土壤硝酸盐也与铁还原关系密切。一方面硝酸盐(7.5 mmol·L-1)可作为竞争电子受体而抑制铁的还原[33], 其抑制作用可因铁氧化物结晶程度和比表面不同而异[33], 亦可因外加碳源而缓解[34]。另一方面硝酸盐可作为微生物可利用之氮源而被同化吸收[35-36]。本试验条件下(硝酸盐含量2.11~13.56 mg·kg-1)土壤中铁还原潜势和最大还原速率与培养前土壤硝酸盐含量、培养前后硝酸盐减少量之间的显著正相关关系(表 2和表 5)显示了硝酸盐对铁还原的促进作用。此外, 研究也发现硝酸盐还原菌Pseudogulbenkiania sp. strain 2002在还原硝酸盐的同时伴随着细胞的生长, 细胞蛋白质含量由20 μg·mL-1增加至30 μg·mL-1[37], 表明了硝酸盐还原菌对氮的同化需求。低浓度(< 2.0 mmol·L-1)硝酸盐可以通过促进细胞活性而促进铁的还原[38]。故而, 硝酸盐可能是在满足微生物自身对氮的同化需要的条件下才可表现为铁氧化物异化还原的竞争电子受体。外加硝酸盐对铁氧化还原过程的浓度效应因土壤而异, 0.59 mmol·L-1硝酸盐可促进Pseudogulbenkiania sp. strain 2002实现氧化Fe(Ⅱ)的功能[37];外加低浓度硝酸盐(0~1.0 mmol·L-1)可促进广东恩平水稻土中铁的还原, 且1.0 mmol·L-1硝酸盐的促进作用高于0.5 mmol·L-1 [39];重复添加1.0 mmol·L-1硝酸盐对意大利维切利水稻土中Fe(Ⅱ)累积并无影响, 对该土壤经过有机质耗竭(30℃厌氧培养3个月)后则表现出轻微不显著的抑制[34]。硝酸盐是否会对本试验所用样品中铁还原过程产生抑制、产生抑制所需硝酸盐的量仍需要进一步研究。
厌氧条件下还原产生Fe(Ⅱ)可被PFO[16]、NDFO微生物氧化[17]。NDFO对Fe(Ⅱ)的氧化受NDFO微生物种类数量、碳源、硝酸盐含量等因素影响, 但不需要光。硝酸盐还原菌Thiobacillus denitrificans和Pseudomonas stutzeri可在将硝酸盐还原为亚硝酸盐的同时氧化Fe(Ⅱ)[37, 40]。Klebsiella pneumoniae L17即可还原硝酸盐亦可还原Fe(Ⅲ)[41]。电子供体受限时硝酸盐还原菌在与铁还原菌竞争电子供体时占优势[34];但也有研究显示江西鹰潭水稻土(pH=5.90)添加生物炭pH升至6.60~7.92后反硝化菌丰度降低而铁还原菌丰度增加[42]。本试验(pH介于7.97~8.17)结果也显示培养前水溶性硝酸盐与铁还原潜势、最大还原速率之间均存在极显著正相关关系(表 3), 但避光培养过程中未见Fe(Ⅱ)氧化现象(图 1 a)。原因可能是:(1)本试验样品中硝酸盐浓度不足以激活硝酸盐依赖型Fe(Ⅱ)氧化微生物, 研究硝酸盐依赖型Fe(Ⅱ)氧化过程采用的硝酸盐浓度多为4.5~10.0 mmol·L-1 [25, 43];(2)无论是DIRB、PFO或者NDFO的繁育均需要氮, 而本试验条件下铁还原微生物与硝酸盐依赖型Fe(Ⅱ)氧化微生物竞争氮源的过程中占优势[42]。
自然条件下耕层以下土壤长期不见光, 可能并不存在光合型Fe(Ⅱ)氧化现象。本试验对耕层及以下土层土壤进行光照刺激的恒温培养试验发现, 采自剖面0~80 cm的土壤样品中均可发生Fe(Ⅱ)的厌氧氧化, 氧化量介于0.26~1.21 mg·g-1。此外, 光合型Fe(Ⅱ)氧化微生物在同化无机碳的同时氧化Fe(Ⅱ)[44]。本试验结果也显示光照时水溶性无机碳显著降低, 且Fe(Ⅱ)氧化量与水溶性无机碳减少量显著正相关(表 2), 与孙丽蓉等[29]研究结果一致。由此, 本试验数据可在侧面显示光合型Fe(Ⅱ)氧化微生物可能随水分的淋溶入渗而在0~80 cm土层存在。80~100 cm土层未见Fe(Ⅱ)氧化现象, 但其光照时铁氧化物还原量显著小于避光时还原量。此外, 虽80~100 cm土层经过光照培养后与避光处理一样增加了水溶性无机碳含量, 但增幅显著小于避光处理(图 5b)。这一差异的原因究竟是光照抑制了铁还原微生物对铁的异化还原过程, 还是虽然存在光合型Fe(Ⅱ)氧化过程, 受限于80~100 cm土壤有机碳、水溶性无机碳含量低或者其他因素而使Fe(Ⅱ)氧化速率小于Fe(Ⅲ)还原速率, 仍需进一步直接分析光合型Fe(Ⅱ)氧化微生物可在土壤剖面淋溶的深度并配合外加碳源、氮源等试验予以证实。
