2. 北京市环境保护监测中心,北京 100048;
3. 安徽农业大学农学院,合肥 230036
土壤N2O的产生途径包括氨氧化、硝化诱导的反硝化(包括硝化细菌反硝化(Nitrifier denitrification,ND)和与硝化相耦合的反硝化(Nitrification-coupled denitrification,NCD))、异养硝化和异养反硝化过程(Heterotrophic denitrification, HD)[1],贡献了全球70%以上的N2O排放[2]。其中,反硝化过程,主要是异养反硝化微生物利用NO3-作为电子受体被逐步还原为NO2-、NO、N2O最终为N2的过程,被普遍认为是土壤N2O产生的主要途径。但是,近年来大量研究表明,含有nirK基因的氨氧化细菌(Ammonia-oxidizing bacteria,AOB)利用NH4+氧化产生电子,还原NO2-为N2O的ND过程,同样对土壤N2O排放具有重要贡献[3],在有些培养条件下,ND过程对土壤N2O的贡献量甚至超过异养反硝化过程[4]。同时,异养反硝化微生物以硝化过程产生的NO2-为电子受体发生反硝化反应,称之为与硝化相耦合的反硝化过程(NCD),同样对N2O排放有重要贡献[1]。此外,在高氧、碳源充足的酸性土壤和污水处理系统中[5],相当数量的异养细菌和真菌可将NH3或有机形态的氨基氧化为NO3-,发生异养硝化作用,许多异养硝化菌同时可以进行好氧反硝化产生N2O,称为同步硝化-反硝化过程(Simultaneous nitrification-denitrification, SND)[6]。
氧分压是影响土壤N2O排放的重要参数。一般认为,厌氧条件下HD是土壤N2O排放的主要途径;氨氧化是高氧分压下土壤N2O的主要来源[7];ND和HD对N2O的贡献量随着土壤氧分压的升高而降低,且ND途径对土壤氧分压的耐性高于HD途径[8]。由于土壤具有高度异质性的特点,低氧条件下各种N2O产生途径可在不同的微域空间同时发生,被认为是促使土壤N2O排放最有利的环境[5],但是,受各种环境因素的影响,低氧条件下各途径对N2O排放的相对贡献存在争议。
为促进作物生长、增加土壤微生物活性以及改善土壤结构,我国设施蔬菜种植体系普遍会投入大量的有机肥[9],这会显著增加土壤碳源的有效性。土壤中充足的碳源有助于发生完全反硝化作用[10],降低土壤N2O排放;但也有研究认为,氧分压而不是碳源的有效性决定了N2O的还原[5, 11]。反之,碳源不足会导致中间产物NO2-的累积[12],NO2-作为硝化和反硝化过程的中间产物,与土壤N2O排放正相关[13]。碳源和氧浓度均会显著影响土壤N2O排放,但二者交互作用对N2O排放的影响缺乏系统研究。
本研究选择典型蔬菜种植区土壤,设置不同的氧分压和有效性碳投入,研究二者对土壤N2O排放的影响;同时设置添加双氰胺(Dicyandiamide, DCD)的处理,分析不同氧分压(0%、1%、3%、10%和21%)和碳源投入下硝化和反硝化途径对土壤N2O排放的相对贡献,以期为设施菜田土壤N2O减排措施的制定提供理论依据。
1 材料与方法 1.1 采样地点及土壤样品供试土壤采自于中国农业大学山东寿光蔬菜研究院(36°85′ N,118°87′ E)的一个日光温室定位试验,该试验设置于2007年,种植系统为一年两季番茄。试验为裂区设计,主处理为滴灌和漫灌,副处理为不添加秸秆、添加小麦秸秆和添加玉米秸秆。本研究选取的土壤样品来自于滴灌和玉米秸秆处理,该处理每个作物生长季平均滴灌量为309 mm,施肥处理N、P2O5、K2O投入量分别为204 kg·hm-2、229 kg·hm-2和526 kg·hm-2;玉米秸秆投入量为8 t·hm-2(相当于109 kg·hm-2氮以及3 406 kg·hm-2碳);商品鸡粪作为基肥,平均施用量相当于210 kg·hm-2氮和1 984 kg·hm-2碳。2014年7月番茄收获后采集0~20 cm土壤样品,充分混匀后过2 mm筛风干备用。该土壤样品NH4+-N、NO3--N含量分别为4.35、75.24 mg·kg-1,全氮1.31 g·kg-1,全碳17.9 g·kg-1,有机碳12.40 g·kg-1,有效磷150 mg·kg-1,速效钾483 mg·kg-1,pH 8.02。有关试验点的更多信息可参见Fan等[14]的研究。
