2. 中国科学院大学,北京 100049
酸沉降带来的土壤酸化是我国南方面临的严重土壤退化类型之一。关于土壤酸化速率的研究已持续数十年,但依然没有准确且高效的测定方法[1-4],主要是因为土壤本身的缓冲性能,使得pH、酸中和容量等难以在短时间内观测到[5-6],而流域元素质量平衡法,可以实地观测到流域内通过各种途径输入和输出的H+以及盐基离子,成为估算当前土壤酸化速率的有效方法[3, 7-8]。但是,从土壤消耗H+的途径来看,H+进入土壤以后,一部分与土壤胶体上吸附的盐基离子(K+、Na+、Ca2+和Mg2+)发生阳离子交换反应,另一部分参与土壤矿物风化而被消耗[9-12],其中只有前者使土壤潜性酸增加,导致土壤酸化[8]。但是这两个途径均会释放盐基离子,导致流域元素质量平衡法所观测的径流水中输出的盐基离子的来源难以区分,据此估算的土壤酸化速率误差较大[13-14]。因此,如何区分酸化过程和矿物风化过程的H+消耗量,是解决问题的关键。由于径流水中的硅主要来自矿物风化,不受阳离子交换的影响,因此Yang等[8]发现利用盐基离子和Si的矿物风化计量关系,能够区分阳离子交换和矿物风化消耗的H+,从而有助于准确定量土壤的酸化速率。
模拟酸雨淋溶是研究土壤地球化学或矿物风化特征的有效方法[15-18],可目前的矿物风化研究以某一种单一的原生矿物或次生矿物为主,重在探索不同控制条件下,不同风化阶段的风化产物和风化机理[19-30],关于风化过程中盐基离子和Si元素的释放特征尚缺乏研究。更重要的是,土壤是一个包括许多不同风化程度矿物的自然综合体,单一矿物的风化特征不能说明处于不同风化阶段自然土壤的风化特征。
我国亚热带地区土壤风化强烈,而且近年来酸沉降严重,土壤酸化问题突出[31-35]。本研究选取亚热带地区花岗岩发育的强风化土壤,通过模拟淋溶实验研究土壤中混合矿物风化后的盐基离子释放特征和矿物风化计量关系,为准确定量该区域土壤的酸化速率提供基础数据。
1 材料与方法 1.1 土壤样品采集土样采自我国广东省广州市天河区(23°9′21″N,113°21′16″E),土壤类型为黏质高岭石型酸性高热性-腐殖黏化湿润富铁土,是我国南方典型的红壤。母质为花岗岩风化物,土地利用类型为自然林地。采样区域属南亚热带季风气候,年平均温度22℃,年降雨量1 983mm[33]。根据不同发育程度,对土壤进行分层取样:A层(0~46 cm)、B1层(46~154 cm)和B2层(154~250 cm)。自然风干后拣去根系等,对于淋溶实验的土样不予碾磨处理以保持土壤混合矿物实际情况;对于进行理化分析和矿物鉴定的土样根据需要分别过10、60、100和200目筛。
1.2 实验设计本实验采用改进后的分批次Batch方法,在常温常压下对土壤进行模拟酸雨淋溶,研究土壤中混合矿物在风化后盐基离子的释放情况[36]。每个供试土样取3份作为重复,预先洗脱盐基;另取1份不予洗脱盐基,每个土样4份,3个层次共计12个样品,每个样品称取待测土样200 g于500 ml离心瓶内。
洗脱盐基处理采用pH为7.0的EDTA-乙酸铵溶液(1 mol·L-1的乙酸铵和0.005 mol·L-1的EDTA),按土液比1:1加入200 ml的EDTA-乙酸铵洗脱液,搅拌均匀震荡后置于离心机中离心10 min,收集上层清液,测定盐基离子含量[36]。重复上述过程,若上层清液中的盐基离子含量连续3次不再有明显的变化,则说明此时土壤中的交换性盐基离子已被完全洗脱。由于风化作用持续释放盐基离子,洗脱液中不可能完全没有盐基离子,故以此作为完全洗脱盐基的标志,本实验的洗脱盐基处理总共13次。洗脱盐基后残留在土壤中的NH4+,则通过无水乙醇反复清洗除去,若收集到的淋出液与纳氏试剂反应无黄色出现,则说明残留的NH4+已被全部洗脱。
