2. 法语鲁汶大学地球与生命研究所, 比利时新鲁汶 B-1348;
3. 西交利物浦大学健康与环境科学系, 江苏苏州 215123;
4. 中国科学院城市环境研究所, 厦门 361021
2. Earth and Life Institute, Environmental sciences, Université catholique de Louvain, Louvain-la-Neuve B-1348, Belgium;
3. Department of Health and Environmental Sciences, Xi'an Jiaotong-Liverpool University, Suzhou, Jiangsu 215123, China;
4. Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China
砷(arsenic,As)是自然界中普遍存在的有毒污染物,在大多数环境下,砷浓度低于对人类健康造成危害的水平。但在特定条件下,砷富集于土壤、沉积物、地下水和生物体等介质中,通过空气、水和食物等形式进入人体并造成显著危害。过量摄入或长期低剂量砷暴露均会对人体健康构成严重危害,典型的地方性砷中毒症状是“乌脚病”,严重时可诱发皮肤、膀胱、肺等器官的癌变以及发育、心血管、代谢性疾病和糖尿病[1-3]。
砷在环境中以多种形态存在,主要有砷酸及其盐(As(Ⅴ))、亚砷酸及其盐(As(Ⅲ))、一甲基胂(MMA)、二甲基胂(DMA)、胂胆碱、胂甜菜碱和胂糖[4-5]。其中,As(Ⅴ)是好氧环境中砷的主要存在形式,而As(Ⅲ)则主要在厌氧环境如淹水土壤中存在,两者之间在微生物作用下更容易发生相互转化[5-7]。甲基化砷尽管含量相对较少,但同样广泛存在[5-6,8-9]。除以上常见砷形态外,硫代砷酸盐也在水稻田土壤溶液和富硫化物温泉中广泛存在[10-11]。氧化还原电位(Eh)和pH是影响砷形态最重要的两个因素[12]。
在土壤环境中,砷的(生物)有效性不仅取决于其在水相中的形态,还受到砷在固液相的分配能力、微生物代谢能力和植物根系作用的显著影响。砷在复杂水-土-生体系的迁移机制依赖于对不同介质中砷界面过程的研究突破。土壤环境微界面是土壤组分(矿物和有机质)、植物根系、微生物、动物区系等微界面的集合体和动态变化的连续体,具有高度的时空异质性,决定着砷在土壤和沉积物环境中的赋存形态、生物有效性和向其他环境介质(如水体和植物)的迁移[13-16]。随着对砷在环境中迁移转化行为的认识越来越充分,研究手段逐渐成为了限制砷有效性和界面过程研究的主要因素。针对这一研究领域,本文首先介绍了砷研究的采样技术以及被动采样技术的必要性,之后介绍被动采样技术在单一水相和土水混合相砷检测中的进展,以及环境微界面砷分布和运移的高分辨表征,最后对今后的研究方向进行了展望。
1 采样技术 1.1 采样技术简介对于土壤环境中的元素行为,相关研究方法经历了一个从粗放到精细,从异位破坏性取样到原位分析的过程[17]。在表 1中总结了常用方法的使用特点和主要限制因素。早期的土壤剖面研究直接破坏性分层取样,现在已很少使用[18-22]。为了尽可能地减少采样过程对样品性质的影响,原位孔隙水采样技术逐渐成为主流。
|
|
表 1 界面过程采样-分析方法的使用特点和主要限制因素 Table 1 Characteristics and main limiting factors of the application of the interfacial process sampling & analysis methods |