1.2 试验设计与方法为缓解土壤中硝态氮(NO3-)背景值含量过高而铵态氮(NH4+)过低的现象,试验开始前用去离子水清洗试验用土以保持NO3--N含量低于20 mg·kg-1,待水分自然蒸发至质量含水量为100~150 g·kg-1之间时,过2 mm筛,随后将过筛土置于4℃冰箱储存备用。洗土后土壤NH4+-N、NO3--N含量分别为3.37 mg·kg-1和8.03 mg·kg-1,全氮1.10 g·kg-1,全碳15.80 g·kg-1,有机碳10.81 g·kg-1,有效磷117 mg·kg-1,速效钾416 mg·kg-1,pH 8.01。
以水洗后的菜田土壤为研究对象,30 mg·kg-1(以N计,下同)NH4NO3为氮源,谷氨酸钠(C5H8NO4Na·H2O)为碳源,设置未添加谷氨酸钠的对照处理(T1)和添加184.8 mg·kg-1(以C计,下同)谷氨酸钠的处理(T2),两种处理土壤同时设置不添加/添加DCD(50 mg·kg-1)的处理,研究DCD在0%、1%、3%、10%和21%氧分压(O2/He混合气体,下同)下对设施菜田土壤N2O的减排效果。为了进一步解释T1土壤有氧条件下N2O产生的原因,根据气体监测结果,选取9个气体产生关键点,同步设置平行试验测定无碳源添加土壤培养过程中NO2-、NO3-和NH4+的动态变化。总培养时间为108 h,每个处理3个重复。
具体的试验操作为:称取相当于10.0 g烘干土重的预处理土样于120 mL血清瓶中,用注射器分别将去离子水或含有添加物(NH4NO3、C5H8NO4Na·H2O或DCD)的溶液均匀喷洒至土壤表面,调节土壤质量含水量为250 g·kg-1。所有血清瓶用铝盖密封后按处理分别用0%、1%、3%、10%、21%的氧气和氦气(O2/He)的混合气(体积比)通过抽真空-洗气系统(帅恩科技有限公司,北京)洗气4次,最后一次抽真空后充入对应浓度的O2/He混合气体,平衡血清瓶内气压后将所有血清瓶置于20℃恒温水浴槽中进行培养,利用Robot自动培养系统每间隔6 h在线连续监测血清瓶内N2O、N2和CO2的浓度变化。培养过程中根据气体监测结果,使用密闭注射器定期向血清瓶中补充纯氧(99.9%,v/v),维持血清瓶中恒定的氧含量。
1.3 土壤气体及相关参数测定恒温培养槽内的样品连接Robot自动培养系统,实时在线监测O2、N2O、N2、CO2的动态变化。该系统由恒温培养系统、自动进样系统和气体分析模块三部分组成,实现了培养、采样、分析过程的全部自动化操作。恒温培养系统主要包括恒温水浴锅(0~40℃)、血清瓶、以及固定血清瓶位置的铁架构成(可放置44个120 mL的血清瓶);自动进样系统由自动进样器(Gilson Model 222,Gilson,法国)和蠕动泵(Gilson Minipuls 3,Gilson,法国)构成,通过Python编程语言控制自动采样功能;气体分析模块由包含热导检测器(TCD)、电子捕获检测器(ECD)和火焰离子化检测器(FID)的气相色谱(Agilent 7890A,美国)组成,能够监测N2O(ECD、TCD)、N2(TCD)、CO2(TCD)和O2(TCD)的含量。该系统最大的优势是能够准确检测N2的变化且采样过程中N2的渗气率较低。相关Robot自动培养系统的运行模块细节可参见Molstad等[15]的描述。
土壤样品中NH4+和NO3-的含量用1 mol·L-1 KCl溶液浸提后用连续流动分析仪(TRACCS2000,德国)测定。土壤亚硝态氮、全氮、全碳、有机碳、有效磷、速效钾、pH测定参照文献[16]。
1.4 数据处理每批测量试验开始时,放置3个已知浓度的标气瓶,根据在线监测的血清瓶中气体数值和标气浓度,利用挪威生命科学大学NMBU研究小组的计算方法(https://www.nmbu.no/en/research/groups/nitrogen/approaches-and-infrastructure)计算上述气体产生量,计算过程中同时考虑了采样过程中由于回注等量He气造成的稀释效应、N2和O2的漏气影响、气体在各温度和pH下的溶解度以及在气液两相之间的扩散率,具体请参见Molstad等[15]的研究。
不同氧分压下气态产物的产物比用N2O/(N2O+N2)指数(IN2O)计算求出,其计算公式如下[17]:
