微生物种间电子传递(Interspecies electron transfer,IET)是指电子供体微生物与电子受体微生物之间通过直接或间接方式传递电子形成互营生长关系,从而共同完成单一微生物不能完成的代谢过程的现象[1-3]。例如,在以乙醇为电子供体、富马酸为电子受体的地杆菌共培养体系中,金属还原地杆菌Geobacter metallireducens不能以富马酸为电子受体氧化乙醇,硫还原地杆菌G. sulfurreducens无法以乙醇为电子供体还原富马酸,但当两微生物同时存在时,G. metallireducens可将乙醇氧化产生的电子传给G. sulfurreducens,G. sulfurreducens再将电子传至富马酸,实现G. metallireducens与G. sulfurreducens的电子互营[4]。微生物IET分为间接种间电子传递(Mediated IET,MIET)和直接种间电子传递(Direct IET,DIET)。其中,MIET是指微生物间通过氧化还原性内源(细菌自身产生的)小分子物质如氢/甲酸、核黄素等或外源(天然存在或人工合成)小分子物质如腐殖质等实现电子传递的方式,是一种经典的互营生长机制[5]。DIET是指微生物间通过自身导电结构如导电菌毛和细胞色素c等实现电子交换的过程[6]。此外,外源导电物质如活性炭[7]和磁铁矿[8]也能介导微生物间的IET。由于它们介导的IET不需要能量载体的协助,而是直接依靠介体的导电性实现电子传递,因此将这类IET归为DIET。
微生物IET起源最早可追溯至20世纪初。1906年Omelianski[9]分离得到的乙醇氧化产甲烷奥氏杆菌(Methanobacillus omelianskii)。之后,Bryant等[10]发现M. omelianskii其实是产乙酸菌与产甲烷菌的共培养物,产乙酸菌与产甲烷菌间以氢气为电子载体实现电子互营。除氢气外,1988年Thiele和Zeikus[11]发现甲酸也能协助微生物间的电子传递,并且在互营产甲烷消化器中甲酸对IET的贡献远大于氢气的贡献。在很长一段时间内,微生物间以氢/甲酸为电子载体进行电子传递被认为是微生物间电子互营的主要方式,直至2010年Summers等[4]在地杆菌G. metallireducens与G. sulfurreducens的共培养体系中发现细胞色素c和菌毛介导的DIET,才打破了微生物互营代谢的传统认识,为微生物IET研究打开了新视角。
微生物IET普遍存在且方式多样。产甲烷反应器、厌氧沉积物中广泛存在的产甲烷菌Methanosaeta与地杆菌Geobacter之间既能借助氢/甲酸[12]和氧化还原性小分子物质[13-15]等实现电子转移,也能利用自身导电菌毛和氧化还原蛋白进行IET。目前微生物间利用氢/甲酸实现共培养的研究已比较成熟,但DIET研究仍处于初级阶段。为进一步理解厌氧微生物间的电子互营,本文在论述MIET机制的同时,重点阐述了DIET机制及研究现状,列举了代表性IET微生物,介绍了IET在环境修复、温室气体减排等过程中的作用,并就IET未来研究方向提出了展望。
1 微生物IET机制微生物IET方式分为MIET和DIET。其中,MIET包括:种间氢/甲酸转移(即微生物间以氢/甲酸为电子载体实现IET的方式,图 1a)和电子穿梭机制(即微生物利用具有氧化还原特性的小分子物质如吩嗪、核黄素、腐殖质等实现电子转移的过程,图 1b);DIET包括:纳米导线机制(微生物间利用自身导电菌毛实现电子互营,图 1c)、氧化还原蛋白机制(主要以细胞色素c为电子载体进行电子传递,图 1d)和导电物质介导机制(利用外源导电颗粒如活性炭、磁铁矿等进行电子传递,图 1e)。此外,近年来在海洋沉积物中发现的电缆细菌(Cable bacteria)与硫氧化菌间的长距离氧化还原耦合也被认为是微生物IET的一种(图 1f):电缆细菌利用自身的长纤毛将硫氧化菌传来的电子传至沉积表面的含氧层,将氧气还原生成水,与硫氧化菌实现电子互营[16]。

|
(a)种间氢/甲酸转移Exchange of electrons between species via hydrogen/formate,(b)电子穿梭体机制Electron transfer shuttle,(c)纳米导线机制Electrically conductive pili,(d)氧化还原蛋白机制Proteins associated with outer cell surfaces,(e)导电颗粒介导机制Conductive materials,(f)长距离氧化还原耦合Long-distance electron transport via redox coupling 图 1 微生物IET机制[16-18] Fig. 1 Mechanisms of microbial interspecies electron transfer[16-18] |