土壤溶液采样器(Rhizon sampler,图 1a))因其简单快速,应用最为广泛。土壤溶液采样器主要由一根中空纤维膜和一个泵或者注射器组成。土壤孔隙水在泵或者注射器的负压下,透过0.22或0.45 μm孔径的中空纤维膜进入收集管或注射器中。当多根采样器按深度不同平行放置时,可采集不同深度的土壤孔隙水,分析结果能反应出土壤孔隙水的元素空间变化。土壤溶液采样器法的采样过程对样品干扰太大,很少用于分析土水界面元素分布。此外,经多次使用后,其中空纤维膜上的滤孔会逐渐被堵塞,造成取样困难,因此其对元素动态变化的研究能力有限。
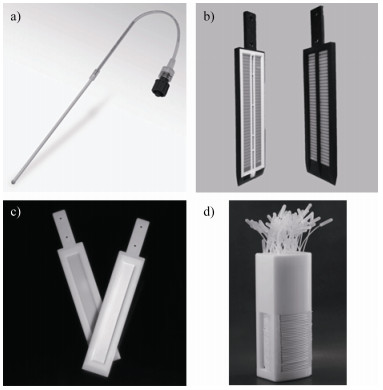
|
a).土壤孔隙水采样器,图片来自Soilmoisture Equipment公司网站;b). Peeper采样器,图片来自Easysensor公司网站;c). DET/DGT探针,图片来自DGT Research公司网站;d). IPI采样器) a). Rhizon sampler; b). Peeper sampler; c). DET/DGT probe; d). IPI sampler) 图 1 常用主动和被动土壤溶液采样器 Fig. 1 Commercially available active/passive soil porewater samplers |
与土壤溶液采样器相比,平衡式孔隙水采样(Peeper,图 1b))是一种原位的孔隙水被动采样技术,其主要结构为一块具有多个采样槽的平板,采样槽中预先填满水,通过一张滤膜密封。使用时,Peeper采样器插入水饱和的土壤中,孔隙水中的化学物质通过扩散进入采样槽中。待平衡一定时间后,将采样器取出,小心地移除滤膜,可去除采样槽的溶液用于后续分析。Peeper的采样过程对环境影响小,其空间分辨率取决于采样槽的宽度,最小可达2 mm。但随着空间分辨率的增加,采样难度加大,可供分析的溶液体积减小[19, 39-40]。
在需求更高的空间分辨率的情况下,薄膜扩散平衡(DET)和薄膜扩散梯度(DGT)探针(图 1c))是更为可靠的技术,各种吸附层的发展使得DET/DGT探针技术可有效分析总砷和无机砷形态的分布[18, 21, 30, 41]。DET探针的原理与Peeper类似,但是采样槽被水凝胶层代替,水凝胶层较采样槽有更好的稳定性和操作性,尤其是平衡时间和空间精度上,具有更大的优势。DGT探针和DET探针的外观类似,但是功能更加强大。DGT探针在DET的水凝胶扩散层后面加入了一个吸附层,吸附层可随着时间逐渐富集待测污染物,因此在对于痕量污染物的分析方面,具有其他方法所不可比拟的优势。
原位反复孔隙水采样器(IPI采样器,图 1d))是唯一可原位重复采样的孔隙水采样器。单根的IPI采样器在结构上类似于土壤孔隙水采样器,不同之处在于IPI采样器在纳米中空纤维膜采样管的两端均连有导管。IPI采样器的取样原理类似于Peeper,使用时,先在导管和纳米中空纤维膜中预先填充除氧纯净水,插入待测的土壤体系,待平衡后,通过两侧导管取出采样管中的平衡溶液。与Peeper相比,IPI采样器不仅具有更高且准确的空间精度,而且可重复采样,同时大幅降低了操作难度和样品污染的可能性。
1.2 被动采样技术的必要性基于不同的采样过程,被动采样相对于主动采样而言,有着采样设备体积相对较小、不需额外电源驱动、可长期使用等特点,但是也具有难以监测短期变化、低富集效率等缺点。然而,对于砷的界面过程和有效性的研究,被动采样相对于主动采样具有巨大的优势,主要表现在:
(1)被动采样对土壤和沉积物介质干扰少。主动采样虽然采样速度快,采样量大,常用于大气和水体等相对均匀且流动性大的体系,但是与大气和水体相比,土壤和沉积物体系具有静态且异质性的特点,选择干扰小的被动采样技术对于研究原位的土壤和沉积物过程非常重要。