为避免其他离子的影响,模拟酸雨淋溶液用NH4Cl和稀HCl配置而成,NH4+浓度为0.1 mol·L-1,根据我国南方酸雨的平均酸度[37],将模拟酸雨的pH调为4.5。供试土样包括洗脱盐基处理后和未洗脱盐基处理的样品,每次按土液比1:1加入200 mL酸雨淋溶液,搅拌均匀后,置于震荡机中震荡24 h,确保土壤和溶液充分反应[36]。随后离心(转速设定为7 000 r·min-1)并收集上清液测定K+、Na+、Ca2+、Mg2+、Si含量和pH,此为一次淋溶过程。为了尽可能接近自然降水过程以及矿物风化的真实情况,给予充分的反应时间并保证土壤的干湿交替过程,实验中采用间歇式酸雨淋溶法,淋溶一次,间歇6d,一周为一次淋溶周期。根据当地实际降雨量,总共淋溶13次即可模拟一年降雨情况。
1.3 测定方法土壤的矿物组成,先用MgCl2进行阳离子饱和以及甘油吸附,制成玻片后通过X射线衍射仪进行鉴定;有机质用重铬酸钾-硫酸消化法测定;矿质全量用碳酸锂-硼酸熔融法制备待测液后,用ICP测定;阳离子交换量选用乙酸铵(pH7.0)交换法测定;交换性盐基离子K+、Na+、Ca2+和Mg2+经乙酸铵(pH7.0)提取后,用ICP测定;盐基饱和度为土壤交换性盐基总量占阳离子交换量的百分比;土壤pH用pH计测定(土:水=1:2.5)。土壤淋出液的盐基离子K+、Na+、Ca2+、Mg2+和Si均用ICP法测定,pH则用pH计测定[38]。
2 结果与讨论 2.1 土壤基本特性矿物鉴定结果表明,土壤中的混合矿物主要有高岭石、三水铝石、石英、蛭石、水云母、蛭石-水云母混层矿物、长石以及针铁矿或赤铁矿(表 1)。已有研究表明,广东省基底岩石均以中生代燕山期花岗岩侵入体为主,土壤母岩多为斑状黑云母二长花岗岩,主要原生矿物为长石、石英和黑云母[39-40],因为研究土壤所在区域降水量丰富,气温较高,强烈的风化作用使长石和云母等易风化的原生矿物大部分被水解,能够检测出的原生矿物含量较少,土壤以1:1型次生硅酸盐黏土矿物为主。矿物风化过程会释放K+、Na+、Ca2+和Mg2+,对于花岗岩发育的土壤,这些盐基离子最初来源于易风化的长石和云母等原生矿物。长石的矿物组成通式为OrxAbyAnz(x+y+z=100),Or、Ab和An分别代表KAlSi3O8、NaAlSi3O8和CaAl2Si2O8,因此根据组分差异,可将长石分为钾长石和斜长石,后者包括钠长石和钙长石。长石风化水解,产生高岭石或水云母等次生矿物[39]。
|
|
表 1 土壤的矿物组成 Table 1 Mineral composition of the soil/% |
土壤在发育过程中,长石和云母类原生矿物水解释放盐基离子并产生水溶性Si,形成以高岭石、水云母和蛭石为主的次生矿物[26, 41]。由于矿物晶层堆垛,也会形成一定比例的蛭石-水云母混层矿物(表 1)。在强风化作用下,高岭石会进一步脱Si形成三水铝石。本研究的供试土壤矿物组分中含有较高比例的三水铝石说明土壤矿物风化强烈,土壤处于强发育阶段。
在亚热带良好的雨热条件下,风化层深厚。从A、B1和B2三层的硅铝率上可以看出(表 2),整个土体的风化较为强烈。整个土体的可交换性盐基和盐基饱和度均较低(表 3),因为盐基离子随着雨水向下淋溶,B2层淋溶作用较上层弱,所以盐基含量一般较其上层土壤高。但A层土壤的K+却高于B2层,可能是因为植物根系从深层吸收K+,并通过落叶归还的方式使得K+在表层出现富集,这与A层高有机质含量是一致的。从盐基饱和度指标来看,其高低顺序为:A < B1 < B2。
|
|
表 2 土壤矿质全量 Table 2 Totals of the mineral elements in the soil/% |
|
|
表 3 土壤的基本化学性质 Table 3 Basic chemical properties of the soil |
土壤胶体上吸附一定量的可交换性盐基离子,它们在洗脱过程中与NH4+发生阳离子交换反应(式(1)),并随洗脱液淋出土体。当该反应趋于平衡,即交换性盐基被洗脱完全时,淋滤液中的盐基离子主要来源于土壤混合矿物风化反应释放(式(2)):
| $ {\rm{X - }}{{\rm{M}}^{\rm{ + }}}{\rm{ + NH}}_4^ + \to {\rm{X - NH}}_4^ + {\rm{ + }}{{\rm{M}}^{\rm{ + }}} $ | (1) |
| $ 土壤混合矿物 + {\rm{H}}^+ → {\rm{M}}^+ + {\rm H_4SiO_4} + 次生 产物 $ | (2) |
