2. 中国科学院大学资源与环境学院,北京 100049
大气氮沉降是陆地生态系统重要的氮输入过程,对维持生态系统氮平衡和生产力至关重要。然而,人类活动加速了氮的输入过程,显著改变陆地生态系统的过程和功能。据估计,过去145年(1860—2005),全球氮沉降增加了近3倍,预计到2050年将达到200 Tg·a-1[1]。由于大多数陆地生态系统普遍受到氮的限制,大气氮沉降增加会显著促进植物生长和生态系统碳固定,是正确解释“失踪碳汇”(missing carbon sink)的重要途径。目前有关氮沉降驱动陆地生态系统固碳效率的研究结果存在很大的不确定性,不同学者估计的“氮促碳汇”范围为每千克沉降氮固碳16~470 kg[2-3]。相似地,氮素富集条件下土壤碳储量的演变方向也存在分歧,包括增加、降低和不变等三种结论,施氮引起土壤碳汇增量变化范围为每千克沉降氮固碳0~70 kg[4-5],同样存在着很大的不确定性。
陆地生态系统净初级生产力(NPP)除了受氮限制外,还会受磷限制或氮磷共同限制。大气氮沉降会改变生态系统磷的赋存形态和生态化学计量平衡,进而影响生态系统碳的转化和累积过程。研究表明,氮沉降增加促使植物根系和土壤微生物合成更多的胞外磷酸酶,加速土壤有机质(SOM)的矿化和磷酸盐的释放[6];而且,氮沉降增加也会导致土壤酸化和Fe3+、Al3+浓度升高,增强土壤有效磷的吸附,从而降低土壤磷的可利用性[7]。此外,全球大气氮磷沉降的摩尔比(46.5)远高于陆地植物最佳生长的N/P摩尔比(16~22),不利于植物的生长和生物量积累[8]。中国区域湿沉降的N/P比更大(77±40),与土壤N/P比负相关[9]。因此,氮沉降的持续增加会促使生态系统由氮限制转变为磷限制,随着土壤N/P比持续升高,磷对生态系统碳循环的限制作用逐渐增强。目前关于氮沉降增加以及不同形态氮添加对陆地生态系统碳过程和碳平衡的研究较多,在响应格局和驱动机制方面已取得一系列普遍的共识。遗憾的是,有关外源性磷输入以及氮磷交互作用对陆地生态系统碳循环的影响研究还十分薄弱,导致陆地生态系统固碳潜力估算存在很大的不确定性。
全球人工林面积约1.4×109 hm2,总碳储量约为11.8 Pg C,每年增汇0.18 Pg C,在陆地生态系统碳固定中具有举足轻重的地位[10]。我国人工林面积高达0.62亿hm2,约占世界人工林总面积的1/3,居世界之首;中国森林碳汇的80%来源于人工林碳固定[11]。南方亚热带常绿针叶林分布于全国氮沉降最高的地区(> 30 kg·hm-2·a-1),其面积占全国人工森林面积的54%[12]。此外,80%的亚热带常绿针叶林为幼龄纯林,群落结构单一,系统稳定性差,对外源性氮磷输入响应敏感。过去30年,国际上有关氮沉降和氮磷交互作用的影响研究主要集中于地带性的自然林,对人工林尤其是亚热带常绿针叶林关注较少,基础性研究数据十分缺乏,无法准确评估大气氮磷沉降对亚热带人工林碳汇变化的贡献;此外,有关氮磷富集情景下土壤有机碳(SOC)累积过程与功能微生物群落演变的研究相对独立,有必要将两者耦合起来。
大气氮磷沉降输入一方面增加土壤养分的可利用性,刺激植物的光合、植物生长和碳分配,改变叶凋落物和根系的数量和质量,进而影响底物的可利用性(图 1);同时,底物的数量和质量的改变又会影响土壤微生物和动物群落的组成和功能,直接影响土壤有机质转化速率、CO2和溶解性碳(DOC和DIC)的交换通量以及土壤有机质(SOM)的化学组成与储量(图 1)。本文从森林土壤碳转化的主要路径出发,围绕“森林土壤碳循环对氮磷交互的非线性响应及机制”这一前沿命题,从生物地球化学和分子生物学角度,分别综述氮磷富集对森林(尤其是人工林)土壤碳通量、原有SOC的激发效应、SOM的稳定性以及功能微生物群落组成的影响及其潜在机理,并指出各个研究领域的薄弱环节,明确未来可能的研究重点。
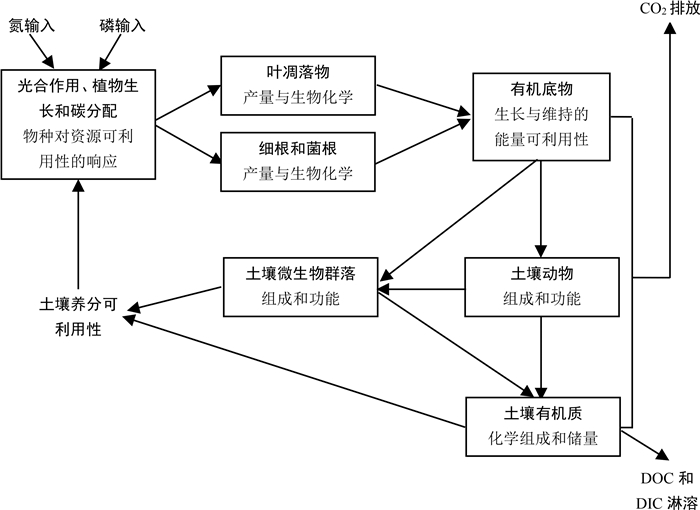
|
图 1 氮磷富集对森林生态系统地下碳循环过程的影响 Fig. 1 Effects of nitrogen and phosphorus enrichment on underground carbon recycling processes in forest ecosystems |