1967年Bryant等[10]在研究乙醇氧化产甲烷奥氏杆菌(M. omelianskii)时首次发现以氢气为电子载体的IET。该奥氏杆菌是产乙酸菌S菌株与产甲烷菌Methanobacterium ruminantium的共培养物。在该共培养体系中,S菌株氧化乙醇产氢气,M. ruminantium利用氢气还原CO2产甲烷。前者属于吸能反应,不能自发发生,必须依赖后者不断消耗氢气降低氢分压(理论上需小于0.1kPa),才能持续反应[19]。除氢外,Thiele和Zeikus[11]在互营产甲烷消化器中发现甲酸也能促进微生物IET,并且甲酸对IET的贡献(大于90%)远高于氢气对IET的贡献(小于10%)。1989年Boone等[20]在互营菌(Syntrophomonas wolfei)与产甲烷菌(M. formicicum)的共培养体系中,采用扩散模型进一步证实了种间甲酸转移的存在,并且发现种间甲酸转移速率是种间氢转移的100倍。2002年Plugge等[21]发现丙酸盐氧化菌与产甲烷菌共培养体系在甲酸和氢气同时存在的情况下生长更好,表明种间氢和甲酸转移可同时发生。此外,对于既可消耗氢气又可产生氢气的互营体系,微生物IET甚至存在电子反向传递的可能。例如在Desulfovibrio vulgaris和Methanosarcina barkeri的共培养体系中,D. vulgaris降解乳酸生成乙酸、二氧化碳和氢气,电子经由氢气传递至M. barkeri将二氧化碳还原成甲烷[22]。但当氢气浓度较低时,M. barkeri自身会释放出氢气,氢气能被D. vulgaris利用,因此D. vulgaris和M.barkeri之间存在电子反向传递(电子由M. barkeri传至D. vulgaris)的可能[23]。此外,2008年Kosaka等[24]研究发现在以氢气为电子载体的互营体系中,甲酸脱氢酶会表达生成甲酸,推测甲酸是微生物种间氢转移的临时电子载体。De Bok等[25]根据费克扩散定律,对比氢气和甲酸的扩散速率和溶解度,提出氢气主要参与近距离的电子传递,而甲酸参与远距离的电子转移的理论。2013年Felchner-Zwirello等[26]研究认为微生物间可以通过缩短距离来达到增加种间氢转移通量的目的。
在种间氢/甲酸转移体系中,微生物氧化有机物产氢通常与NAD+/NADH、FAD/FADH2或辅酶F420/F420-H2以及Fd(ox)/Fd(red)(Fd,铁氧化还原蛋白)等氧化还原中间体的相互转化相耦联[27]。这些耦联反应在标准状态下的吉布斯自由能通常为正值,反应无法自发发生。当氢气分压小于某值时,吉布斯自由能由正值变为负值,反应可自发发生,方便电子供体微生物传出电子(表 1)。
|
|
表 1 种间氢/甲酸转移中氢/甲酸相关反应能量变化[5] Table 1 Energy variation of hydrogen/formate-related reactions in interspecies hydrogen/formate transfer[5] |
电子穿梭机制是指微生物间借助自身分泌、天然存在或人工合成的具有氧化还原活性的小分子物质(电子穿梭体)进行电子传递的方式[28]。1978年Biebl和Pfennig[29]在脱硫单胞杆菌Desulfuromonas acatoxidans和突柄绿菌Prosthecochloris aestuarii的共培养体系中发现硫化物能够介导微生物IET。其中,D. acatoxidans以乙醇为电子供体将硫单质还原成硫化物,P. aestuarii通过光合作用固定CO2并将硫化物氧化生成硫单质,如此循环往复实现IET。2002年Kaden等[30]在以乙酸为电子供体、硝酸盐为电子受体的地杆菌G. sulfurreducens与沃林氏菌Wolinella succinogenes共培养体系中发现了L-胱氨酸/半胱氨酸介导的IET。G. sulfurreducens在氧化乙酸的同时将L-胱氨酸还原成L-半胱氨酸,W.succinogenes利用L-半胱氨酸将硝酸盐还原,而L-半胱氨酸被氧化成L-胱氨酸再次参与乙酸氧化。2015年Smith等[15]发现在外源添加腐殖质类似物AQDS的情况下,G. sulfurreducens与G. metallireducens的互营代谢速率得到促进,并且突变株共培养实验证明AQDS协助的IET能为受体微生物G. sulfurreducen提供足够能量用于生长。
1.2 DIET机制及研究现状2010年Summers等[4]在以乙醇为电子供体、富马酸为电子受体的G. metallireducens与G.sulfurreducens共培养体系中发现了细胞色素c和菌毛介导的DIET。DIET的发现打破了对微生物IET的传统认知,是微生物IET研究的一大里程碑。目前地杆菌参与的DIET研究最为透彻,本节将主要总结地杆菌参与的DIET机制研究。