| $ {I_{{\rm{N}}_2{\rm{O}}}} = \int_0^T {{{\rm{N}}_2}{\rm{O}}} \left( t \right)dt/\int_0^T {\left[ {{\rm{N}}_2{\rm{O}}\left( t \right) + {{\rm{N}}_2}\left( t \right)} \right]dt} $ | (1) |
式中,无氧处理的N2O(t)、N2(t)为N2O测量值达到高峰时间(t)下的累积产生量,mg·kg-1;有氧处理的时间节点是培养结束时。
质量平衡是指培养结束时土壤无机氮(NH4+、NO3-)与气体产生量(N2O、N2)之和与培养开始时土壤无机氮(NH4+、NO3-)与气体产生量(N2O、N2)之和两者间的差值。数据利用SPSS 20.0进行单因素、多因素方差分析。采用SigmaPlot 12.5作图,图表中数据均为:平均值±标准误差。文中所有涉及的NO3-、NO2-、NH4+、N2O、N2、无机氮投入等均以N计。
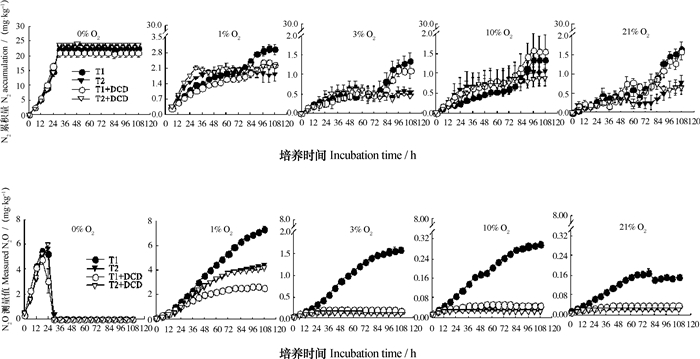
2 结果 2.1 不同氧分压下碳源添加对设施菜田土壤N2O、N2产生量的影响由图 1可见,在厌氧条件下,未添加和添加碳源土壤N2O的排放量分别于在线培养后的18、24 h达到5.42 mg·kg-1和6.02 mg·kg-1的排放峰值,并于30 h全部转化为N2,且硝化抑制剂是否添加对N2O产生量无显著影响。
|
注:T1、T2、T1+DCD、T2+DCD分别表示无碳源添加的处理、添加碳源的处理、无碳源添加但添加DCD的处理、添加碳源和DCD的处理,下同 Note: T1, T2, T1+DCD and T2+DCD stands for treatment without carbon added, treatment with carbon added, treatment with DCD, but no carbon added, treatment with both DCD and carbon added, respectively. The same below 图 1 不同氧分压下不同处理土壤培养过程中N2O和N2浓度的动态变化 Fig. 1 Dynamics of the concentrations of N2O and N2 in the soil under incubation relative to treatment and oxygen partial pressure (OPP) |
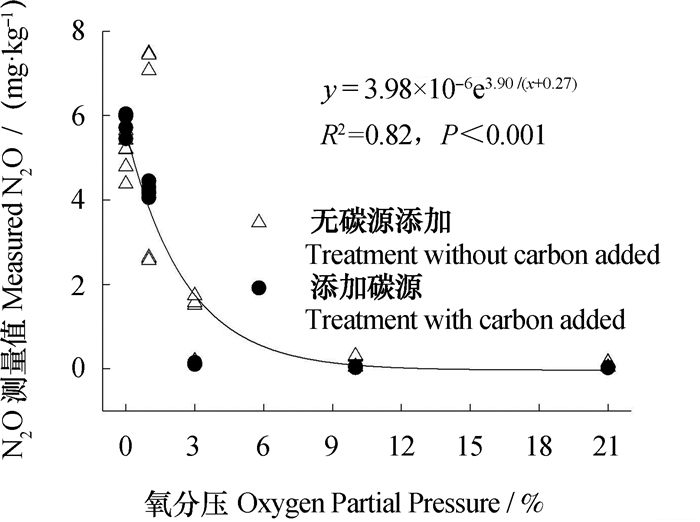
随着土壤氧分压的升高,N2O排放量呈指数下降(图 2)。1%氧分压下未添加硝化抑制剂和碳源的处理N2O累积排放量显著高于厌氧处理N2O的排放量(P < 0.01)。在氧分压大于等于1%的条件下,未添加碳源土壤N2O排放量均高于添加碳源的处理,但是在1%氧分压同时添加DCD和碳源的条件下,土壤N2O排放量显著高于仅添加DCD的土壤(P < 0.01),分别为4.21 mg·kg-1和2.61 mg·kg-1。DCD的添加显著降低了有氧条件下未添加碳源土壤N2O和N2产生量,在1%、3%、10%和21%氧分压下,与未添加DCD的处理相比,添加DCD后未添加碳源的处理N2O产生量分别降低了64.4%、88.8%、83.5%和76.3%;添加DCD同时导致1%和3%氧分压下N2累积产生量分别降低了23.4%、18.6%。DCD的添加对添加碳源处理的N2O和N2产生量均无显著影响。
|
图 2 氧分压与N2O排放量间的相关性(n=60) Fig. 2 Relationship between oxygen partial pressure and N2O emission (n=60) |
厌氧条件下N2O和N2排放量显著高于有氧处理(P < 0.01),最高达到23.5 mg·kg-1。随着氧分压的增加,土壤N2O和N2排放量迅速下降。从1%氧分压时的10.3 mg·kg-1(未添加碳源处理)和6.74 mg·kg-1(添加碳源处理),分别下降至3%氧分压时的2.94 mg·kg-1和0.68 mg·kg-1。同时,培养瓶顶空氧分压高于3%后,添加碳源土壤的N2O和N2排放峰值显著低于未添加碳源的土壤(P < 0.01)。