土壤CO2排放是陆地生态系统碳循环的重要环节,主要由三个生物过程(土壤微生物呼吸、根系呼吸和土壤动物呼吸)和一个非生物过程(含碳物质的化学氧化)组成。基于单站点和全球增氮控制试验数据的整合(Meta)分析,森林土壤CO2排放对外源性氮输入的响应有促进、抑制和无显著影响等三种结论,与森林生态系统类型、初始有效氮状态、施氮类型与剂量以及持续时间等因素有关,取决于森林生态系统“氮饱和”阶段。在时间序列上,森林土壤CO2排放对增氮的响应呈现非线性,包括促进→不变→抑制三个演变阶段。施氮初期,植物地下碳分配增加刺激了土壤微生物活性,凋落物C/N比的下降提高了其易分解性[13]。随着土壤无机氮的累积,植物根系自养呼吸仍被促进,而土壤微生物异养呼吸则被抑制,两者之间的消长导致土壤CO2排放对增氮响应不显著[14]。施氮后期,氮素富集降低根系生物量、地下碳分配以及植物根/冠比,同时抑制有机质降解的胞外酶活性,从而降低凋落物和SOM的分解[15]。同样,磷添加对森林土壤CO2通量的影响也有促进、抑制和不变等三种结论,氮磷交互效应更加多元化。例如,Zeng和Wang[16]发现单独添加磷不改变塞罕坝樟子松林土壤呼吸(Rs)及其组分,但是氮磷配施会显著促进樟子松林土壤的碳排放。相反,Kang等[17]研究表明,氮磷添加降低13种北方硬木林土壤呼吸,细根生物量或根际碳通量决定了土壤呼吸对养分添加的响应。Wang等[18]研究也发现氮磷添加显著降低亚热带杉木林土壤呼吸及其组分(自养呼吸Ra和异养呼吸Rh),并且施磷提高了Rh对土壤呼吸下降的贡献率。可见,土壤呼吸不同组分对氮磷添加差异性响应可能是导致土壤呼吸多样性响应的根源,下一步应该通过同位素技术等方法分离出呼吸组分,进一步进行机理性解析。
溶解性有机质(DOM)是SOC循环和氮、磷、硫等养分运移的重要媒介。据估计,每天由土壤呼吸损失的碳量相当于溶解性有机碳(DOC)库的70%,占总土壤碳库的0.06%[19]。森林土壤DOC主要来源于根际沉积和枯枝落叶的降解,其损耗主要通过生物降解和物理淋溶。氮沉降/施氮能够增加、降低和不改变土壤DOC含量和流失通量。氮素输入倾向于增加森林流域DOC流失量,与植被生产力增加和pH变化有关[20]。少数案例研究结果刚好相反。例如,Lu等[21]研究发现,施氮7年显著降低亚热带常绿阔叶林土壤溶液中DOC的含量和主根区土壤DOC的年流失通量,溶液酸化改变土壤吸附能力是导致亚热带老龄林土壤持续固碳的一个新机制。施氮促进或抑制土壤DOC流失的潜在机制是:矿质氮抑制木质素降解的白腐菌活性,增加水溶性产物的释放[22];相反,微生物同化氮刺激了对碳的需求,以及土壤矿物的物理吸附,两者均会降低森林土壤DOC的流失通量[21]。除了改变土壤DOC的数量,外源性氮输入还会改变土壤DOM的组成和化学结构。施氮倾向于增加DOM的腐殖化系数,增加生物的可降解性[23]。施磷对森林土壤DOC的影响研究较少。Cleveland等[24]报道,虽然施磷未改变热带森林凋落物的质量损失,但是刺激了微生物固持凋落物分解释放的磷,降低DOM的C/P比,进而促进DOM的微生物矿化。相反,Mori等[25]发现施磷显著增加泰国热带森林土壤DOC含量和CO2排放量,认为热带森林土壤受磷限制,外源性磷添加会改变土壤微生物群落。由于大多数研究观测时间较短(< 3年),只能观测到土壤碳通量对增氮的线性响应特征。与非线性方程相比,土壤碳通量与氮添加剂量之间的线性方程在不同的氮饱和阶段会低估或高估土壤碳通量的平衡[26]。综上所述,氮、磷输入对森林土壤碳通量及其组分的影响迥异,导致这种差异性响应的原因和潜在机制尚不清楚。目前未能构建亚热带常绿针叶林土壤CO2排放和DOC流失通量对氮磷输入的非线性响应函数,引起森林土壤碳损失急剧转变的氮沉降临界负荷也尚未确定。
2 氮磷富集对原有SOC激发效应的影响激发是指有机质输入促使SOC周转发生的变化,方向可正可负,导致SOC矿化增加或降低[27]。正负激发的平衡非常复杂,在时间和空间上可以相互转化。全球尺度上,根系分泌物、凋落物和动物残体输入能够导致SOM矿化增加380%(正激发)或降低50%(负激发)[28],显著影响陆地生态系统和土壤的碳平衡。与微生物活性相关的激发机制包括:(1)共代谢(co-metabolism)和活化理论:添加易降解的能源物质刺激了微生物活性,增加微生物矿化SOM的能力[27];(2)刺激/抑制作用:添加的底物改变了土壤环境(如pH),提高或降低微生物活性[28];(3)底物优先利用:添加的底物更容易被微生物作为能源利用,导致SOM分解下降[29];(4)稳定机制:添加的底物与SOM相互作用或被SOM吸附,降低微生物的可利用性[28];(5)微生物挖掘(microbial mining):在氮受限制而活性碳充足时,微生物利用碳和能源来获取难分解有机质中的氮、磷养分[30]。本质上,负激发是微生物从利用SOM转变为固持活性有机质,在微生物活性受碳限制时发生[31]。普遍认为,负激发是底物优先利用的结果,而活化理论与微生物挖掘常被用来解释SOM矿化的正激发效应[32]。