式中,X代表土壤胶体,M+代表盐基离子;土壤混合矿物包括原生矿物和可风化的次生矿物。
在盐基洗脱过程的初期,盐基的淋出量比较高,随着洗脱持续进行,淋出液中的盐基含量愈来愈低(图 1),说明土壤通过阳离子交换释放盐基离子的能力逐渐减弱,尤其在盐基洗脱的初期,盐基释放量下降得很快,当洗脱液体积达到1 600 ml时,继续予以洗脱处理,淋出的盐基离子含量亦不再有显著的变化。这表明在土液比为1:1的条件下,予以8次洗盐处理时,土壤胶体上的吸附态盐基几乎全被NH4+取代,故可认为此时土壤胶体上的盐基离子被洗脱完全。虽然无法避免被淋出的盐基中有少量来自矿物风化(式(2)),但土壤矿物在中性洗脱液中的水解速率极小,绝大部分盐基离子还是来源于土壤胶体所吸附盐基的阳离子交换作用(式(1))。
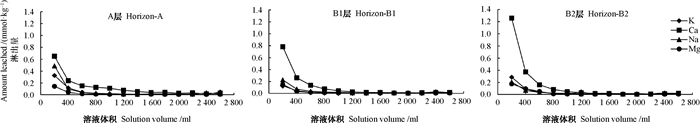
|
图 1 洗脱盐基过程中土壤盐基离子释放特征 Fig. 1 Base cation releasing characteristics of the soil during elution |
从盐基离子的淋出量来看,A、B1和B2层土壤在洗脱初期,淋出液中均为Ca2+的淋出量最大,Na+、K+和Mg2+的释放量较低(图 1),而土壤的交换性盐基中也属Ca2+含量最高(表 3),表明土壤胶体上吸附最多的盐基离子,在盐基洗脱时的淋出量也最大。从K+的淋出量来看,A层在淋溶初期的淋出量约为B1和B2层的2倍,这与表层土壤中交换性K+的含量约为B1和B2层的2倍相一致(表 3)。可见洗脱出的盐基离子主要来自土壤胶体所吸附的盐基。
2.3 模拟酸雨淋溶下盐基离子释放特征在模拟酸雨淋溶情况下,未洗脱盐基土壤K+、Na+、Ca2+和Mg2+均具有明显的变化趋势:淋溶初期的淋出量较高,随着H+的持续输入,盐基离子淋出量迅速下降,直至酸雨淋溶量达到1 600 ml时趋于平缓,之后便以稳定的速率释放(图 2),这与盐基洗脱过程的盐基淋出特征和淋洗液的用量基本一致。这证明当外源H+输入时,土壤首先通过以阳离子交换为主的缓冲机制来消耗H+(式(1)),但随着土壤胶体吸附的交换性盐基数量下降,该反应就会减弱直至平衡,之后土壤便以混合矿物风化反应为主的机制来消耗H+(式(2)),各盐基离子的释放量大大降低,并且随着酸雨继续淋溶,盐基离子释放趋于平缓。
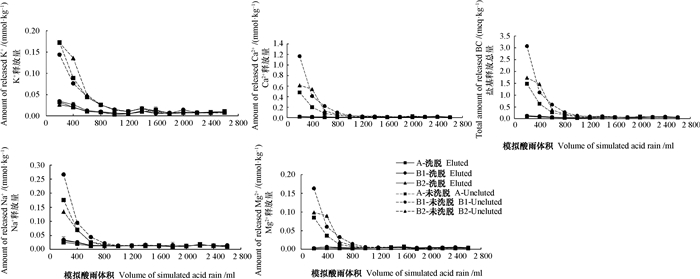
|
图 2 模拟酸雨淋溶过程中盐基离子释放特征 Fig. 2 Base cation releasing characteristics of the soil during leaching with simulated acid rain |
未洗脱盐基土壤在模拟酸雨淋溶后,盐基离子中释放量最多的是Ca2+,其次是Na+和K+,Mg2+相对较少(图 2)。这是因为未洗脱土壤在淋溶初期发生的反应主要为阳离子交换,这与供试土壤的交换性盐基含量有关(表 3)。与未洗脱盐基土壤相比,洗脱盐基土壤的释放量更低而且较为平缓(图 2),盐基离子的释放量从大到小依次为:Na+ > Ca2+ > K+ > Mg2+,这不同于未洗脱盐基土壤的释放特征。
洗脱盐基土壤与未洗脱盐基土壤之间,不同层次的盐基释放情况也存在差异。未洗脱盐基土壤在酸雨淋溶时,A层释放的盐基总量低于B1层和B2层,这与土壤的发育过程有关,强烈的淋溶作用使表层土壤的交换性盐基向下层迁移。而洗脱盐基土壤,不同层次的盐基释放量及特征非常相似,这与A、B1和B2三层的矿物组分非常相似是一致的。
2.4 盐基离子化学计量关系在亚热带地区充沛的雨热条件下,土壤中的原生矿物高度水解,产生高岭石、蛭石、蛭石-水云母混层矿物和水云母等层状硅酸盐黏土矿物,以及三水铝石、针铁矿和赤铁矿等非硅酸盐次生矿物,并剩余极少量未完全风化的长石和难风化的石英。因此,在模拟酸雨淋溶过程中,土壤中能继续水解风化的矿物主要为长石、水云母、蛭石、蛭石-水云母混层矿物和高岭石,而石英在本实验pH4.5的酸雨作用下,短时间内不易分解。