当氧分压高于3%以后,土壤N2排放量很低且可能存在较大的误差,因此,仅计算了前三个氧分压处理的N2O/(N2O+N2)(IN2O)值(表 1)。1%氧分压下全部处理,以及未添加DCD和碳源的3%氧分压处理的IN2O普遍高于其余各氧分压处理,其中,未添加碳源土壤在3%与1%氧分压下IN2O无显著差异,均显著高于厌氧处理。碳源的添加显著降低了土壤的IN2O,而DCD的添加则仅显著降低了未添加碳源土壤的IN2O。添加碳源和DCD的处理IN2O对氧分压的响应类似,即在1%氧分压下IN2O最高,0%氧次之。
|
|
表 1 不同氧分压下以N2O/(N2O+N2)(IN2O)指数表示的产物比 Table 1 Gaseous product ratio [N2O/(N2O+N2) index (IN2O)] relative to oxygen partial pressure (OPP) |
质量平衡计算结果显示,在有氧和无氧条件下均有氮的净矿化,而且厌氧条件下两种处理中土壤的矿化量显著低于有氧处理(P < 0.01),最高达到17.0 mg·kg-1。DCD有效抑制了土壤的氨氧化,未添加碳源处理的土壤尤为显著(P < 0.01)。同时,在不同氧分压下,添加碳源的处理培养前后NH4+含量无显著降低现象,即使在21%氧分压下,培养结束时不添加/添加DCD的处理NH4+-N含量分别为50.0 mg·kg-1和55.2 mg·kg-1(谷氨酸钠添加带入了43.12 mg·kg-1的氮)。
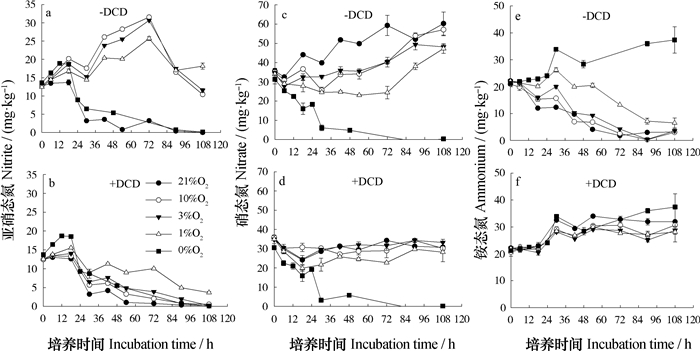
2.2 不同氧分压下无碳源添加处理培养过程中无机氮动态未添加碳源土壤培养过程中无机氮动态监测的结果(图 3)显示,厌氧条件下NO2-也会发生短暂的积累,累积量在12 h达到峰值,为18.9 mg·kg-1。在1%、3%和10%氧分压下,未添加DCD处理NO2-的累积时间、累积量显著高于厌氧和21%氧分压处理,三种氧分压下最高的NO2-累积量出现在培养开始后72 h,分别为25.7、30.6和31.5 mg·kg-1(图 3a)。
|
图 3 无碳源添加处理培养过程中无机氮的动态变化 Fig. 3 Dynamics of inorganic nitrogen in the soil under incubation without available carbon added |
由图 3c可见,培养开始后,厌氧条件下NO3-含量显著降低(P < 0.01),90 h时NO3-含量低于检测限。有氧条件下,培养结束时NO3-含量显著增加(P < 0.05)。在1%浓度下,NO3-含量呈现先降低后增加的趋势,培养结束时NO3-的增加量显著低于10%和21%氧分压下的处理(P < 0.01)。
厌氧条件下NH4+含量呈增加趋势,培养结束时NH4+增加了16.6 mg·kg-1。有氧条件下NH4+整体呈下降趋势,且1%氧分压下NH4+的降低速率显著低于其他有氧处理(P < 0.01)。
DCD的添加对厌氧条件下NO2-、NO3-、NH4+的变化无影响,但其显著降低了有氧条件下NH4+的氧化速率,导致整个培养过程中土壤NH4+、NO3-含量无显著变化(图 3d和图 3f)。NH4+氧化速率的降低同时显著降低了1%、3%和10%氧分压下中间产物NO2-的累积量(图 3b),而在1%氧分压下添加DCD的处理NO2-累积量显著高于其他有氧处理(P < 0.01)。
3 讨论随着土壤氧分压的升高,N2O排放量呈指数下降,土壤N2O排放主要发生在厌氧和1%氧分压下(图 2)。厌氧条件下土壤N2O主要来自HD过程,且在本试验条件下,厌氧处理土壤N2O的排放量被明显低估,这是因为在封闭培养体系中,大量的N2O很快会被进一步还原为N2,而在田间实际条件下,大部分N2O将被释放进入大气,这也是室内培养试验普遍存在的问题[18],大量N2O被还原导致本研究中无碳源和DCD添加的处理在微量氧(1% O2)下N2O累积产生量显著高于厌氧处理(P < 0.01)。在持续微量氧供应条件下,各处理土壤中N2O排放量以较高的速率不断增加;当土壤氧分压增加至3% O2后,仅在未添加碳源的处理中表现出N2O的持续排放,与相同处理条件下的1%氧分压处理相比,N2O和N2O加N2排放量仍分别降低了78.2%和71.3%,表明土壤氧分压高于3%后,即使是在碳源充足的条件下,N2O排放量、氮素气态损失显著降低[19]。