激发效应的发生还会受到土壤养分可利用性的制约。氮添加对SOM降解具有负激发、正激发和零激发等三种效应。当土壤养分缺乏时,微生物可“投资”1%~5%的同化产物用于产生胞外酶,通过解聚作用来分解部分难以利用的有机质,获取其所需的养分[33]。在氮磷富集条件下,微生物偏好利用易分解的有机质,而无需通过分解难以利用的SOM来获取养分,从而减少对胞外酶的投资。同时,较高的可利用性氮磷会减少植物向地下碳的分配[34],降低土壤微生物数量和活性,发生负激发效应,抑制SOM的矿化[35]。最近,Nottingham等[36]构建添加13C标记的葡萄糖培养实验,研究发现磷添加促进巴拿马热带森林土壤中活性碳(葡萄糖)的矿化,而氮添加抑制了SOC的矿化,氮磷同时添加对活性碳矿化的促进作用和对SOC矿化的抑制效应最大。同样,基于13C标记凋落物的培养实验,Wang等[18]发现氮磷单独或联合添加均抑制华南亚热带马尾松林土壤呼吸,促进土壤碳截存。相反,Poeplau等[37]发现施磷导致氮限制土壤有机碳库耗竭,其潜在的机制为:施磷降低了植物根/茎比和丛枝菌根真菌的丰度,导致更高的根源碳输入到土壤中,刺激了土壤异养呼吸;此外,磷输入增加了氮的输出,导致微生物氮的挖掘作用和有机质矿化增加。可见,过去有关激发效应的研究几乎全部集中于碳,鲜有研究关注氮磷等其他养分的影响。虽然已认识到养分可利用性会影响激发效应的强度和方向,但是对影响的程度和潜在的机制知之甚少,限制了对森林土壤碳氮循环驱动力的预测。
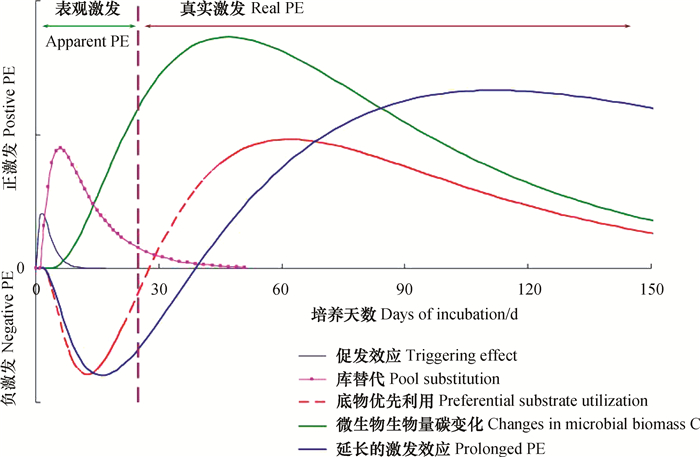
|
图 2 土壤激发效应的时间演变及其主控机理 Fig. 2 Temporal evolution of soil priming effect and its major control mechanisms |
SOM库由不同分解阶段的有机物组成,包括完全分解的腐殖质、半分解有机残体、微生物及其排泄物。在操作上,按照密度可将SOM分为游离态轻组(free light fraction)、包裹态轻组(intra-aggregate light fraction)和重组(heavy fraction)[38]等组分。按照SOM在土壤结构中的分布,可分为溶解性有机质(DOM)、游离态颗粒有机质(free POM)、闭蓄态颗粒有机质(occluded POM)和矿质结合态有机质(MAOM)[39]。SOC不同组分对外源性氮输入的响应并不一致,取决于生态系统类型、施氮剂量与类型以及持续时间。例如,Hagedorn等[40]发现氮沉降显著促进瑞士亚高山森林土壤轻组碳分解,却使重组碳变得更加稳定。Zhong等[41]研究指出,施氮降低亚热带马尾松林土壤微生物活性,阻碍SOC分解,导致团聚体中DOC、热水提取有机碳(HWOC)含量增加,暗示团聚体的物理保护作用会促进土壤碳的积累。此外,受氮限制的森林和草地生态系统SOC对施氮剂量的响应似乎存在一个临界阈值。基于加拿大草地27年的施氮实验,Malhi等[42]研究表明,低剂量氮输入增加SOC和轻组碳含量,施氮水平为N336和224 kg·hm-2·a-1时,表层和亚表层SOC和轻组碳含量分别达到最高值,随着施氮剂量的持续增加,SOC和轻组碳反而下降。相似地,低剂量氮添加显著增加大兴安岭北方森林和青藏高原高寒草甸SOC和颗粒态有机碳(POC)含量,而中、高剂量氮添加却导致土壤碳的耗竭,SOC由截存转变为损耗的大气氮沉降临界负荷为20 kg·hm-2·a-1,并且铵态氮肥对SOC的损耗高于硝态氮肥[43-44]。
SOC的截存与稳定是一个物理、化学和生物共同作用的过程。SOM的稳定机制分为三类:(1)土壤团聚体的物理保护。(2)金属氧化物、无机氮离子和黏土矿物/有机碳的结合。例如,NH4+和NO3-能够结合到SOM骨架中,生成微生物难以降解的化合物(如杂环氮化合物)[45],或通过氮键生成酚聚合物[46],进而促进SOC的积累。(3)SOM的化学稳定性。SOM由多种复杂的有机分子单体和化合物组成,不同组分化学结构的差异导致SOM化学稳定性千差万别。新增的SOC能否在土壤中稳定持留很大程度上取决于SOM的化学结构。利用1H-NMR波谱和PLFA技术,Feng等[47]研究发现施氮降低美国杜克森林矿质层土壤真菌/细菌比和革兰氏阴性/阳性细菌(G-/G+)比,增加木质素酚中的酸/醛比,提高矿质层土壤木质素的降解;同时,施氮促进木质素、脂肪类有机质的微生物降解,导致植物源难分解结构(如烷基碳)在土壤中富集。同样,Pisani等[48]也发现氮素富集增加北美哈佛森林凋落物层植物源碳输入,提高了木质素的氧化程度,导致矿质层土壤植物源烷基碳和微生物源有机质富集。