地杆菌参与的DIET体系如G. metallireducens与G. sulfurreducens的共培养中,通常能看到大量菌毛形成的缠绕结构[4]。在菌毛的作用下,互营微生物双方形成紧密的团聚体,以缩短电子传递距离,减少能量损耗。而对于无导电菌毛介导的互营体系通常无法实现DIET。如G. metallireducens在缺失菌毛表达能力的情况无法与产甲烷菌Methanosaeta或Methanosarcina实现IET[31]。并且G. metallireducens与G. sulfurreducens中无论哪一方被敲除菌毛表达基因均无法与对方进行电子互营[4, 6],表明了菌毛在DIET中的重要地位。
菌毛是从细胞表面延伸出来的丝状蛋白,不仅能帮助细菌附着,促进细胞运动,也能将电子传出至胞外电子受体。2005年Reguera等[32]利用改造的原子力显微镜发现G. sulfurreducens菌毛具有导电欧姆响应。Lovley等[33]纯化菌毛检测发现G. sulfurreducens菌毛电导率能达到188Ms·cm-1,类似于金属导电性,并提出了菌毛的类金属导电模型[34](见图 2a):电子通过菌毛芳香环π-π轨道折叠形成的电子云进行传递,而细胞色素c在菌毛电子传递过程中只是充当中间体或电子储容器。2011年Strycharz等[35]在G. sulfurreducens传递电子至电极的过程中,通过基因敲除等手段提出了不同于类金属导电模型的电子跃迁理论(见图 2b):电子沿着菌毛上毗邻的氧化还原蛋白(细胞色素c)逐步跃迁至电子受体实现电子转移,其中菌毛起着电子传递支架的作用。2013年Bonanni等[36]结合类金属导电模型和电子跃迁模型,提出了Stepping stone理论(见图 2c):电子沿着菌毛上的芳香环簇进行传导,当芳香环簇距离大于电子可直接传递距离时,电子依靠菌毛上的细胞色素c进行传递。
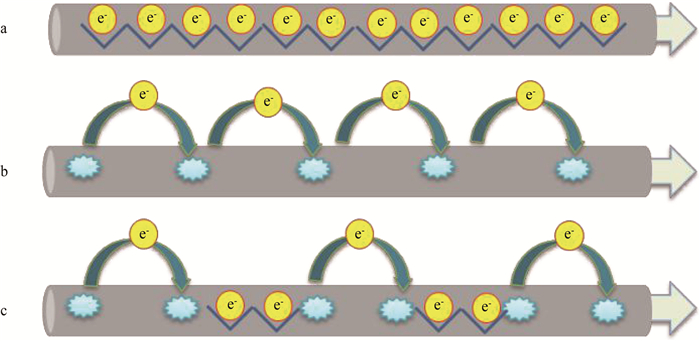
|
(a)类金属导电性模型Metalloid conductivity model[34],(b)电子跃迁模型Electron hopping model[35],(c)Stepping stone模型Stepping stone model[36] 图 2 纳米导线导电模型 Fig. 2 Models of electron flow via nanowires |
微生物周质和膜上通常分布有多种氧化还原蛋白,且在不同胞外电子传递模式下参与的细胞色素c种类存在差异[37]。根据2016年Shi等[38]提出的地杆菌G. sulfurreducens电子由内膜向外膜传递的模型(图 3):胞内电子通过氢醌的氧化反应将电子传递给内膜上的ImcH(高电位)或CbcL(低电位)蛋白,ImcH或CbcL上的电子经周质蛋白PpcA家族蛋白的介导传递至外膜蛋白如OmaB、OmcB、OmaC、OmcC。其中OmaB、OmcB与OmbB以及OmaC、OmcC与OmbC形成跨膜蛋白复合体介导电子传递,在胞外电子传递过程中起着重要作用。在以Fe3+为电子受体的胞外电子传递过程中,细胞色素OmcS是电子由细胞表面传递至胞外电子受体的重要介体蛋白[39]而参与较薄的阳极生物膜胞外电子传递的蛋白主要是OmcS和OmcE[40],当阳极生物膜变厚时,参与胞外电子传递过程的细胞色素主要是菌毛蛋白PilA和细胞色素OmcZ[41]。
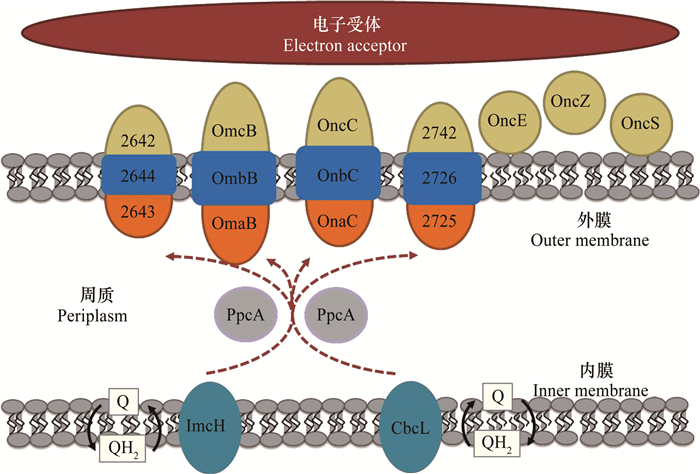
|
图 3 地杆菌电子由内膜向外膜的转移[38-41] Fig. 3 Electron transfer from inner membrane to outer membrane in Geobacter[38-41] |
在地杆菌参与的DIET过程中,发挥重要作用的氧化还原蛋白是外膜细胞色素OmcS。2010年Summers等[4]在G. metallireducens与G. sulfurreducens的互营体系中发现一种与G. sulfurreducens导电菌毛相结合的氧化还原蛋白-细胞色素OmcS得到了大量表达,并且G. sulfurreducens在敲除掉OmcS的表达基因后无法与G. metallireducens实现共培养,表明OmcS在DIET中担当着重要角色。此外,跨膜蛋白在IET也发挥了一定的作用。2017年Phuc等[42]在突柄绿菌P. aestaurii与地杆菌G. sulfurreducens的互营厌氧光合成体系中发现,两者外膜紧密接触,且G. sulfurreducens在缺失跨膜孔蛋白与细胞色素复合体(ombB-omaB-omcB-orfS-ombC-omaC-omcC)的情况下无法与P. aestaurii达成共培养关系,表明外膜蛋白影响了微生物间的DIET过程。2015年Benomar等[43]通过蛋白荧光标记发现,脱硫弧菌Desulfovibrio vulgaris和梭菌Clostridium acetobutylicum共培养时进行了蛋白交换,表明微生物间存在通过交换蛋白实现IET的可能。