模拟酸雨淋溶后,洗脱盐基土壤释放的K+、Na+、Ca2+和Mg2+累积总量(A、B1和B2三层)低于未洗脱盐基土壤,因为前者释放的盐基离子仅来自土壤矿物风化,后者还包括了土壤胶体上发生阳离子交换反应的盐基离子。从盐基离子的释放量来看(表 4),未洗脱盐基土壤的Ca2+释放量最大,主要受土壤交换性Ca2+含量影响;而交换性盐基被洗脱后,土壤主要通过混合矿物的风化,释放不同比例的盐基离子。根据洗脱盐基土壤的计量关系(表 4),土壤混合矿物在风化过程中释放的Na+较多,Ca2+和K+次之,Mg2+释放量较低,这与土壤中的可风化矿物组成有关。本研究土壤在发育过程中,原生矿物长石和云母主要会发生以下不同阶段的风化[26, 41-43]:
|
|
表 4 土壤在酸雨淋溶过程中的盐基释放量及化学计量关系 Table 4 Released amount and stoichiometry of base cations from the soil during leaching with simulated acid rain |
| $ 长石 \xrightarrow{{{\rm 脱Si 脱盐基(K \;Na \;Ca)}}} 高岭石+水云母 $ |
| $ 黑云母 \xrightarrow{{{\rm 脱Si 脱盐基(K \;Mg)}}}针铁矿 +蛭石 $ |
| $ 水云母\xrightarrow{{{\rm 脱Si\; 脱K \;脱水}}}蛭石 $ |
| $ 高岭石\xrightarrow{{{\rm 脱Si}}}三水铝石 $ |
在原生矿物和次生矿物同时存在的情况下,前者更易发生风化。研究土壤的原生矿物中,石英难以水解,长石风化会释放不同比例的Na+、K+和Ca2+。次生黏土矿物中,高岭石的化学式为Al2Si2O5(OH)4,水解产物只有三水铝石,不会释放盐基离子;蛭石-水云母混层矿物会向蛭石相或水云母相转化,蛭石水解释放的盐基离子为Mg2+,水云母水解释放的盐基离子为K+。综上所述,本研究土壤混合矿物在风化过程中释放的K+主要由长石和水云母分解得到,Na+和Ca2+来源于长石的风化,Mg2+则来自蛭石和黑云母的水解。已有的皖南花岗岩发育土壤的研究中,洗脱盐基土壤同样予以酸雨淋溶,盐基离子释放最多的为K+[36]。这主要是因为皖南属于北亚热带,土壤弱发育,为湿润雏形土,易风化矿物类型和总量不同。因此,相同母质不同风化程度土壤的盐基释放特征会有较大差异。
2.5 盐基离子与硅风化计量关系硅酸盐矿物水解是土壤释放硅的主要途径。在模拟酸雨淋溶过程中,土壤中不同风化程度的矿物会发生水解(式(2)),释放出水溶性的H4SiO4,同时释放盐基离子[44]。本实验中,模拟酸雨淋溶时洗脱盐基土壤淋溶液中的Si含量一直处于 < 0.100 mmol·kg-1这一低值范围内(图 3),表明土壤矿物风化水解反应不剧烈,Si的释放量低,原因是富铁土经过了较强的脱硅富铝化作用,易风化矿物少。随着酸雨淋溶量的增加,Si释放量的特征曲线未有明显波动,呈现平缓变化的趋势(图 3)。对A、B1和B2层的Si释放量进行差异性分析,由P < 0.01可知不同土层间存在显著差异,因为土体表层的风化作用较深层强烈,风化程度更高,土壤演化过程中已经产生并随雨水淋失了大量的Si,因此实验中Si释放量的高低顺序为A < B1 < B2。
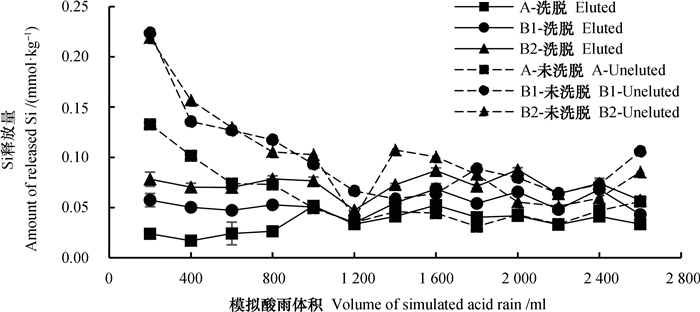
|
图 3 土壤矿物风化过程中的Si释放特征 Fig. 3 Si release characteristics of the soil during mineral weathering processes /(mmol·kg-1) |
根据模拟酸雨淋溶后的盐基离子(BC)和Si的释放总量,分别计算出A、B1和B2三层土壤的矿物风化计量关系(表 5)。从未洗脱盐基和洗脱盐基土壤各层次的盐基离子与硅的化学计量关系(BC:Si)可以看出,未予以洗脱处理的土壤,由于交换性盐基的影响,其BC:Si为3.4~4.0:1,这与经过盐基洗脱的土壤(0.8~1.4:1)相比,存在较大差异。因此,要获取准确的土壤混合矿物风化计量关系,必须先洗脱土壤胶体上吸附的交换性盐基。