有效氮不同的森林对增氮响应差异很大。Cusack等[49]研究指出施氮增加低海拔热带森林土壤革兰氏阴性细菌丰度,导致活性的烷氧基碳显著下降;相反,施氮增加高海拔热带森林真菌生物量,进而降低了烷基碳的含量,胞外酶活性提供了微生物群落结构与SOM化学结构在功能上的联系。相似地,Xu等[50]研究发现培养前期氮硫添加促进亚热带马尾松叶凋落物分解,土壤CO2排放增加与烷氧基碳的损失有关;培养后期土壤CO2排放减少,伴随着甲氧基碳含量的增加;凋落物烷基碳、芳香碳与土壤缓性碳库的半衰期正相关,暗示长期氮硫沉降会增加凋落物残体的耐分解性和植物残体碳积累。然而,关于磷添加对SOM化学结构的影响结果极少。Guo等[51]研究表明,氮磷添加显著降低高寒草甸土壤微生物活性,增加有机底物的芳香度和脂肪度,且Q10值与SOM的复杂度呈正相关,长期氮磷添加会增加青藏高原变暖情景下土壤净碳损失预测的不确定性。就有机质化学结构测定方法而言,CP MAS 13C-NMR技术擅长表达复杂大分子化学结构的总体特征,而热裂解-色谱-质谱(Py-GC/MS)技术通过将大分子化合物降解为小分子的化合物,能够更加精确地表征有机质的分子结构。综上所述,物理分组能够认识有机-矿物颗粒空间排列对SOC动态的影响,而化学分析可以从分子水平上理解不同有机单体和化合物之间的联系。因此,在SOM物理分组的基础上,结合化学分析技术(13C NMR和Py-GC/MS),能够进一步阐明氮磷交互作用对SOM组成和化学稳定性的影响机制。
4 氮磷富集对土壤微生物群落组成和功能的影响通俗而言,土壤微生物群落组成就是谁发生了变化,变化了多少?微生物功能即是它们正在干什么?SOM的积累通常与真菌系统发育多样性、木质纤维素水解胞外酶活性呈正相关,而细菌多样性和功能基因组成通常受土壤pH支配[52]。氮沉降增加导致森林土壤无机氮富集,理论上会产生以下三种效应:(1)施氮抑制腐生真菌分泌木质纤维素水解酶,降低腐生微生物群落获取碳源(如纤维素、半纤维素)的能力。根据密歇根糖枫林近20年的模拟氮沉降控制试验结果,长期NO3-沉降显著抑制了白腐担子菌酚氧化酶的产生[22],降低担子菌漆酶基因lcc丰度和基因表达[53]。除了降低真菌的生物量和群落组成外,施氮还会影响真菌的进化轨迹,氮富集会显著降低真菌的降解速率[54]。(2)施氮改变微生物群落之间的交互作用和组装(assemble)方式,从而改变分解微生物群落的组成[55]。长期施氮改变了密歇根糖枫林土壤真菌群落组成(担子菌OTUs/子囊菌OTUs增加),并且随着施氮时间的延长其抑制效应更加显著[56]。Eisenlord等[57]利用GeoChip4.0基因芯片分析土壤放线菌和真菌群落功能基因,发现施氮显著改变放线菌和真菌功能基因组成,降低淀粉、半纤维素、纤维素、几丁质、木质素解聚功能基因丰富度和多样性,在一定程度上解释了凋落物分解下降和SOM积累。细菌类漆酶-多铜氧化酶基因(LMCOs)能够氧化木质素中酚类化合物的β-O-4链接二聚体,产生DOC。Freedman和Zak[58]研究发现,模拟氮沉降增加细菌LMCO酶活性,有利于产生LMCO酶的细菌生存,解释了氮素富集条件下DOC流失增加的微生物学机理。(3)降低凋落物分解速率和程度,增加类木质素化合物的氧化程度和稳定性,进而促进SOM的积累[59]。相反,也有研究认为施氮只改变植物源烷基化合物的比例,提高或不影响木质素的氧化程度[60]。
土壤有效磷动态也会强烈影响微生物群落组成。无机磷含量较低的温带森林土壤,微生物通过增加磷酸酶活性获得有机磷,克服生物地球化学磷的限制。相对于氮素输入而言,土壤微生物群落组成对磷添加的研究案例不多,研究结果也不够系统深入。例如,Huang等[61]研究发现,施磷2年增加了亚高山云杉林土壤微生物生物量,主要归因于土壤有效碳含量和pH增加的间接影响。Jing等[62]基于2年的氮磷添加实验,得出施氮未显著改变青藏高原高寒草甸土壤微生物生物量、土壤呼吸和胞外酶活性,但是施磷显著抑制了0~10 cm土层碳循环相关的胞外酶活性。丛枝菌根真菌(AMF)丰度对氮磷沉降的响应取决于养分的可利用性。通过分析根系长度、菌根浸染和AMF生物量,Camenzind等[63]发现,施磷显著增加而施氮则降低不同海拔热带低地森林土壤AMF丰度。然而,上述研究结果是建立在全体微生物群落基础上的认识,未区分活性微生物群落的代谢作用。通过基于RNA的宏基因组学分析,Freedman等[64]研究发现,施氮不影响温带森林凋落物层真菌群落的组成、多样性和丰富度,但是其多样性降低8%,改变了活性真菌群落的组成;相反,施氮不改变全体和活性细菌的群落组成。