除依靠自身导电结构进行DIET之外,微生物也能借助外源的非生物导电材料如磁铁矿、颗粒活性炭或生物炭等实现DIET。2012年Kato等[44]在水稻土中添加(半)导体铁氧化物如磁铁矿和赤铁矿发现Geobacter与Methanosarcina间的电子传递得到了促进,而添加非导电性铁氧化物对Geobacter与Methanosarcina间的电子传递不具备促进作用。2015年Li等[45]在水稻土中分别加入二氧化硅包裹的磁铁矿和裸露的磁铁矿发现,二氧化硅包裹的磁铁矿不能像裸露磁铁矿一样促进微生物DIET,表明磁铁矿对DIET的促进作用与导电性相关。同年,Liu等[46]发现原本无法实现电子互营的G. sulfurreducens的细胞色素OmcS缺陷株与G. metallireducens在外源添加磁铁矿的作用下能够形成共培养,但G. sulfurreducens的菌毛PilA突变株与G. metallireducens不能恢复电子互营,表明磁铁矿可以弥补细胞色素OmcS的缺失,但不能替代菌毛的功能。导电碳材料如颗粒活性炭、生物炭和碳布等也可以介导微生物IET[47]。2012年Liu等[7]发现,在颗粒活性炭的介导下,G. sulfurreducens细胞色素OmcS缺陷株或菌毛蛋白PilA缺陷株能够与G. metallireducens实现电子互营,证明颗粒活性炭具有介导DIET的能力,且颗粒活性炭介导下的DIET无需菌毛和细胞色素c参与。此外,Chen等[48-49]发现导电性生物炭和碳布能促进G. metallireducens与G. sulfurreducens及M. barkeri间的DIET,但对D. vulgaris与G. sulfurreducens的种间氢转移无促进作用。
1.3 IET微生物MIET常见形式有种间氢/甲酸转移和电子穿梭体介导的IET,其中,种间氢/甲酸转移常见于产甲烷微生物群落,如产甲烷菌与产乙酸菌[10]、产甲烷菌与脱硫弧菌[22]、地杆菌与甲醇降解菌[50]。此外,电子穿梭体如硫化物、L-半胱氨酸、AQDS等也能协助微生物MIET,如脱硫菌与光合菌[29]、地杆菌与沃林氏菌[30]等之间的电子传递。
DIET由于需要微生物导电菌毛和细胞色素c的介导,常见于地杆菌参与的共培养体系,如地杆菌与地杆菌[46]、地杆菌与产甲烷菌[31, 48, 51]、地杆菌与光合菌[42]、地杆菌与脱氮菌[52]等。外源添加的(半)导电材料如磁铁矿、活性炭等也能协助微生物DIET(表 2)。此外,脱氯菌与互营菌[53]、厌氧甲烷氧化菌与硫酸盐还原菌[54]间也能以DIET形式进行电子传递。在厌氧甲烷氧化古菌ANME-1与硫酸盐还原菌HotSeep-1的互营代谢过程中,ANME-1与HotSeep-1的胞外细胞色素c和菌毛相关基因得到了高度表达,表明菌毛和细胞色素c在两者互营过程中扮演着重要角色[54]。
|
|
表 2 部分参与IET微生物及其IET方式 Table 2 Microorganisms participating in IET and their IET patterns |
厌氧消化技术是指厌氧环境中,微生物分工合作将大分子有机废物水解并最终生成可再生生物质能源氢气和甲烷的过程[55]。常规厌氧消化系统具有启动缓慢、处理能效低、反应时间长等缺点,提高产甲烷菌的代谢活性和产甲烷速率是提高厌氧消化效能的关键所在。2014年Cruz等[8]发现磁铁矿颗粒能够更快速地引发丙酸降解产甲烷,表明磁铁矿对微生物有机物氧化产甲烷具有促进作用。2016年Dang等[56]发现往厌氧消化池添加导电碳材料如碳布、碳毡和活性炭等可促进厌氧消化池中有机物的降解。Zhao等[57]通过往厌氧消化池添加导电碳布发现微生物IET方式由IHT转变为DIET,缓解了消化池中酸对种间氢转移的抑制作用。Yang等[58]发现厌氧消化池中的微生物量与有机物降解速率密切相关,因此外源添加产甲烷微生物,可加快微生物厌氧消化产甲烷过程。
2.2 甲烷厌氧氧化甲烷是一种重要的能源物质,也是引起地球温室效应的重要气体。微生物主导的甲烷厌氧氧化能够有效地减少甲烷的排放[59]。目前为止发现的甲烷厌氧氧化类型主要有硫酸盐还原型甲烷氧化和反硝化型甲烷氧化,并且认为是微生物进行IET的结果[60]。2015年McGlynn等[54]发现增强菌毛和细胞色素c的表达可促进甲烷厌氧氧化。此外,Egger[61]和Ettwig[62]等发现在铁氧化物如磁铁矿纳米颗粒等协助下,微生物甲烷厌氧氧化得到促进,表明外源添加(半)导体材料是提高甲烷厌氧氧化速率的重要手段。
2.3 微生物脱氯含氯污染物通常具有持久性,对环境及人类的健康威胁大。微生物厌氧脱氯是自然环境下含氯污染物的主要分解方法,但脱氯微生物在总微生物群中占的比重较低,对含氯污染物的降解有限。2015年Mao等[53]发现微生物IET能够促使污染物脱氯。并且添加磁铁矿纳米颗粒可以促进微生物IET脱氯反应[63],表明导电材料如磁铁矿纳米颗粒等能够加快含氯污染物的降解。