农田土壤N2O排放主要发生在施肥和灌水后的脉冲式排放,特别是设施菜田土壤,在全年17.5%的时间内排放了约78.8%的N2O[20]。由此可见,田间状态下,土壤N2O排放量中的绝大部分来自于厌氧和低氧的微域环境,当土壤空气中的氧分压普遍高于3%后,以氨氧化及其相关过程等途径来源的N2O表现为脉冲式排放后的土壤N2O背景排放。氨氧化过程中N2O排放主要源于中间产物NH2OH氧化,但是土壤中几乎无NH2OH累积,且仅有1/3的NH2OH氧化酶活性位点可能会将NH2OH氧化为N2O[21],导致该过程N2O产生量很低,一般不超过氨氧化量的1‰~2‰[22]。NH2OH或NO2-的化学分解也会造成N2O排放,但该途径主要发生在低pH土壤中[23]。
在碳源较为充足的条件下,HD过程对设施菜田土壤N2O排放的贡献量更大,只是随着氧分压的升高,其贡献的比例显著降低。添加碳源的处理中,DCD对土壤N2O、N2产生量和IN2O均无显著影响,且在添加DCD的1%氧分压下,添加碳源的处理N2O排放量显著高于无碳源添加的处理(P < 0.01)(图 1)。此外,与无碳源添加的土壤相比,氨氧化量显著降低,即使在21%氧分压下,也无显著的氨氧化现象发生。由此可见,碳源的添加可能会造成有氧条件下异养菌对自养菌生长产生竞争优势[24],导致有氧条件下N2O可能主要来自HD过程。添加碳源后可能会有部分N2O来自异养硝化-反硝化过程(SND),因为有氧且碳源充足的条件有利于异养硝化作用的发生[25],但本研究所用土壤pH较高,且添加DCD的处理培养前后硝酸盐基本平衡,异养的同步硝化-反硝化过程或许很弱。
在低氧(1%~3% O2)且无碳源添加的土壤中,异养反硝化菌可能会通过HD或NCD途径对土壤N2O排放产生重要贡献。DCD的添加显著降低了无碳源添加处理土壤N2O排放量(P < 0.01),这与田间通量观测结果一致[26-28],且大量研究结果也表明,低氧且低碳源环境会显著增加ND途径对N2O的贡献量[29],但不能以此推测ND途径是微量氧条件下N2O排放的主导途径。例如,在微量氧1% O2分压下,无DCD添加的处理产物比IN2O指数为0.69,添加DCD后IN2O为0.57,根据产物比下降的幅度,可以推算出在微量氧供应情况下,约有82.6%的N2O来自HD和NCD过程,17.8% N2O来自于ND等过程。即在微量氧供应条件下,N2O排放中的绝大部分来自于分别以NO3-和NO2-为底物的异养反硝化过程。以同样方式计算,在3%O2分压下HD和NCD过程对N2O排放的贡献量为57.4%,42.6%的N2O主要来自ND过程。硝化抑制剂同样会降低广义的HD途径氮素损失,主要原因可能是DCD添加后NCD过程的底物NO2-含量降低[30]。NCD过程在本质上仍然是HD过程[31],在氧扩散受阻的情况下好氧微生物对土壤孔隙氧的消耗会导致微域土壤产生厌氧微位点,造成氨氧化过程产生的NO2-通过异养反硝化途径损失。Petersen等[32]研究结果表明,有氧-厌氧的土壤环境会导致70%的硝化产物通过反硝化途径损失,Lee等[30]也观察到土壤中硝化与反硝化潜势呈正相关。由此可见,与自养的氨氧化微生物相比,在微量氧和低氧且无碳源添加的土壤中,异养菌可能对土壤N2O排放和氮素气态损失更为重要。对比1%和3%氧分压下N2O排放量也可以看出,尽管氨氧化和ND过程在3%氧分压下对N2O贡献量显著增加,但N2O的排放量较1%氧分压时下降了78.2%,因而,总体上ND过程对设施菜田土壤N2O排放的贡献不大。
有些条件下,土壤中ND途径对N2O的贡献量有可能被高估。例如,本研究表明,在1%、3%和10%氧分压下,均存在NO2-较长时间的过渡性积累(图 3a),但仅在1%氧分压下出现大量的N2O排放。通常认为,硝化微生物进行ND的目的是解除NO2-对其细胞的毒害作用[8]。由此可以推论,当土壤中出现NO2-大量积累时,就有可能伴随着通过ND途径排放大量N2O的现象。这一假设显然与本研究的结果不符。认为有氧条件下ND过程是土壤N2O排放主导途径之一的结论,主要来自于在低氧条件下利用同位素标记方法进行的试验[3-4]。然而,利用双同位素技术计算排放途径的前提,是假定氨氧化产生的NO2-仅能被氨氧化菌所利用,进行反硝化过程并释放出N2O[33]。这种假设或许存在下列不足:首先,土壤中有许多异养反硝化菌对NO3-和NO2-有相同的异养还原能力;其次,部分异养菌由于缺少NO3-还原基因仅能以NO2-为底物进行反硝化作用,因此,利用双同位素标记法得到的结果可能会高估ND途径对N2O排放的贡献[1, 34]。