宏转录组学方法可整体评估真核微生物和细菌的基因表达,适合于活性真核生物和细菌相互作用的研究。Freedman等[65]利用胞外酶活性和宏基因组鸟枪测序方法评价施氮对密歇根糖枫林土壤微生物群落功能的影响,发现施氮显著降低降解植物细胞壁的胞外酶活性,伴随着碳水化合物、芳香族化合物的代谢过程以及微生物呼吸等宏基因组功能基因表达路径的变化,与腐生细菌功能基因相对丰度增加有关;相反,施氮不影响降解凋落物的真菌功能基因的相对丰度和组成,说明未来氮沉降增加将提升腐生细菌的降解功能,而腐生真菌的代谢潜力对氮富集的响应具有一定的弹性和恢复力。总体上,土壤无机氮富集抑制真菌功能基因表达和氧化酶活性、改变群落组成等方面已初步形成共识,但是细菌群落组成的变化如何代谢有机底物的机制仍然不清楚,土壤活性微生物群落组成、SOM化学结构的变化与SOC积累之间的耦联关系尚不清晰。
5 问题与展望考虑到氮磷富集条件下森林土壤碳转化过程研究的不确定性,未来的研究重点和研究思路如下:(1)亚热带人工林土壤碳排放与流失通量对氮磷添加的非线性响应特征。基于野外氮磷添加长期控制试验和室内培养实验平台,利用静态箱—气相色谱、大型自由排水采集器分别测定土壤碳排放和流失通量以及相关环境因子,研究土壤碳通量对氮磷添加的非线性响应特征及主要驱动因子。(2)氮磷交互作用对亚热带人工林土壤有机碳储量、组成和稳定性的影响。利用SOM物理分组以及13C-NMR和Py-GC/MS方法测定不同SOM组分的碳含量、单体有机化合物和主要官能团比例,研究氮磷富集对SOC组成、来源、降解程度和化学稳定性的影响。(3)氮磷交互作用对亚热带人工林土壤分解微生物群落组成和功能的影响。利用rt-qPCR、高通量测序和群落谱系分析等方法测定土壤真菌、细菌以及SOM降解功能基因丰度和群落组成,探讨氮磷富集对介导土壤碳循环微生物丰度、群落组成及碳降解功能的影响;(4)氮磷富集条件下凋落物输入对亚热带人工林原有SOC的激发作用。构建13C标记凋落物的室内培养实验,测定底物和土壤CO2的碳浓度及其δ13C,计算底物添加引起的激发效应强度和微生物底物利用效率,分析氮磷富集条件下微生物激发效应的方向及其对SOC动态的影响。内容(1)和(2)共同阐明氮磷富集条件下土壤碳转化与SOM稳定性的演变特征,内容(3)和(4)共同阐明主要分解菌丰度和群落组成与土壤碳转化之间的耦联关系,最终揭示氮磷交互作用影响亚热带人工林土壤碳积累与损耗的微生物学机制。
6 结论外源性氮磷输入通过改变微生物群落组成和功能直接影响森林土壤碳转化过程和净碳交换通量,同时通过改变底物化学组成和有效性,进而影响土壤碳固定,取决于生态系统类型、氮磷添加形态、剂量和持续时间。总体而言,有关氮输入对森林土壤碳通量、SOM激发效应、SOM稳定性和功能微生物群落的影响研究相对较为深入,有关磷富集以及氮磷交互作用的研究尚未形成一致的研究结论。而且,过去相关研究主要集中在自然森林生态系统中,对固碳效应更加显著的人工林关注不足。针对这些研究的薄弱环节,今后的研究应该从生物地球化学和分子生物学角度出发,重点探讨氮磷添加对亚热带人工林土壤碳排放与流失通量、SOC激发效应、SOM组成与稳定性、功能微生物群落组成的影响及其潜在的机制。研究结果有助于完善陆地生态系统碳-氮-磷耦合循环模型,控制森林土壤碳损失,有效降低陆地“氮促碳汇”评估的不确定性,并可为亚热带人工林生态系统应对全球变化提供科学依据。
| [1] |
Galloway J N, Townsend A R, Erisman J W, et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions . Science, 2008, 320(5878): 889-892. DOI:10.1126/science.1136674
(  0) 0) |
| [2] |
Magnani F, Mencuccini M, Borghetti M, et al. The human footprint in the carbon cycle of temperate and boreal forests . Nature, 2007, 447(7146): 849-851. DOI:10.1038/nature05847
(  0) 0) |
| [3] |
de Vries W, Solberg S, Dobbertin M, et al. Ecologically implausible carbon response? . Nature, 2008, 451(7180): E1-E3.