3 展望微生物IET研究不仅有助于深入理解厌氧微生物互营代谢,也可为生物能源生产、温室气体减排以及环境修复提供技术支撑。微生物IET的核心是电子在微生物之间的传递,研究IET的微观机制,发掘高效IET微生物,寻找提高微生物IET效率的方法,是将微生物IET用于实践的重点研究方向。目前微生物IET微观机制的研究仍处于初级阶段,电子究竟如何进行传递、自然界中是否存在更高效的IET微生物以及是否存在更为经济高效的方法加速微生物IET等这些问题尚待进一步明晰。地杆菌作为土壤中广泛存在的电活性菌已有大量研究报道其胞外电子传递机制[64]:1)以Fe3+为电子受体的胞外电子传递过程中,G. sulfurreducens的细胞色素OmcS是参与胞外电子传递的主要蛋白[39];2)在以电极为电子受体的情况下,参与胞外电子传递的主要蛋白是OmcS、OmcE[40]以及OmcZ[41];3)在DIET过程中,外膜细胞色素OmcS是参与胞外电子传递的主要氧化还原蛋白[4]。受限于生物手段及技术水平,目前尚无法对微生物IET的电子传递路径进行全程追踪,无法明确参与IET的所有蛋白及结构。研究微生物IET微观机制,确定参与IET的蛋白及结构,通过基因改造等手段,可提高微生物IET。2013年Leang等[65]发现,改造G. sulfurreducens的基因使其表达更多的导电菌毛,能够提高G. sulfurreducens的产电能力。2016年Tan等[66]发现改造菌毛上的氨基酸能够使菌毛的导电性能增强,提高胞外电子传递效率。此外,往IET体系中添加导电材料如活性炭等物质能够有效的促进胞外电子传递。本课题组2017年Chen等[67]通过外源添加信号分子发现能够促进微生物产电,增强生物膜电化学活性。Ye等[68]发现赤泥能够增加混菌培养体系的导电性,促进微生物产甲烷。由于外源添加这些物质增强微生物胞外电子传递能力的成本较高,寻找廉价高效的微生物IET介导材料是将IET用于实际的可行方向。
| [1] |
Kouzuma A, Kato S, Watanabe K. Microbial interspecies interactions: Recent findings in syntrophic consortia . Frontiers in Microbiology, 2015. DOI:10.3389/fmicb.2015.00477
(  0) 0) |
| [2] |
Storck T, Virdis B, Batstone D J. Modelling extracellular limitations for mediated versus direct interspecies electron transfer . ISME Journal, 2016, 10(3): 621-631. DOI:10.1038/ismej.2015.139
(  0) 0) |
| [3] |
Usher K M, Kaksonen A H, Cole I, et al. Critical review: Microbially influenced corrosion of buried carbon steel pipes . International Biodeterioration & Biodegradation, 2014, 93: 84-106.
(  0) 0) |
| [4] |
Summers Z M, Fogarty H E, Leang C, et al. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria . Science, 2010, 330(6009): 1413-1415. DOI:10.1126/science.1196526
(  0) 0) |
| [5] |
Stams A J, Plugge C M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea . Nature Reviews Microbiology, 2009, 7: 568-577. DOI:10.1038/nrmicro2166
(  0) 0) |
| [6] |
Shrestha P M, Rotaru A E, Summers Z M, et al. Transcriptomic and genetic analysis of direct interspecies electron transfer . Applied & Environmental Microbiology, 2013, 79(7): 2397-2404.
(  0) 0) |
| [7] |
Liu F, Rotaru A E, Shrestha P M, et al. Promoting direct interspecies electron transfer with activated carbon . Energy & Environmental Science, 2012, 5(10): 8982-8989.