(2)被动采样可用于模拟植物吸收过程。被动采样可通过控制滤膜的孔径,调控可进入采集管/槽/凝胶层的元素分子和离子的大小,过滤掉大部分胶体或更大的颗粒物,模拟元素在根际的扩散行为,更接近植物吸收的真实过程,进而更准确地评估有效性。此外,基于动力学的被动采样技术(如DGT)通过持续吸收间隙水中离子浓度,促进易解离络合物的解离以及固相吸附态向液相的迁移,可更好地模拟根际微区元素的活化与迁移过程。
2 被动采样技术在单一水相和土水混合相砷检测中的进展 2.1 水相中总砷及砷形态浓度的被动检测目前已有的被动采样技术均可用于水相中砷的检测,其原理主要分为扩散和扩散后富集两类。以单纯扩散为机理的被动采样技术以Peeper、DET和IPI采样器为代表,其检测限和检测目标主要决定于后续分析手段;扩散后富集以DGT技术为代表,其检测限取决于被动采样时间,而检测目标取决于固定相的组成结构。
DGT可在pH为4~8、离子强度为0.1~500 mmol·L-1的环境条件下有效测定砷。目前可有效吸附砷的DGT吸附层包括单一吸附材质吸附层和混合吸附材质吸附层。吸附层中起吸附作用的主要是对砷具有较强吸附性能的材料,如水铁矿[42]、二氧化钛[43]和氧化锆[32]。这类材料对砷及其他含氧阴离子(如磷酸根、钒酸根、钼酸根、锑酸根等)均具有良好的吸附性能。混合吸附层主要是用于同时阴阳离子(如水铁矿-Chelex[44])或阴离子与S2-(如氧化锆-AgI[45])的测定。氧化铁类的吸附层还可吸附部分有机态的砷(一甲基胂和二甲基胂)[46-47]。多功能的吸附层在研究砷与其他元素的交互作用时具有很大优势。
被动采样测定砷形态有2种主要的思路:1)采样端调控,通过调控选择性通过的扩散层,或者选择性吸附的吸附层,使采样器仅采集特定形态的砷。2)分析端调控,采样器广泛采集,通过后续的仪器分析实现砷形态的分离和定量。
在实际的采样器设计中,可利用带负电荷的Nafion®膜作为扩散层,使电中性的As(Ⅲ)(H3AsO3)可以通过,而带负电的As(Ⅴ)(H2AsO4-和HAsO42-)难以通过,从而实现DGT对As(Ⅲ)的选择性累积[48]。该方法也有望用于DET、IPI和Peeper等被动采样器,但目前尚无这样的尝试。需要注意的是,此方法无法区分As(Ⅲ)和同为电中性的其他有机砷形态,如甲基胂和胂糖。
可选择性捕获砷的吸附材质种类较多,各有侧重。比如利用3-巯基丙基功能化硅胶(3MFS)特异性吸附As(Ⅲ)[49],利用Amberlite IRA 910树脂特异性吸附As(Ⅴ)[50]。因常见水环境中砷的形态主要为无机的As(Ⅲ)和As(Ⅴ),将3MFS-DGT与非选择性DGT联用,分别测出As(Ⅲ)以及As(Ⅲ)和As(Ⅴ)总量,这是目前利用DGT进行砷形态分析最常用的方法。
对于水样中砷形态分析,离子色谱(IC)或高效液相色谱(HPLC)与质谱联用是最成熟的方法。Peeper和IPI采样器可直接获取溶液样品用于HPLC/IC-ICP-MS分析。然而,常用的Peeper和IPI采样器采集的样品较少(~0.5 mL),通常仅能满足总量元素测试需求。最近,Yuan等[37]使用IPI采样器和优化的NH4HCO3淋洗液,通过手动向25 μL进样环中加载样品,在仅需消耗50 μL样品的条件下,实现了快速(10 min内)测试孔隙水中的常见砷形态,包括As(Ⅲ)、As(Ⅴ)、MMA和DMA,并且该方法可同步测试孔隙水中P和S的形态。当应用于DGT时需要注意洗脱提取砷的过程对砷形态的影响[51]。此外,基于同步辐射的X射线吸收近边光谱分析则可准确测定水铁矿吸附层中多种砷形态[51],但是由于同步辐射大科学装置的稀缺性,该分析方法难以作为日常形态分析手段。