(  0) 0) |
| [4] |
de Vries W I M, Reinds G J, Gundersen P E R, et al. The impact of nitrogen deposition on carbon sequestration in European forests and forest soils . Global Change Biology, 2006, 12(7): 1151-1173. DOI:10.1111/gcb.2006.12.issue-7
(  0) 0) |
| [5] |
Janssens I A, Dieleman W, Luyssaert S, et al. Reduction of forest soil respiration in response to nitrogen deposition . Nature Geoscience, 2010, 3(5): 315-322. DOI:10.1038/ngeo844
(  0) 0) |
| [6] |
Ratliff T J, Fisk M C. Phosphatase activity is related to N availability but not P availability across hardwood forests in the northeastern United States . Soil Biology & Biochemistry, 2016, 94: 61-69.
(  0) 0) |
| [7] |
Matson P A, McDowell W H, Townsend A R, et al. The globalization of N deposition: Ecosystem consequences in tropical environments . Biogeochemistry, 1999, 46(1/3): 67-83. DOI:10.1023/A:1006152112852
(  0) 0) |
| [8] |
Peñuelas J, Sardans J, Rivas-ubach A, et al. The human-induced imbalance between C, N and P in Earth's life system . Global Change Biology, 2012, 18(1): 3-6.
(  0) 0) |
| [9] |
Zhu J, Wang Q, He N, et al. Imbalanced atmospheric nitrogen and phosphorus depositions in ChinaImplications for nutrient limitation . Journal of Geophysical Research-Biogeosciences, 2016, 121(6): 1605-1616. DOI:10.1002/2016JG003393
(  0) 0) |
| [10] |
Winjum J K, Schroeder P E. Forest plantations of the worldTheir extent, ecological attributes, and carbon storage . Agricultural and Forest Meteorology, 1997, 84(1/2): 153-167.
(  0) 0) |
| [11] |
Fang J, Chen A, Peng C, et al. Changes in forest biomass carbon storage in China between 1949 and 1998 . Science, 2001, 292(5525): 2320-2322. DOI:10.1126/science.1058629
(  0) 0) |
| [12] |
Piao S, Fang J, Ciais P, et al. The carbon balance of terrestrial ecosystems in China . Nature, 2009, 458(7241): 1009-1013. DOI:10.1038/nature07944
(  0) 0) |
| [13] |
McDowell W H, Magill A H, Aitkenhead-Peterson J A, et al. Effects of chronic nitrogen amendment on dissolved organic matter and inorganic nitrogen in soil solution . Forest Ecology and Management, 2004, 196(1): 29-41. DOI:10.1016/j.foreco.2004.03.010
(  0) 0) |
| [14] |
Wang Y, Cheng S, Fang H, et al. Contrasting effects of ammonium and nitrate inputs on soil CO2 emission in a subtropical coniferous plantation of southern China . Biology and Fertility of Soils, 2015, 51(7): 815-825. DOI:10.1007/s00374-015-1028-x
(  0) 0) |
| [15] |
Mo J, Zhang W E I, Zhu W, et al. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China . Global Change Biology, 2008, 14(2): 403-412.
(  0) 0) |
| [16] |
Zeng W, Wang W. Combination of nitrogen and phosphorus fertilization enhance ecosystem carbon sequestration in a nitrogen-limited temperate plantation of Northern China . Forest Ecology and Management, 2015, 341: 59-66. DOI:10.1016/j.foreco.2015.01.004
(  0) 0) |
| [17] |
Kang H Z, Fahey T J, Bae K, et al. Response of forest soil respiration to nutrient addition depends on site fertility . Biogeochemistry, 2016, 127(1): 113-124. DOI:10.1007/s10533-015-0172-6
(  0) 0) |
| [18] |
Wang Q K, Zhang W D, Sun T, et al. N and P fertilization reduced soil autotrophic and heterotrophic respiration in a young Cunninghamia lanceolata forest . Agricultural and Forest Meteorology, 2017, 232: 66-73. DOI:10.1016/j.agrformet.2016.08.007
(  0) 0) |
| [19] |
Jones D L, Hughes L T, Murphy D V, et al. Dissolved organic carbon and nitrogen dynamics in temperate coniferous forest plantations . European Journal of Soil Science, 2008, 59(6): 1038-1048. DOI:10.1111/ejs.2008.59.issue-6
(  0) 0) |
| [20] |
Rowe E C, Tipping E, Posch M, et al. Predicting nitrogen and acidity effects on long-term dynamics of dissolved organic matter . Environmental Pollution, 2014, 184(1): 271-282.
(  0) 0) |
| [21] |
Lu X, Gilliam F S, Yu G, et al. Long-term nitrogen addition decreases carbon leaching in a nitrogen-rich forest ecosystem . Biogeosciences, 2013, 10(6): 3931-3941. DOI:10.5194/bg-10-3931-2013
(  0) 0) |
| [22] |
DeForest J L, Zak D R, Pregitzer K S, et al. Atmospheric nitrate deposition and enhanced dissolved organic carbon leaching . Soil Science Society of America Journal, 2005, 69(4): 1233-1237. DOI:10.2136/sssaj2004.0283
(  0) 0) |
| [23] |
Fang H, Cheng S, Yu G, et al. Experimental nitrogen deposition alters the quantity and quality of soil dissolved organic carbon in an alpine meadow on the Qinghai-Tibetan Plateau . Applied Soil Ecology, 2014, 81: 1-11. DOI:10.1016/j.apsoil.2014.04.007
(  0) 0) |
| [24] |
Cleveland C C, Reed S C, Townsend A R. Nutrient regulation of organic matter decomposition in a tropical rain forest . Ecology, 2006, 87(2): 492-503.