4 结论随着土壤氧分压的升高,N2O排放量呈指数下降,土壤N2O排放主要发生在厌氧和微量氧(1% O2)情况下,氧分压大于等于3%后,土壤N2O排放量、氮素气态损失显著降低。在低氧条件下(0%~3% O2),总体上异养反硝化菌对设施菜田土壤N2O排放更为重要,特别是在碳源较为充足的条件下。田间实际条件下,在保证作物产量的前提下,适当的碳源投入、通过水分管理控制土壤空气中氧分压以及减少低氧与厌氧的“热点”,并结合施用硝化抑制剂,对降低菜田土壤N2O排放有重要意义。
| [1] |
Shi X Z, Hu H W, Xia Z, et al. Nitrifier-induced denitrification is an important source of soil nitrous oxide and can be inhibited by a nitrification inhibitor 3, 4-dimethylpyrazole phosphate . Environmental Microbiology, 2017, 19(12): 4851-4865. DOI:10.1111/1462-2920.13872
(  0) 0) |
| [2] |
Butterbach-Bahl K, Baggs E M, Dannenmann M, et al. Nitrous oxide emissions from soils:how well do we understand the processes and their controls? . Philosophical Transactions of the Royal Society, 2013, 368(1621): 20130122. DOI:10.1098/rstb.2013.0122
(  0) 0) |
| [3] |
Kool D M, Dolfing J, Wrage N, et al. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil . Soil Biology and Biochemistry, 2011, 43(1): 174-178. DOI:10.1016/j.soilbio.2010.09.030
(  0) 0) |
| [4] |
Zhu X, Burger M, Doane T A, et al. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability . Proceedings of the National Academy of Sciences, 2013, 110(16): 6328-6333. DOI:10.1073/pnas.1219993110
(  0) 0) |
| [5] |
Ju X T, Lu X, Gao Z, et al. Processes and factors controlling N2O production in an intensively managed low carbon calcareous soil under sub-humid monsoon conditions . Environmental Pollution, 2011, 159(4): 1007-1016. DOI:10.1016/j.envpol.2010.10.040
(  0) 0) |
| [6] |
Cui F, Yan G, Zhou Z, et al. Annual emissions of nitrous oxide and nitric oxide from a wheat-maize cropping system on a silt loam calcareous soil in the North China Plain . Soil Biology and Biochemistry, 2012, 48(4): 10-19.