(  0) 0) |
| [8] |
Cruz V C, Rossetti S, Fazi S, et al. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation . Environmental Science & Technology, 2014, 48(13): 7536-7543.
(  0) 0) |
| [9] |
Omelianski W. Über methanbildung in der natur bei biologischen prozessen . Zentralblatt Fuèr Bakteriologie Parasitenkunde Ⅱ, 1906, 15: 673-687.
(  0) 0) |
| [10] |
Bryant M P, Wolin E A, Wolin M J, et al. Methanobacillus omelianskii, a symbiotic association of two species of bacteria . Archiv Für Mikrobiologie, 1967, 59(3): 20-31.
(  0) 0) |
| [11] |
Thiele J H, Zeikus J G. Control of interspecies electron flow during anaerobic digestion: Significance of formate transfer versus hydrogen transfer during syntrophic methanogenesis in flocs . Applied & Environmental Microbiology, 1988, 54(1): 20-29.
(  0) 0) |
| [12] |
Liu Y, Xu H L, Yang S F, et al. Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor . Water Research, 2003, 37(3): 661-673. DOI:10.1016/S0043-1354(02)00351-2
(  0) 0) |
| [13] |
Harald V C, Jun O, Sakayu S, et al. Secretion of flavins by Shewanella species and their role in extracellular electron transfer . Applied & Environmental Microbiology, 2008, 74(3): 615-623.
(  0) 0) |
| [14] |
Min D, Cheng L, Zhang F, et al. Enhancing extracellular electron transfer of Shewanella oneidensis MR-1 through coupling improved flavin synthesis and metal-reducing conduit for pollutant degradation . Environmental Science & Technology, 2017, 51(9): 5082-5089.
(  0) 0) |
| [15] |
Smith J A, Nevin K P, Lovley D R. Syntrophic growth via quinone-mediated interspecies electron transfer . Frontiers in Microbiology, 2015, 6: 121.
(  0) 0) |
| [16] |
Vasquezcardenas D, Vossenberg J V D, Polerecky L, et al. Microbial carbon metabolism associated with electrogenic sulphur oxidation in coastal sediments . ISME Journal, 2015, 9: 1966-1978. DOI:10.1038/ismej.2015.10
(  0) 0) |
| [17] |
Lovley D R. Happy together: Microbial communities that hook up to swap electrons . ISME Journal, 2016, 11(2): 327-336.
(  0) 0) |
| [18] |
Lovley D R. Syntrophy goes electric: Direct interspecies electron transfer . Annual Review of Microbiology, 2017, 71: 643-664. DOI:10.1146/annurev-micro-030117-020420
(  0) 0) |
| [19] |
Wintermute E H, Silver P A. Dynamics in the mixed microbial concourse . Genes & Development, 2010, 24(23): 2603-2614.
(  0) 0) |
| [20] |
Boone D R, Johnson R L, Liu Y. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake . Applied & Environmental Microbiology, 1989, 55(7): 1735-1741.
(  0) 0) |
| [21] |
Plugge C M, Balk M, Stams A J. Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum subsp. nov., a thermophilic, syntrophic, propionate-oxidizing, spore-forming bacterium . International Journal of Systematic & Evolutionary Microbiology, 2002, 52(2): 391-399.
(  0) 0) |
| [22] |
Bryant M P, Campbell L L, Reddy C A, et al. Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria . Applied & Environmental Microbiology, 1977, 33(5): 1162-1169.
(  0) 0) |
| [23] |
Valentine D L, Blanton D C, Reeburgh W S. Hydrogen production by methanogens under low-hydrogen conditions . Archives of Microbiology, 2000, 174(6): 415-421. DOI:10.1007/s002030000224
(  0) 0) |
| [24] |
Kosaka T, Kato S, Shimoyama T, et al. The genome of Pelotomaculum thermopropionicum reveals niche-associated evolution in anaerobic microbiota . Genome Research, 2008, 18(3): 442-448. DOI:10.1101/gr.7136508
(  0) 0) |
| [25] |
De Bok F A, Plugge C M, Stams A J. Interspecies electron transfer in methanogenic propionate degrading consortia . Water Research, 2004, 38(6): 1368-1375. DOI:10.1016/j.watres.2003.11.028
(  0) 0) |
| [26] |
Felchner-Zwirello M, Winter J, Gallert C. Interspecies distances between propionic acid degraders and methanogens in syntrophic consortia for optimal hydrogen transfer . Applied Microbiology & Biotechnology, 2013, 97(20): 9193-9205.
(  0) 0) |
| [27] |
Zhang J, Lu Y H. A review of interspecies electron transfer in syntrophic-methanogenic associations . Microbiology China, 2015, 42(5): 920-927.