(  0) 0) |
| [25] |
Mori T, Wachrinrat C, Staporn D, et al. Contrastive effects of inorganic phosphorus addition on soil microbial respiration and microbial biomass in tropical monoculture tree plantation soils in Thailand . Agriculture and Natural Resources, 2016, 50(5): 327-330. DOI:10.1016/j.anres.2016.04.004
(  0) 0) |
| [26] |
Gomez-Casanovas N, Hudiburg T W, Bernacchi C J, et al. Nitrogen deposition and greenhouse gas emissions from grasslands: Uncertainties and future directions . Global Change Biology, 2016, 22(4): 1348-1360. DOI:10.1111/gcb.2016.22.issue-4
(  0) 0) |
| [27] |
Kuzyakov Y, Friedel J K, Stahr K. Review of mechanisms and quantification of priming effects . Soil Biology & Biochemistry, 2000, 32(11): 1485-1498.
(  0) 0) |
| [28] |
Cheng W, Parton W J, Gonzalez-Meler M A, et al. Synthesis and modeling perspectives of rhizosphere priming . New Phytologist, 2014, 201(1): 31-44. DOI:10.1111/nph.12440
(  0) 0) |
| [29] |
Zimmerman A R, Gao B, Ahn M Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils . Soil Biology & Biochemistry, 2011, 43(6): 1169-1179.
(  0) 0) |
| [30] |
Meyer N, Welp G, Bornemann L, et al. Microbial nitrogen mining affects spatio-temporal patterns of substrate-induced respiration during seven years of bare fallow . Soil Biology & Biochemistry, 2017, 104: 175-184.
(  0) 0) |
| [31] |
Hamer U, Marschner B. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions . Soil Biology & Biochemistry, 2005, 37(3): 445-454.
(  0) 0) |
| [32] |
Craine J M, Morrow C, Fierer N. Microbial nitrogen limitation increases decomposition . Ecology, 2007, 88(8): 2105-2113. DOI:10.1890/06-1847.1
(  0) 0) |
| [33] |
Burns R G, DeForest J L, Marxsen J, et al. Soil enzymes in a changing environmentCurrent knowledge and future directions . Soil Biology & Biochemistry, 2013, 58: 216-234.
(  0) 0) |
| [34] |
Phillips R P, Finzi A C, Bernhardt E S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation . Ecology Letters, 2011, 14(2): 187-194.
(  0) 0) |
| [35] |
Dijkstra F A, Carrillo Y, Pendall E, et al. Rhizosphere primingA nutrient perspective . The Microbial Regulation of Global Biogeochemical Cycles, 2014, 4(1): 216.
(  0) 0) |
| [36] |
Nottingham A T, Turner B L, Stott A W, et al. Nitrogen and phosphorus constrain labile and stable carbon turnover in lowland tropical forest soils . Soil Biology & Biochemistry, 2015, 80: 26-33.
(  0) 0) |
| [37] |
Poeplau C, Bolinder M A, Kirchmann H, et al. Phosphorus fertilisation under nitrogen limitation can deplete soil carbon stocksEvidence from Swedish meta-replicated long-term field experiments . Biogeosciences, 2016, 13(19): 1119-1127.
(  0) 0) |
| [38] |
Roscoe R, Buurman P. Tillage effects on soil organic matter in density fractions of a Cerrado Oxisol . Soil and Tillage Research, 2003, 70(2): 107-119. DOI:10.1016/S0167-1987(02)00160-5
(  0) 0) |
| [39] |
Six J, Conant R T, Paul E A, et al. Stabilization mechanisms of soil organic matterImplications for C-saturation of soils . Plant and Soil, 2002, 241(2): 155-176. DOI:10.1023/A:1016125726789
(  0) 0) |
| [40] |
Hagedorn F, Spinnler D, Siegwolf R. Increased N deposition retards mineralization of old soil organic matter . Soil Biology & Biochemistry, 2003, 35(12): 1683-1692.
(  0) 0) |
| [41] |
Zhong X L, Li J T, Li X J, et al. Physical protection by soil aggregates stabilizes soil organic carbon under simulated N deposition in a subtropical forest of China . Geoderma, 2017, 285: 323-332. DOI:10.1016/j.geoderma.2016.09.026
(  0) 0) |
| [42] |
Malhi S S, Harapiak J T, Nyborg M, et al. Total and light fraction organic C in a thin Black Chernozemic grassland soil as affected by 27 annual applications of six rates of fertilizer N . Nutrient Cycling in Agroecosystems, 2003, 66(1): 33-41. DOI:10.1023/A:1023376905096
(  0) 0) |
| [43] |
Fang H J, Cheng S L, Yu G R, et al. Nitrogen deposition impacts on the amount and stability of soil organic matter in an alpine meadow ecosystem depend on the form and rate of applied nitrogen . European Journal of Soil Science, 2014, 65(4): 510-519. DOI:10.1111/ejss.2014.65.issue-4
(  0) 0) |
| [44] |
Cheng S, He S, Fang H, et al. Contrasting effects of NH4+ and NO3- amendments on amount and chemical characteristics of different density organic matter fractions in a boreal forest soil . Geoderma, 2017, 293: 1-9. DOI:10.1016/j.geoderma.2017.01.023
(  0) 0) |
| [45] |
Thorn K A, Mikita M A. Ammonia fixation by humic substancesa nitrogen-15 and carbon-13 NMR study . Science of the Total Environment, 1992, 113(1): 67-87.