|
|
表 5 土壤矿物的风化计量关系(BC:Si) Table 5 Stoichiometry of base cations and silicon (BC:Si) during soil mineral weathering |
pH为7.0的EDTA-乙酸铵溶液能够有效地洗脱土壤中吸附的盐基离子。针对高度风化的富铁土,按照土液比1:1,经过8次洗脱处理时,土壤中的吸附态盐基能够被完全洗脱。洗脱盐基土壤和未洗脱盐基土壤同时进行模拟酸雨淋溶,两者盐基离子释放特征存在显著差异。洗脱盐基土壤酸雨淋出液中的盐基主要来自矿物风化,盐基释放量低而平稳。未洗脱盐基土壤在酸雨淋溶初期的盐基释放,以胶体吸附的盐基离子与H+交换释放为主,释放量较大;随着土壤可交换盐基的减少,淋出液的盐基量迅速降低。根据洗脱盐基土壤和未洗脱盐基土壤的酸雨淋出液中K+:Na+:Ca2+:Mg2+比例关系,结合花岗岩强发育土壤的矿物风化特征,判断出风化过程中的盐基离子来源为长石、水云母、蛭石的水解;洗脱盐基土壤的BC:Si为0.8~1.4:1,未洗脱盐基土壤为3.4~4.0:1,两者存在较大差异,说明要获取准确的矿物风化计量关系,必须先洗脱土壤胶体上吸附的交换性盐基。
| [1] |
张新民, 柴发合, 王淑兰, 等. 中国酸雨研究现状. 环境科学研究, 2010, 23(5): 527-532. Zhang X M, Cai F H, Wang S L, et al. Research progress of acid precipitation in China (In Chinese). Research of Environmental Sciences, 2010, 23(5): 527-532. (  0) 0) |
| [2] |
Paces T. Source of acidification in Central Europe estimated from elemental budgets in small basins . Nature, 1985, 315(2): 31-36.
(  0) 0) |
| [3] |
Forsius M, Kleemola S, Starr M, et al. Ion mass budgets for small forested catchments in Finland . Water Air & Soil Pollution, 1995, 79(1/4): 19-38.
(  0) 0) |
| [4] |
Sanclements M D, Fernandez I J, Norton S A. Soil chemical and physical properties at the Bear Brook Watershed in Maine, USA . Environmental Monitoring & Assessment, 2010, 171(1/4): 111-128.
(  0) 0) |
| [5] |
Blake L, Goulding K W T, Mott C J B, et al. Changes in soil chemistry accompanying acidification over more than 100 years under woodland and grass at Rothamsted Experimental Station, UK . European Journal of Soil Science, 2010, 50(3): 401-412.
(  0) 0) |
| [6] |
Jönsson U, Rosengren U, Thelin G, et al. Acidification-induced chemical changes in coniferous forest soils in southern Sweden 1988-1999 . Environmental Pollution, 2003, 123(1): 75-83.