(  0) 0) |
| [28] |
Roden E E, Kappler A, Bauer I, et al. Extracellular electron transfer through microbial reduction of solid-phase humic substances . Nature Geoscience, 2010, 3(6): 417-421. DOI:10.1038/ngeo870
(  0) 0) |
| [29] |
Biebl H, Pfennig N. Growth yields of green sulfur bacteria in mixed cultures with sulfur and sulfate reducing bacteria . Archives of Microbiology, 1978, 117(1): 9-16. DOI:10.1007/BF00689344
(  0) 0) |
| [30] |
Kaden J, Galushko A S, Schink B. Cysteine-mediated electron transfer in syntrophic acetate oxidation by cocultures of Geobacter sulfurreducens and Wolinella succinogenes . Archives of Microbiology, 2002, 178(1): 53-58. DOI:10.1007/s00203-002-0425-3
(  0) 0) |
| [31] |
Rotaru A E, Shrestha P M, Liu F, et al. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane . Energy & Environmental Science, 2014, 7(1): 408-415.
(  0) 0) |
| [32] |
Reguera G, Mccarthy K D, Mehta T, et al. Extracellular electron transfer via microbial nanowires . Nature, 2005, 435(7045): 1098-1101. DOI:10.1038/nature03661
(  0) 0) |
| [33] |
Lovley D, Malvankar N S, Vargas M, et al. Tunable metallic-like conductivity in microbial nanowire networks . Nature Nanotechnology, 2011, 6(9): 573-579. DOI:10.1038/nnano.2011.119
(  0) 0) |
| [34] |
Malvankar N S, Tuominen M T, Lovley D R. Biofilm conductivity is a decisive variable for high-current-density Geobacter sulfurreducens microbial fuel cells . Energy & Environmental Science, 2012, 5(2): 5790-5797.
(  0) 0) |
| [35] |
Strycharz S M, Glaven R H, Coppi M V, et al. Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens . Bioelectrochemistry, 2011, 80(2): 142-150. DOI:10.1016/j.bioelechem.2010.07.005
(  0) 0) |
| [36] |
Bonanni P S, Massazza D, Busalmen J P. Stepping stones in the electron transport from cells to electrodes in Geobacter sulfurreducens biofilms . Physical Chemistry Chemical Physics, 2013, 15(25): 10300-10306. DOI:10.1039/c3cp50411e
(  0) 0) |
| [37] |
Pan M, Zhang Y, Shan C, et al. Flat graphene enhanced electron transfer involved in redox reactions . Environmental Science & Technology, 2017, 51(15): 8597-8605.
(  0) 0) |
| [38] |
Shi L, Dong H, Reguera G, et al. Extracellular electron transfer mechanisms between microorganisms and minerals . Nature Reviews Microbiology, 2016, 14(10): 651-662. DOI:10.1038/nrmicro.2016.93
(  0) 0) |
| [39] |
Mehta T, Coppi M V, Childers S E, et al. Outer membrane c-type cytochromes required for Fe (Ⅲ) and Mn (Ⅳ) oxide reduction in Geobacter sulfurreducens . Applied & Environmental Microbiology, 2005, 71(12): 8634-8638.
(  0) 0) |
| [40] |
Holmes D, Chaudhuri S, Nevin K, et al. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens . Environmental Microbiology, 2006, 8(10): 1805-1815. DOI:10.1111/emi.2006.8.issue-10
(  0) 0) |
| [41] |
Lovley D, Nevin K P, Kim B C, et al. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells . PLoS ONE, 2009. DOI:10.1371/journal.pone.0005628
(  0) 0) |
| [42] |
Phuc T H, Stephen R L, Shi L, et al. Syntrophic anaerobic photosynthesis via direct interspecies electron transfer . Nature Communications, 2017. DOI:10.1038/ncomms13924
(  0) 0) |
| [43] |
Benomar S, Ranava D, Trably E, et al. Nutritional stress induces exchange of cell material and energetic coupling between bacterial species . Nature Communications, 2015. DOI:10.1038/ncomms7283
(  0) 0) |
| [44] |
Kato S, Hashimoto K, Watanabe K. Methanogenesis facilitated by electric syntrophy via(semi)conductive iron-oxide minerals . Environmental Microbiology, 2012, 14(7): 1646-1654. DOI:10.1111/j.1462-2920.2011.02611.x
(  0) 0) |
| [45] |
Li H, Chang J, Liu P, et al. Direct interspecies electron transfer accelerates syntrophic oxidation of butyrate in paddy soil enrichments . Environmental Microbiology, 2015, 17(5): 1533-1547. DOI:10.1111/emi.2015.17.issue-5
(  0) 0) |
| [46] |
Liu F, Rotaru A E, Shrestha P M, et al. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange . Environmental Microbiology, 2015, 17(3): 648-655. DOI:10.1111/emi.2015.17.issue-3
(  0) 0) |
| [47] |
Zee F P, Bisschops I A, Lettinga G, et al. Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes . Environmental Science & Technology, 2003, 37(2): 402-408.
(  0) 0) |
| [48] |
Chen S, Rotaru A E, Shrestha P M, et al. Promoting interspecies electron transfer with biochar . Scientific Reports, 2014. DOI:10.1038/srep05019
(  0) 0) |
| [49] |
Chen S, Rotaru A E, Liu F, et al. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures . Bioresource Technology, 2014, 173: 82-86. DOI:10.1016/j.biortech.2014.09.009
(  0) 0) |
| [50] |
Rotaru A E, Shrestha P M, Liu F, et al. Interspecies electron transfer via hydrogen and formate rather than direct electrical connections in cocultures of Pelobacter carbinolicus and Geobacter sulfurreducens . Applied & Environmental Microbiology, 2012, 78(21): 7645-7651.