(  0) 0) |
| [7] |
Sutka R L, Ostrom N E, Ostrom P H, et al. Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances . Applied and Environmental Microbiology, 2006, 72(1): 638-644. DOI:10.1128/AEM.72.1.638-644.2006
(  0) 0) |
| [8] |
Hu H W, Chen D, He J Z. Microbial regulation of terrestrial nitrous oxide formation:Understanding the biological pathways for prediction of emission rates . FEMS Microbiology Reviews, 2015, 39(5): 729-749. DOI:10.1093/femsre/fuv021
(  0) 0) |
| [9] |
Yan Z, Chen S, Li J, et al. Manure and nitrogen application enhances soil phosphorus mobility in calcareous soil in greenhouses . Journal of Environmental Management, 2016, 181: 26-35.
(  0) 0) |
| [10] |
Saito T, Ishii S, Otsuka S, et al. Identification of novel Betaproteobacteria in a succinate-assimilating population in denitrifying rice paddy soil by using stable isotope probing . Microbes & Environments, 2008, 23(3): 192-200.
(  0) 0) |
| [11] |
Morley N, Baggs E M. Carbon and oxygen controls on N2O and N2 production during nitrate reduction . Soil Biology and Biochemistry, 2010, 42: 1864-1871. DOI:10.1016/j.soilbio.2010.07.008
(  0) 0) |
| [12] |
Yang X, Wang S, Zhou L. Effect of carbon source, C/N ratio, nitrate and dissolved oxygen concentration on nitrite and ammonium production from denitrification process by Pseudomonas stutzeri D6 . Bioresource Technology, 2012, 104(1): 65-72.
(  0) 0) |
| [13] |
Maharjan B, Venterea R T. Nitrite intensity explains N management effects on N2O emissions in maize . Soil Biology and Biochemistry, 2013, 66(11): 229-238.
(  0) 0) |
| [14] |
Fan Z B, Lin S, Zhang X M, et al. Conventional flooding irrigation causes an overuse of nitrogen fertilizer and low nitrogen use efficiency in intensively used solar greenhouse vegetable reduction . Agricultural Water Management, 2014, 144(2): 11-19.
(  0) 0) |
| [15] |
Molstad L, Dorsch P, Bakken L R. Robotized incubation system for monitoring gases(O2, NO, N2O, N2)in denitrifying cultures . Journal of Microbiological Methods, 2007, 71(3): 202-211. DOI:10.1016/j.mimet.2007.08.011
(  0) 0) |
| [16] |
鲍士旦. 土壤农化分析. 3版. 北京: 中国农业出版社, 1999: 25-111. Bao S D. Soil agrochemical analysis (In Chinese). 3rd ed. Beijing: China Agriculture Press, 1999: 25-111. (  0) 0) |
| [17] |
Qu Z, Wang J G, Almøy T, et al. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2)product ratio of denitrification, primarily due to acidification of the soils . Global Change Biology, 2014, 20(5): 1685-1698. DOI:10.1111/gcb.2014.20.issue-5
(  0) 0) |
| [18] |
Wu D, Dong W, Oenema O, et al. N2O consumption by low-nitrogen soil and its regulation by water and oxygen . Soil Biology and Biochemistry, 2013, 60(1): 165-172.
(  0) 0) |
| [19] |
杨艳菊, 蔡祖聪, 张金波. 氧气浓度对水稻土N2O排放的影响. 土壤, 2016, 48(3): 539-545. Yang Y J, Cai Z C, Zhang J B. Effect of oxygen concentration on N2O emission from paddy soil (In Chinese). Soils, 2016, 48(3): 539-545. (  0) 0) |
| [20] |
Diao T, Xie L, Guo L, et al. Measurements of N2O emissions from different vegetable fields on the North China Plain . Atmospheric Environment, 2013, 72(2): 70-76.