(  0) 0) |
| [7] |
Miegroet H V, Cole D W. The impact of nitrification on soil acidification and cation leaching in a red alder ecosystem . Journal of Environmental Quality, 1984, 13(4): 586-590.
(  0) 0) |
| [8] |
Yang J L, Zhang G L, Huang L M, et al. Estimating soil acidification rate at watershed scale based on the stoichiometric relations between silicon and base cations . Chemical Geology, 2013, 337(1): 30-37.
(  0) 0) |
| [9] |
廖柏寒, 李长生. 土壤对酸沉降缓冲机制探讨. 环境科学, 1989, 10(1): 30-34. Liao B H, Li C S. Study on buffer mechanism of soil to acid deposition (In Chinese). Environmental Science, 1989, 10(1): 30-34. DOI:10.3321/j.issn:0250-3301.1989.01.001 (  0) 0) |
| [10] |
廖柏寒, 戴昭华. 土壤对酸沉降的缓冲能力与土壤矿物的风化特征. 环境科学学报, 1991, 11(4): 425-430. Liao B H, Dai S H. Soil buffering capacity to acid precipitation and weathering characteristics of soil minerals (In Chinese). Acta Scientiae Circumstantiae, 1991, 11(4): 425-430. (  0) 0) |
| [11] |
潘根兴. 土壤酸化过程的土壤化学分析. 生态学杂志, 1990, 9(6): 48-52. Pan G X. Soil chemical analysis on the process of soil acidification (In Chinese). Journal of Ecology, 1990, 9(6): 48-52. (  0) 0) |
| [12] |
鲁艳红, 廖育林, 聂军, 等. 长期施用氮磷钾肥和石灰对红壤性水稻土酸性特征的影响. 土壤学报, 2016, 53(1): 202-212. Lu Y H, Liao Y L, Nie J, et al. Effect of long-term fertilization and lime application on soil acidity of Reddish Paddy Soil (In Chinese). Acta Pedologica Sinica, 2016, 53(1): 202-212. (  0) 0) |
| [13] |
Frey J, Frey T, Pajuste K. Input-output analysis of macroelements in ICP-IM catchment area, Estonia . Landscape and Urban Planning, 2004, 67(1): 217-223.
(  0) 0) |
| [14] |
Fujii K, Hartono A, Funakawa S, et al. Acidification of tropical forest soils derived from serpentine and sedimentary rocks in East Kalimantan, Indonesia . Geoderma, 2011, 160(3): 311-323.
(  0) 0) |
| [15] |
Zhang J E, Ouyang Y, Ling D J. Impacts of simulated acid rain on cation leaching from the Latosol in south China . Chemosphere, 2007, 67(11): 2131-2137. DOI:10.1016/j.chemosphere.2006.12.095
(  0) 0) |
| [16] |
樊后保, 林德喜. 模拟酸雨对福建四种山地土壤的淋溶与风化作用. 山地学报, 2002, 20(5): 570-577. Fan H B, Lin D X. Leaching and weathering effects of simulated acid rain on four types of mountain soils in Fujian, China (In Chinese). Journal of Mountain Science, 2002, 20(5): 570-577. DOI:10.3969/j.issn.1008-2786.2002.05.011 (  0) 0) |
| [17] |
冯志刚, 马强, 李石朋, 等. 模拟不同气候条件下碳酸盐岩风化作用的淋溶实验研究. 中国岩溶, 2012, 31(4): 361-376. Feng Z G, Ma Q, Li S P, et al. Leaching experiment for the weathering of carbonate rocks by simulating different climates (In Chinese). Carsologica Sinica, 2012, 31(4): 361-376. DOI:10.3969/j.issn.1001-4810.2012.04.003 (  0) 0) |
| [18] |
仇荣亮, 杨平. 南方土壤酸沉降敏感性研究Ⅴ——模拟酸雨条件下土壤矿物风化特征. 中山大学学报(自然科学版), 1998, 37(4): 89-93. Qiu R L, Yang P. Study of sensitivity of soil to acid deposition in south China V. Weathering characteristics of soil minerals under simulated acid rain (In Chinese). Acta Scientiarum Naturalium Universitatis Sunyatseni, 1998, 37(4): 89-93. DOI:10.3321/j.issn:0529-6579.1998.04.020 (  0) 0) |
| [19] |
Busenberg E. The products of the interaction of feldspars with aqueous solution at 25℃ . Geochimica et Cosmochimica Acta, 1978, 42(11): 1679-1683. DOI:10.1016/0016-7037(78)90256-9
(  0) 0) |
| [20] |
Kawano M. Formation of clay minerals during low temperature experimental alteration of obsidian . Clays & Clay Minerals, 1993, 33(4): 431-441.