4 结论黄河中下游旱地土壤剖面0~100 cm土层存在铁氧化物的厌氧还原过程, 铁还原潜势随剖面深度增加显著降低, 0~40 cm土层的还原速率常数、最大还原速率和最大速率出现时间均显著高于40 cm以下土壤。旱地土壤剖面0~80 cm土层存在光合型Fe(Ⅱ)氧化现象, 且0~40 cm土层Fe(Ⅱ)氧化量和氧化速率显著高于40~80 cm土层。剖面土壤铁氧化还原过程不仅受到有机碳含量的影响, 也受到硝酸盐、钾等养分元素的影响, 硝酸盐和钾不仅促进光照时Fe(Ⅱ)的氧化, 亦促进避光时铁的还原。
| [1] |
Vink J P M, van Zomeren A, Dijkstra J J, et al. When soils become sediments:Large-scale storage of soils in sandpits and lakes and the impact of reduction kinetics on heavy metals and arsenic release to groundwater . Environmental Pollution, 2017, 227: 146-156. DOI:10.1016/j.envpol.2017.04.016
(  0) 0) |
| [2] |
Yu H Y, Li F B, Liu C S, et al. Iron redox cycling coupled to transformation and immobilization of heavy metals:Implications for paddy rice safety in the red soil of South China . Advances in Agronomy, 2016, 137: 279-317. DOI:10.1016/bs.agron.2015.12.006
(  0) 0) |
| [3] |
Li X, Li Y, Li F, et al. Interactively interfacial reaction of iron-reducing bacterium and goethite for reductive dechlorination of chlorinated organic compounds . Chinese Science Bulletin, 2009, 54(16): 2800-2804.
(  0) 0) |
| [4] |
Li X M, Zhou S G, Li F B, et al. Fe(Ⅲ)oxide reduction and carbon tetrachloride dechlorination by a newly isolated Klebsiella pneumoniaestrain L17 . Journal of Applied Microbiology, 2009, 106(1): 130-139. DOI:10.1111/jam.2008.106.issue-1
(  0) 0) |
| [5] |
Roden E E, Mcbeth J M, Blothe M, et al. The microbial ferrous wheel in a neutral pH groundwater seep . Frontiers in Microbiology, 2012.
(  0) 0) |
| [6] |
Weber K A, Urrutia M M, Churchill P F, et al. Anaerobic redox cycling of iron by freshwater sediment microorganisms . Environmental Microbiology, 2006, 8(1): 100-113. DOI:10.1111/EMI.2006.8.issue-1
(  0) 0) |
| [7] |
Emerson D, Roden E, Twining B S. The microbial ferrous wheel:Iron cycling in terrestrial, freshwater, and marine environments . Frontiers in Microbiology, 2012.