(  0) 0) |
| [46] |
Nӧmmik H, Vahtras K. Retention and fixation of ammonium and ammonia in soils//Nitrogen in Agricultural Soils, 1982123-171
(  0) 0) |
| [47] |
Feng X J, Simpson A J, Schlesinger W H, et al. Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the duke forest . Global Change Biology, 2010, 16(7): 2104-2116. DOI:10.1111/gcb.2010.16.issue-7
(  0) 0) |
| [48] |
Pisani O, Frey S D, Simpson A J, et al. Soil warming and nitrogen deposition alter soil organic matter composition at the molecular-level . Biogeochemistry, 2015, 123(3): 391-409. DOI:10.1007/s10533-015-0073-8
(  0) 0) |
| [49] |
Cusack D F, Torn M S, McDowell W H, et al. The response of heterotrophic activity and carbon cycling to nitrogen additions and warming in two tropical soils . Global Change Biology, 2010, 16(9): 2555-2572.
(  0) 0) |
| [50] |
Xu Y H, Fan J L, Ding W X, et al. Characterization of organic carbon in decomposing litter exposed to nitrogen and sulfur additionsLinks to microbial community composition and activity . Geoderma, 2017, 286: 116-124. DOI:10.1016/j.geoderma.2016.10.032
(  0) 0) |
| [51] |
Guo H, Ye C L, Zhang H, et al. Long-term nitrogen & phosphorus additions reduce soil microbial respiration but increase its temperature sensitivity in a Tibetan alpine meadow . Soil Biology & Biochemistry, 2017, 113: 26-34.
(  0) 0) |
| [52] |
Cline L C, Zak D R. Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession . Ecology, 2015, 96(12): 3374-3385. DOI:10.1890/15-0184.1
(  0) 0) |
| [53] |
Hassett J E, Zak D R, Blackwood C B, et al. Are basidiomycete laccase gene abundance and composition related to reduced lignolytic activity under elevated atmospheric NO3- deposition in a northern hardwood forest? . Microbial Ecology, 2009, 57(4): 728-739. DOI:10.1007/s00248-008-9440-5
(  0) 0) |
| [54] |
van Diepen L T A, Frey S D, Landis E A, et al. Fungi exposed to chronic nitrogen enrichment are less able to decay leaf litter . Ecology, 2017, 98(1): 5-11.
(  0) 0) |
| [55] |
Freedman Z B, Zak D R. Atmospheric N deposition alters connectance, but not functional potential among saprotrophic bacterial communities . Molecular Ecology, 2015, 24(12): 3170-3180. DOI:10.1111/mec.13224
(  0) 0) |
| [56] |
Entwistle E M, Zak D R, Edwards I P. Long-term experimental nitrogen deposition alters the composition of the active fungal community in the forest floor . Soil Science Society of America Journal, 2013, 77(5): 1648-1658. DOI:10.2136/sssaj2013.05.0179
(  0) 0) |
| [57] |
Eisenlord S D, Freedman Z, Zak D R, et al. Microbial mechanisms mediating increased soil C storage under elevated atmospheric N deposition . Applied and Environmental Microbiology, 2013, 79(4): 1191-1199. DOI:10.1128/AEM.03156-12
(  0) 0) |
| [58] |
Freedman Z B, Zak D R. Atmospheric N deposition increases bacterial laccase-like multicopper oxidases: Implications for organic matter decay . Applied and Environmental Microbiology, 2014, 80(14): 4460-4468. DOI:10.1128/AEM.01224-14
(  0) 0) |
| [59] |
Whittinghill K A, Currie W S, Zak D R, et al. Anthropogenic N deposition increases soil C storage by decreasing the extent of litter decayAnalysis of field observations with an ecosystem model . Ecosystems, 2012, 15(3): 450-461. DOI:10.1007/s10021-012-9521-7
(  0) 0) |
| [60] |
Thomas D C, Zak D R, Filley T R. Chronic N deposition does not apparently alter the biochemical composition of forest floor and soil organic matter . Soil Biology & Biochemistry, 2012, 54(6): 7-13.
(  0) 0) |
| [61] |
Huang J S, Hu B, Qi K B, et al. Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation . European Journal of Soil Biology, 2016, 72: 35-41. DOI:10.1016/j.ejsobi.2015.12.007
(  0) 0) |
| [62] |
Jing X, Yang X X, Ren F, et al. Neutral effect of nitrogen addition and negative effect of phosphorus addition on topsoil extracellular enzymatic activities in an alpine grassland ecosystem . Applied Soil Ecology, 2016, 107: 205-213. DOI:10.1016/j.apsoil.2016.06.004
(  0) 0) |
| [63] |
Camenzind T, Homeier J, Dietrich K, et al. Opposing effects of nitrogen versus phosphorus additions on mycorrhizal fungal abundance along an elevational gradient in tropical montane forests . Soil Biology & Biochemistry, 2016, 94(11): 37-47.
(  0) 0) |
| [64] |
Freedman Z B, Romanowicz K J, Upchurch R A, et al. Differential responses of total and active soil microbial communities to long-term experimental N deposition . Soil Biology & Biochemistry, 2015, 90(7): 275-282.
(  0) 0) |
| [65] |
Freedman Z B, Upchurch R A, Zak D R, et al. Anthropogenic N deposition slows decay by favoring bacterial metabolismInsights from metagenomic analyses . Frontiers in Microbiology, 2016, 7(259). DOI:10.3389/fmicb.2016.00259
(  0) 0) |
2. College of Resources and Environment, University of Chinese Academy of Sciences, Beijing 100049, China
 2019, Vol. 56
2019, Vol. 56