(  0) 0) |
| [21] |
Kalinowski B E, Schweda P. Kinetics of muscovite, phlogopite and biotite dissolution and alteration at pH 1-4, room temperature . Geochimica et Cosmochimica Acta, 1996, 60(3): 367-385. DOI:10.1016/0016-7037(95)00411-4
(  0) 0) |
| [22] |
张治伟, 许娟娟, 严焕德, 等. 海拔与岩性变异对石灰岩发育土壤黏土矿物组成的影响. 土壤学报, 2017, 54(2): 535-542. Zhang Z W, Xu J J, Yan H D, et al. Effect of elevation and lithology on clay mineral composition of soils derived from limestone (In Chinese). Acta Pedologica Sinica, 2017, 54(2): 535-542. (  0) 0) |
| [23] |
王彦华, 谢先德, 罗立峰. 花岗岩中黑云母风化的矿物变化机制. 地球化学, 1999, 28(3): 239-247. Wang Y H, Xie X D, Luo L F. The mechanism of compositional and structural change of biotite in granite during weathering process (In Chinese). Geochimica, 1999, 28(3): 239-247. DOI:10.3321/j.issn:0379-1726.1999.03.005 (  0) 0) |
| [24] |
向廷生, 蔡春芳, 付华娥. 不同温度与羧酸溶液中长石溶解模拟实验. 沉积学报, 2004, 22(4): 597-602. Xiang T S, Cai C F, Fu H E. Dissolution of microcline by carboxylic acids at different temperatures and complexing reaction of Al anion with carboxylic acid in aqueous solution (In Chinese). Acta Sedimentologica Sinica, 2004, 22(4): 597-602. DOI:10.3969/j.issn.1000-0550.2004.04.007 (  0) 0) |
| [25] |
刘莉红, 胡雪峰, 叶玮, 等. 皖南第四纪红土伊利石结晶度值与风化强度的关系. 土壤学报, 2015, 52(5): 991-1001. Liu L H, Hu X F, Ye W, et al. Relationship between illite crystallinity(IC)value and weathering degree of Quaternary red clay in southern Anhui Province, Southeast China (In Chinese). Acta Pedologica Sinica, 2015, 52(5): 991-1001. (  0) 0) |
| [26] |
李福春, 李莎, 杨用钊, 等. 原生硅酸盐矿物风化产物的研究进展——以云母和长石为例. 岩石矿物学杂志, 2006, 25(5): 440-448. Li F C, Li S, Yang Y Z, et al. Advances in the study of weathering products of primary silicate minerals, exemplified by mica and feldspar (In Chinese). Acta Petrologica et Mineralogica, 2006, 25(5): 440-448. DOI:10.3969/j.issn.1000-6524.2006.05.008 (  0) 0) |
| [27] |
Kawano M. Growth of smectite from leached layer during experimental alteration of albite . Clays & Clay Minerals, 1994, 42(1): 7-17.
(  0) 0) |
| [28] |
Ferrow E A. Experimental weathering biotite, muscovite and vermiculite:A Mössbauer spectroscopy study . European Journal of Mineralogy, 2002, 14(1): 85-95. DOI:10.1127/0935-1221/2002/0014-0085
(  0) 0) |
| [29] |
孙德四, 陈晔, 曹飞. 组成复杂铝土矿微生物风化作用和模拟实验. 矿物岩石, 2012, 32(3): 6-12. Sun D S, Chen Y, Cao F. Degradation of bauxite with complicate mineral compositions by microorganisms in culture experiments (In Chinese). Journal of Mineralogy and Petrology, 2012, 32(3): 6-12. DOI:10.3969/j.issn.1001-6872.2012.03.002 (  0) 0) |
| [30] |
肖奕, 王汝成, 陆现彩, 等. 低温碱性溶液中微纹长石溶解性质研究. 矿物学报, 2003, 23(4): 333-340. Xiao Y, Wang R C, Lu X C, et al. Experimental study on the low-temperature dissolution of microperthite in alkaline solution (In Chinese). Acta Mineralogica Sinica, 2003, 23(4): 333-340. DOI:10.3321/j.issn:1000-4734.2003.04.009 (  0) 0) |
| [31] |
郝吉明, 段雷, 谢绍东. 中国土壤对酸沉降的相对敏感性区划. 环境科学, 1999, 4(1): 1-5. Hao J M, Duan L, Xie S D. Mapping the relative sensitivity of soils to acid deposition in China (In Chinese). Environmental Science, 1999, 4(1): 1-5. (  0) 0) |
| [32] |