(  0) 0) |
| [8] |
孙丽蓉, 王旭刚, 郭大勇, 等. 旱作褐土中铁氧化物的厌氧还原动力学特征. 土壤学报, 2013, 50(1): 106-112. Sun L R, Wang X G, Guo D Y, et al. Dynamics of anaerobic reduction of iron oxides in upland cinnamon soils (In Chinese). Acta Pedologica Sinica, 2013, 50(1): 106-112. (  0) 0) |
| [9] |
Huang B, Yu K, Gambrell R P. Effects of ferric iron reduction and regeneration on nitrous oxide and methane emissions in a rice soil . Chemosphere, 2009, 74(4): 481-486. DOI:10.1016/j.chemosphere.2008.10.015
(  0) 0) |
| [10] |
何群, 陈家坊, 许祖诒. 土壤中氧化铁的转化及其对土壤结构的影响. 土壤学报, 1981, 18(4): 326-334. He Q, Chen J F, Xu Z Y. Transformation of iron oxides and its influence on soil structure (In Chinese). Acta Pedologica Sinica, 1981, 18(4): 326-334. (  0) 0) |
| [11] |
田娟, 刘凌, 丁海山, 等. 淹水土壤土-水界面磷素迁移转化研究. 环境科学, 2008, 29(7): 1818-1823. Tian J, Liu L, Ding H S, et al. Mobilization and transformation of phosphorus from water-soil interface of flooded soil (In Chinese). Environmental Science, 2008, 29(7): 1818-1823. DOI:10.3321/j.issn:0250-3301.2008.07.010 (  0) 0) |
| [12] |
李宪文, 魏克循. 河南黄土母质褐土碳酸盐特征的研究. 土壤通报, 1997, 28(6): 265-267. Li X W, Wei K X. Study on the carbonate characteristics of loess soil in Henan (In Chinese). Chinese Journal of Soil Science, 1997, 28(6): 265-267. DOI:10.3321/j.issn:0564-3945.1997.06.009 (  0) 0) |
| [13] |
季峻峰, 陈骏, Balsam W, 等. 黄土剖面中赤铁矿和针铁矿的定量分析与气候干湿变化研究. 第四纪研究, 2007, 27(2): 221-229. Ji J F, Chen J, Balsam W, et al. Quantitative analysis of hematite and goethite in the Chinese loess-paleosol sequences and its implication for dry and humid variability (In Chinese). Quaternary Sciences, 2007, 27(2): 221-229. DOI:10.3321/j.issn:1001-7410.2007.02.007 (  0) 0) |
| [14] |
Jaisi D P, Dong H L, Liu C X. Kinetic analysis of microbial reduction of Fe(Ⅲ)in nontronite . Environmental Science & Technology, 2007, 41(7): 2437-2444.
(  0) 0) |
| [15] |
Kostka J E, Wu J, Nealson K H, et al. The impact of structural Fe(Ⅲ)reduction by bacteria on the surface chemistry of smectite clay minerals . Geochimica et Cosmochimica Acta, 1999, 63(22): 3705-3713. DOI:10.1016/S0016-7037(99)00199-4
(  0) 0) |
| [16] |
Caiazza N C, Lies D P, Newman D K. Phototrophic Fe(Ⅱ)oxidation promotes organic carbon acquisition by Rhodobacter capsulatus SB1003 . Applied and Environmental Microbiology, 2007, 73(19): 6150-6158. DOI:10.1128/AEM.02830-06
(  0) 0) |
| [17] |
Weber K A, Pollock J, Cole K A, et al. Anaerobic nitrate-dependent iron(Ⅱ)bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002 . Applied and Environmental Microbiology, 2006, 72(1): 686-694. DOI:10.1128/AEM.72.1.686-694.2006
(  0) 0) |
| [18] |
陈娅婷, 李芳柏, 李晓敏. 水稻土嗜中性微好氧亚铁氧化菌多样性及微生物成矿研究. 生态环境学报, 2016, 25(4): 547-554. Chen Y T, Li F B, Li X M. Diversity and biomineralization of microaerophilic iron-oxidizing bacteria in paddy soil (In Chinese). Ecology & Environmental Sciences, 2016, 25(4): 547-554. (  0) 0) |
| [19] |
Lack J G, Chaudhuri S K, Kelly S D, et al. Immobilization of radionuclides and heavy metals through anaerobic bio-oxidation of Fe(Ⅱ) . Applied and Environmental Microbiology, 2002, 68(6): 2704-2710. DOI:10.1128/AEM.68.6.2704-2710.2002
(  0) 0) |
| [20] |
孙丽蓉, 王旭刚, 徐晓峰, 等. 旱作褐土中氧化铁的厌氧还原与光合型亚铁氧化特征. 土壤学报, 2015, 52(6): 1291-1300. Sun L R, Wang X G, Xu X F, et al. Anaerobic redox of iron oxides and photosynthetic oxidation of ferrous iron in upland cinnamon soils (In Chinese). Acta Pedologica Sinica, 2015, 52(6): 1291-1300. (  0) 0) |