(  0) 0) |
| [51] |
Rotaru A E, Shrestha P M, Liu F, et al. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri . Applied & Environmental Microbiology, 2014, 80(15): 4599-4605.
(  0) 0) |
| [52] |
Kato S, Hashimoto K, Watanabe K. Microbial interspecies electron transfer via electric currents through conductive minerals . Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(25): 10042-10046. DOI:10.1073/pnas.1117592109
(  0) 0) |
| [53] |
Mao X, Stenuit B, Polasko A, et al. Efficient metabolic exchange and electron transfer within a syntrophic TCE degrading co-culture of Dehalococcoides mccartyi 195 and Syntrophomonas wolfei . Applied & Environmental Microbiology, 2015, 81(6): 2015-2024.
(  0) 0) |
| [54] |
McGlynn S E, Chadwick G L, Kempes C P, et al. Single cell activity reveals direct electron transfer in methanotrophic consortia . Nature, 2015, 526(7574): 531-535. DOI:10.1038/nature15512
(  0) 0) |
| [55] |
Chynoweth D P, Owens J M, Legrand R. Renewable methane from anaerobic digestion of biomass . Renewable Energy, 2001, 22(3): 1-8.
(  0) 0) |
| [56] |
Dang Y, Holmes D E, Zhao Z, et al. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials . Bioresource Technology, 2016, 220: 516-522. DOI:10.1016/j.biortech.2016.08.114
(  0) 0) |
| [57] |
Zhao Z, Zhang Y, Li Y, et al. Potentially shifting from interspecies hydrogen transfer to direct interspecies electron transfer for syntrophic metabolism to resist acidic impact with conductive carbon cloth . Chemical Engineering Journal, 2017, 313: 10-18. DOI:10.1016/j.cej.2016.11.149
(  0) 0) |
| [58] |
Yang Y, Tsukahara K, Yagishita T, et al. Performance of a fixed-bed reactor packed with carbon felt during anaerobic digestion of cellulose . Bioresource Technology, 2004, 94(2): 197-201. DOI:10.1016/j.biortech.2003.11.025
(  0) 0) |
| [59] |
沈李东, 胡宝兰, 郑平. 甲烷厌氧氧化微生物的研究进展. 土壤学报, 2011, 48(2): 619-628. Shen L D, Hu B L, Zheng P. Progress in study on microorganisms responsible for anaerobic oxidation of methane (In Chinese). Acta Pedologica Sinica, 2011, 48(3): 619-628. (  0) 0) |
| [60] |
Wegener G, Krukenberg V, Riedel D, et al. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria . Nature, 2015, 526(7574): 587-590. DOI:10.1038/nature15733
(  0) 0) |
| [61] |
Egger M, Rasigraf O, Sapart C J, et al. Iron-mediated anaerobic oxidation of methane in Brackish coastal sediments . Environmental Science & Technology, 2014, 49(1): 277-283.
(  0) 0) |
| [62] |
Ettwig K F, Zhu B, Speth D, et al. Archaea catalyze iron-dependent anaerobic oxidation of methane . Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(45): 12792-12796. DOI:10.1073/pnas.1609534113
(  0) 0) |
| [63] |
Aulenta F, Rossetti S, Amalfitano S, et al. Conductive magnetite nanoparticles accelerate the microbial reductive dechlorination of trichloroethene by promoting interspecies electron transfer processes . ChemSusChem, 2013, 6(3): 433-436. DOI:10.1002/cssc.201200748
(  0) 0) |
| [64] |
吴云当, 李芳柏, 刘同旭. 土壤微生物-腐殖质-矿物间的胞外电子传递机制研究进展. 土壤学报, 2016, 53(2): 277-291. Wu Y D, Li F B, Liu T X. Mechanism of extracellular electron transfer among microbe-humus-mineral in soil: A review (In Chinese). Acta Pedologica Sinica, 2016, 53(2): 277-291. (  0) 0) |
| [65] |
Leang C, Malvankar N S, Franks A E, et al. Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production . Energy & Environmental Science, 2013, 6(6): 1901-1908.
(  0) 0) |
| [66] |
Tan Y, Adhikari R Y, Malvankar N S, et al. Synthetic biological protein nanowires with high conductivity . Small, 2016, 12(33): 4481-4485. DOI:10.1002/smll.201601112
(  0) 0) |
| [67] |
Chen S, Jing X, Tang J, et al. Quorum sensing signals enhance the electrochemical activity and energy recovery of mixed-culture electroactive biofilms . Biosensors & Bioelectronics, 2017, 97: 369-376.
(  0) 0) |
| [68] |
Ye J, Hu A, Ren G, et al. Red mud enhances methanogenesis with the simultaneous improvement of hydrolysis-acidification and electrical conductivity . Bioresource Technology, 2017, 247: 131-137.
(  0) 0) |
 2018, Vol. 55
2018, Vol. 55