郭治兴, 王静, 柴敏, 等. 近30年来广东省土壤pH的时空变化. 应用生态学报, 2011, 22(2): 429-430. Guo Z X, Wang J, Chai M, et al. Spatiotemporal variation of soil pH in Guangdong Province of China in past 30 years (In Chinese). Chinese Journal of Applied Ecology, 2011, 22(2): 429-430. (  0) 0) |
| [33] |
卢瑛. 中国土系志(广东卷). 北京: 科学出版社, 2017. Lu Y. Soil series of China(Guangdong Volume) (In Chinese). Beijing: Science Press, 2017. (  0) 0) |
| [34] |
唐先干, 杨金玲, 张甘霖. 皖南山区降水酸性特征与元素沉降通量. 环境科学, 2009, 30(2): 356-361. Tang X G, Yang J L, Zhang G L. Acidity characteristics and element flux of rainwater in the hilly area of south Anhui, China (In Chinese). Environmental Science, 2009, 30(2): 356-361. DOI:10.3321/j.issn:0250-3301.2009.02.007 (  0) 0) |
| [35] |
徐仁扣. 土壤酸化及其调控研究进展. 土壤, 2015, 47(2): 238-244. Xu R K. Research progresses in soil acidification and its control (In Chinese). Soils, 2015, 47(2): 238-244. (  0) 0) |
| [36] |
庄翔宇, 杨金玲, 张甘霖, 等. 亚热带花岗岩地区土壤矿物风化过程中盐基离子的释放特征. 土壤, 2016, 48(2): 374-380. Zhuang X Y, Yang J L, Zhang G L, et al. Release characteristics of base cations during soil mineral weathering in subtropical granite area (In Chinese). Soils, 2016, 48(2): 374-380. (  0) 0) |
| [37] |
Chang C T, Wang C P, Huang J C, et al. Trends of two decadal precipitation chemistry in a subtropical rainforest in East Asia . Science of the Total Environment, 2017, 605: 88-98.
(  0) 0) |
| [38] |
张甘霖, 龚子同. 土壤调查实验室分析方法. 北京: 科学出版社, 2012. Zhang G L, Gong Z T. Soil survey laboratory methods (In Chinese). Beijing: Science Press, 2012. (  0) 0) |
| [39] |
莫彬彬, 连宾. 长石风化作用及影响因素分析. 地学前缘, 2010, 17(3): 281-288. Mo B B, Lian B. Study on feldspar weathering and analysis of relevant impact factors (In Chinese). Earth Science Frontiers, 2010, 17(3): 281-288. (  0) 0) |
| [40] |
殷细宽, 曾维琪. 广东花岗岩发育几种红壤的矿物组成. 华南农业大学学报, 1987, 8(1): 29-40. Yin X K, Zeng W Q. Mineralogical studies of several major soil types developed from granite in Guangdong (In Chinese). Journal of South China Agricultural University, 1987, 8(1): 29-40. (  0) 0) |
| [41] |
马在平, 姜在兴, 钱峥. 我国热带亚热带部分地区花岗岩和片麻岩中黑云母风化研究. 矿物岩石, 1996, 16(2): 17-24. Ma Z P, Jiang Z X, Qian Z. Weathering of biotite in some weathered granite and gneiss from some subtropical and tropical area of China (In Chinese). Journal of Mineralogy and Petrology, 1996, 16(2): 17-24. (  0) 0) |
| [42] |
杨德湧, 蒋梅茵. 我国东部花岗岩发育的红壤和黄壤的粘粒矿物组成及其演变. 土壤学报, 1991, 28(3): 276-283. Yang D Y, Jiang M Y. Clay mineral composition and evolution of red earths and yellow earths derived from granite in eastern China (In Chinese). Acta Pedologica Sinica, 1991, 28(3): 276-283. (  0) 0) |
| [43] |
章明奎, 何振立, Wilson M J. 红壤中高岭类矿物的鉴定. 土壤, 1998, 30(2): 106-110. Zhang M K, He Z L, Wilson M J. Identification of kaolinic minerals in red soil (In Chinese). Soils, 1998, 30(2): 106-110. (  0) 0) |
| [44] |
吴箐, 仇荣亮, 杨平, 等. 南方土壤酸沉降敏感性研究Ⅲ. Si释放与缓冲作用. 中国环境科学, 1998, 18(4): 302-305. Wu Q, Qiu R L, Yang P, et al. Study on soil sensitivity to acid deposition in south China Ⅲ. Releasing and buffering reaction of Si (In Chinese). China Environmental Science, 1998, 18(4): 302-305. DOI:10.3321/j.issn:1000-6923.1998.04.004 (  0) 0) |
2. University of Chinese Academy of Sciences, Beijing 100049, China
 2019, Vol. 56
2019, Vol. 56