| [21] |
王旭刚, 徐晓峰, 孙丽蓉, 等. 厌氧条件下水稻土中铁硫循环与光照的关系. 土壤学报, 2013, 50(4): 712-719. Wang X G, Xu X F, Sun L R, et al. Relationships of illumination with iron and sulfur cyclings in paddy soil under anaerobic incubation (In Chinese). Acta Pedologica Sinica, 2013, 50(4): 712-719. (  0) 0) |
| [22] |
Zhu Y, Gillings M, Simonet P, et al. Microbial mass movements . Science, 2017, 357(6356): 1099-1100. DOI:10.1126/science.aao3007
(  0) 0) |
| [23] |
夏梦洁, 陈竹君, 刘占军, 等. 黄土高原旱地夏季休闲期15N标记硝态氮的去向. 土壤学报, 2017, 54(5): 1230-1239. Xia M J, Chen Z J, Liu Z J, et al. Fate of 15N labeled nitrate in dryland under summer fallow on the Loess Plateau (In Chinese). Acta Pedologica Sinica, 2017, 54(5): 1230-1239. (  0) 0) |
| [24] |
高亚军, 李云, 李生秀, 等. 旱地小麦不同栽培条件对土壤硝态氮残留的影响. 生态学报, 2005, 25(11): 109-118. Gao Y J, Li Y, Li S X, et al. Effects of different wheat cultivation methods on residual nitrate nitrogen in soil in dryland (In Chinese). Acta Ecologica Sinica, 2005, 25(11): 109-118. (  0) 0) |
| [25] |
Li X M, Zhang W, Liu T X, et al. Changes in the composition and diversity of microbial communities during anaerobic nitrate reduction and Fe(Ⅱ)oxidation at circumneutral pH in paddy soil . Soil Biology & Biochemistry, 2016, 94: 70-79.
(  0) 0) |
| [26] |
鲁如坤. 土壤农业化学分析方法. 北京: 中国农业科学技术出版社, 2000. Lu R K. Analytical methods for soil and agro-chemistry (In Chinese). Beijing: China Agricultural Science and Technology Press, 2000. (  0) 0) |
| [27] |
赵秀娟, 任意, 张淑香. 25年来褐土区土壤养分演变特征. 核农学报, 2017, 31(8): 1647-1655. Zhao X J, Ren Y, Zhang S X. Evolution characteristics of cinnamon soil nutrients in 25 years (In Chinese). Journal of Nuclear Agricultural Sciences, 2017, 31(8): 1647-1655. (  0) 0) |
| [28] |
李金珠, 王旭刚, 袁雪红, 等. pH与石灰性水稻土铁氧化还原过程的关系. 土壤学报, 2014, 51(1): 143-149. Li J Z, Wang X G, Yuan X H, et al. Relationship between pH and iron redox cycle in calcareous paddy soil (In Chinese). Acta Pedologica Sinica, 2014, 51(1): 143-149. (  0) 0) |
| [29] |
孙丽蓉, 黄海霞, 王旭刚, 等. 褐土中铁的氧化还原与碳素转化. 土壤学报, 2013, 50(3): 540-547. Sun L R, Huang H X, Wang X G, et al. Relationship between anaerobic redox of iron oxides and carbon transformation in cinnamon soil (In Chinese). Acta Pedologica Sinica, 2013, 50(3): 540-547. (  0) 0) |
| [30] |
王旭刚, 郭大勇, 张苹, 等. 水稻土中铁氧化还原循环的光照水分效应. 土壤学报, 2014, 51(4): 853-859. Wang X G, Guo D Y, Zhang P, et al. Effect of illumination and water condition on iron redox cycle in paddy soil (In Chinese). Acta Pedologica Sinica, 2014, 51(4): 853-859. (  0) 0) |
| [31] |
盛浩, 周萍, 袁红, 等. 亚热带不同稻田土壤微生物生物量碳的剖面分布特征. 环境科学, 2013, 34(4): 1576-1582. Sheng H, Zhou P, Yuan H, et al. Profile of soil microbial biomass carbon in different types of subtropical paddy soils (In Chinese). Environmental Science, 2013, 34(4): 1576-1582. (  0) 0) |
| [32] |
王旭刚, 孙丽蓉. 土壤剖面胶体中铁氧化物及其厌氧还原特征研究. 河南农业科学, 2009(3): 38-42. Wang X G, Sun L R. Iron oxides in soil colloids and its reduction characters (In Chinese). Journal of Henan Agricultural Sciences, 2009(3): 38-42. DOI:10.3969/j.issn.1004-3268.2009.03.011 (  0) 0) |
| [33] |
Zhang W, Li X, Liu T, et al. Competitive reduction of nitrate and iron oxides by Shewanella putrefaciens 200 under anoxic conditions . Colloids & Surfaces A:Physicochemical & Engineering Aspects, 2014, 445(6): 97-104.