(  0) 0) |
| [21] |
Yamazaki T, Hozuki T, Arai K, et al. Isotopomeric characterization of nitrous oxide produced by reaction of enzymes extracted from nitrifying and denitrifying bacteria . Biogeosciences, 2014, 11(10): 2679-2689. DOI:10.5194/bg-11-2679-2014
(  0) 0) |
| [22] |
Morkved P T, Dorsch P, Bakken L R. The N2O product ratio of nitrification and its dependence on long-term changes in soil pH . Soil Biology and Biochemistry, 2007, 39(8): 2048-2057. DOI:10.1016/j.soilbio.2007.03.006
(  0) 0) |
| [23] |
Lim N Y N, Frostegård Å, Bakken L R. Nitrite kinetics during anoxia:The role of abiotic reactions versus microbial reduction . Soil Biology and Biochemistry, 2018, 119: 203-209. DOI:10.1016/j.soilbio.2018.01.006
(  0) 0) |
| [24] |
Third K A, Burnett N, Cord-Ruwisch R. Simultaneous nitrification and denitrification using stored substrate(PHB)as the electron donor in an SBR . Biotechnology and Bioengineering, 2003, 83(6): 706-720. DOI:10.1002/(ISSN)1097-0290
(  0) 0) |
| [25] |
Zhang J, Müller C, Cai Z. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils . Soil Biology and Biochemistry, 2015, 84: 199-209. DOI:10.1016/j.soilbio.2015.02.028
(  0) 0) |
| [26] |
毛新伟, 程敏, 徐秋芳, 等. 硝化抑制剂对毛竹林土壤N2O排放和氨氧化微生物的影响. 土壤学报, 2016, 53(6): 1528-1540. Mao X W, Cheng M, Xu Q F, et al. Effects of nitrification inhibitors on soil N2O emission and community structure and abundance of ammonia oxidation microorganism in soil under extensively managed Phyllostachys edulis stands (In Chinese). Acta Pedologica Sinica, 2016, 53(6): 1528-1540. (  0) 0) |
| [27] |
陈浩, 李博, 熊正琴. 减氮及硝化抑制剂对菜地氧化亚氮排放的影响. 土壤学报, 2017, 54(4): 938-947. Chen H, Li B, Qiong Z Q. Effects of N reduction and nitrification inhibitor on N2O emissions in intensive vegetable field (In Chinese). Acta Pedologica Sinica, 2017, 54(4): 938-947. (  0) 0) |
| [28] |
孙海军, 闵炬, 施卫明, 等. 硝化抑制剂影响小麦产量、N2O与NH3排放的研究. 土壤, 2017, 49(5): 876-881. Sun H J, Min J, Shi W M, et al. Effects of nitrification inhibitor application on wheat grain yield, N2O emission and NH3 volatilization (In Chinese). Soils, 2017, 49(5): 876-881. (  0) 0) |
| [29] |
Wrage N, Velthof G L, van Beusichem M L, et al. Role of nitrifer denitrifcation in the production of nitrous oxide . Soil Biology and Biochemistry, 2001, 33(12/13): 1723-1732.
(  0) 0) |
| [30] |
Lee A, Winther M, Priemé A, et al. Hot spots of N2O emission move with the seasonally mobile oxic-anoxic interface in drained organic soils . Soil Biology and Biochemistry, 2017, 115: 178-186. DOI:10.1016/j.soilbio.2017.08.025
(  0) 0) |
| [31] |
Bergstrom D W, Tenuta M, Beauchamp E G. Increase in nitrous oxide production in soil induced by ammonium and organic carbon . Biology & Fertility of Soils, 1994, 18(1): 1-6.
(  0) 0) |
| [32] |
Peterson S O, Henriksen K, Blackburn T H. Coupled nitrification-denitrification associated with liquid manure in a gel-stabilized model system . Biology & Fertility of Soils, 1991, 12(1): 19-27.
(  0) 0) |
| [33] |
Kool D M, van Groenigen J W, Wrage N. Source determination of nitrous oxide based on nitrogen and oxygen isotope tracing dealing with oxygen exchange . Methods in Enzymology, 2011, 496(16): 139-160.
(  0) 0) |
| [34] |
Bakken L R, Frostegård Å. Sources and sinks for N2O, can microbiologist help to mitigate N2O emissions? . Environmental Microbiology, 2017, 19(12): 4801-4805. DOI:10.1111/1462-2920.13978
(  0) 0) |
2. Beijing Municipal Environmental Monitoring Center, Beijing 100048, ChinaM;
3. College of Agronomy, Anhui Agricultural University, Hefei 230036, China
 2019, Vol. 56
2019, Vol. 56