(  0) 0) |
| [34] |
Achtnich C, Bak F, Conrad R. Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil . Biology & Fertility of Soils, 1995, 19(1): 65-72.
(  0) 0) |
| [35] |
程谊, 黄蓉, 余云飞, 等. 应重视硝态氮同化过程在降低土壤硝酸盐浓度中的作用. 土壤学报, 2017, 54(6): 1326-1331. Cheng Y, Huang R, Yu Y F, et al. Role of microbial assimilation of soil NO3- in reducing soil NO3- concentration (In Chinese). Acta Pedologica Sinica, 2017, 54(6): 1326-1331. (  0) 0) |
| [36] |
Vitousek P M, Howarth R W. Nitrogen limitation on land and in the sea:How can it occur? . Biogeochemistry, 1991, 13(2): 87-115.
(  0) 0) |
| [37] |
Chen D, Liu T, Li X, et al. Biological and chemical processes of microbially mediated nitrate-reducing Fe(Ⅱ)oxidation by Pseudogulbenkiania sp. strain 2002 . Chemical Geology, 2018, 476: 59-69. DOI:10.1016/j.chemgeo.2017.11.004
(  0) 0) |
| [38] |
Li B B, Cheng Y Y, Wu C, et al. Interaction between ferrihydrite and nitrate respirations by Shewanella oneidensis MR-1 . Process Biochemistry, 2015, 50(11): 1942-1946. DOI:10.1016/j.procbio.2015.07.016
(  0) 0) |
| [39] |
Yu H Y, Wang Y K, Chen P C, et al. Effect of nitrate addition on reductive transformation of pentachlorophenol in paddy soil in relation to iron(Ⅲ)reduction . Journal of Environmental Management, 2014, 132: 42-48.
(  0) 0) |
| [40] |
Straub K L, Benz M, Schink B, et al. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron . Applied and Environmental Microbiology, 1996, 62(4): 1458-1460.
(  0) 0) |
| [41] |
Liu T X, Li X M, Zhang W, et al. Fe(Ⅲ)oxides accelerate microbial nitrate reduction and electricity generation by Klebsiella pneumoniae L17 . Journal of Colloid & Interface Science, 2014, 423(6): 25-32.
(  0) 0) |
| [42] |
Wang N, Chang Z Z, Xue X M, et al. Biochar decreases nitrogen oxide and enhances methane emissions via altering microbial community composition of anaerobic paddy soil . Science of the Total Environment, 2017, 581/582: 689-696. DOI:10.1016/j.scitotenv.2016.12.181
(  0) 0) |
| [43] |
Chakraborty A, Picardal F. Neutrophilic, nitrate-dependent, Fe(Ⅱ)oxidation by a dechloromonas species . World Jounal of Microbiology Biotechnology, 2013, 29(4): 617-623. DOI:10.1007/s11274-012-1217-9
(  0) 0) |
| [44] |
Melton E D, Schmidt C, Kappler A. Microbial iron(Ⅱ)oxidation in littoral freshwater lake sediment:The potential for competition between phototrophic vs. nitrate-reducing iron(Ⅱ)-oxidizers . Frontiers in Microbiology, 2012.
(  0) 0) |
 2018, Vol. 55
2018, Vol. 55