2.2 基于被动采样技术的土壤中砷含量的测定被动采样技术可用于采集土壤孔隙水中的砷。孔隙水中的砷代表土壤中最易移动的砷库,常被用于表征砷在土壤中的迁移性和毒性。常用的被动采样技术,如DET[52]、Peeper[36]和IPI[38]采样器,采样器中的砷浓度可用于表征孔隙水中的砷浓度。在野外,土壤环境动态变化,受环境中复杂的物理、化学和生物过程影响。因此,DET、Peeper和IPI采样器采集的砷反映了准平衡条件下孔隙水中的砷,即溶解态(dissolved)砷浓度。
DGT通过特定的吸附层富集孔隙水中的砷,可表征砷从固相或复杂螯合态向液相补充的动力学过程[53-54]。因此,DGT测定结果将土壤多方面特性整合为一个关键参数,即有效态(labile)砷浓度(CDGT)或通量(FDGT)[55]。与水体中的砷相比,土壤和沉积物孔隙水中的砷一般具有更强的再补充能力,为防止吸附层饱和,水铁矿和二氧化钛DGT在土壤中的推荐放置时间一般为1~3 d[41-42]。容量较大的吸附层适宜用于高浓度砷或长期砷扩散过程研究,比如氧化锆[32, 41],不必担心砷富集或干扰物(如磷酸根)富集而造成DGT吸附层饱和。
使用被动采样技术对土壤孔隙水中砷形态分析的思路与水体中相似,但是不同元素形态在土壤中存在复杂的耦合和解耦合现象,尽可能全面地收集相关元素形态信息将有助于深入理解相关元素在土壤中的生物地球化学过程。由于砷形态多样且化学性质类似,从采样端同步富集所有砷形态难度较大,在分析端优化测试方法将是增强土壤中砷形态分析能力的关键。通过优化的砷形态分析方法,IPI和Peeper有望方便地测试土壤溶液准平衡条件下的砷形态,但所得结果不能用于反映特定砷形态在土壤中的动力学过程。作为对比,特制的DGT可完成相应的研究。在实际应用中,研究者可根据自身需要,单独使用或联用已有的被动采样器技术。
2.3 基于被动采样技术的土壤和沉积物中砷植物有效性分析对于土壤环境中砷含量和形态的分析,可用于推测砷的植物吸收量。植物能够吸收利用的砷在砷总量中通常占比很小,所有潜在的能够被植物吸收利用的砷,被称为“植物有效态(phytoavailability)”,具有“植物有效性(phytoavailable)”。有效砷大致可分成两个部分,一是在土壤孔隙水中以小分子或者离子形式存在的砷,这部分可直接被植物吸收;另外一部分是大颗粒或者土壤固相中可快速释放至土壤孔隙水中的砷,这部分不能直接被植物吸收,但是可快速补充至孔隙水中供植物吸收。
砷在土壤中多吸附于土壤的固相组分,如与铁氧化物结合,同时也与土壤溶液中的有机物存在竞争、络合等复杂关系[56]。当砷自由离子或分子浓度保持不变时,这一复杂体系处于准平衡状态。当植物根系开始从土壤溶液中吸收砷,砷的吸收促使砷从土壤固相中解离,补充至土壤溶液。砷在土壤中的含量低,移动性也相对较差,可利用态占比较低,导致自由态的砷被消耗后,土壤再补给的速度低于砷被植物根系吸收消耗的速度,根表砷浓度几乎为零,形成了从土壤溶液主体至根表的具有浓度梯度的耗竭层,厚度约为0.7~0.8 mm[57]。
现有被动采样技术中,DGT拥有基于动力学的元素形态分析能力,在分析元素的植物有效性方面,较传统的植物有效性分析方式具有独特的优势。自2003年被应用于土壤中砷的植物有效性分析[58]以来,DGT分析预测土壤中植物有效态砷的可靠性已被广泛探讨。DGT技术涉及的动力学过程与植物根系从土壤中吸收砷的过程具有相似性,并形成了相似的贯穿土壤基体(bulk soil)至根表/DGT吸附层表面的具有浓度梯度的扩散层[22]。DGT装置的扩散层和根系周围的耗竭层具有类似的特性。为了对二者进行对比,首先需要了解植物吸收砷所引起的土壤化学过程。
DGT将土壤中各种环境因素对砷的植物有效性的作用,综合反映为CDGT或FDGT,在模拟植物吸收过程的同时也消除了土壤性质造成的测定影响。因此,相比于传统的基于平衡分配的化学提取方式,DGT测定的土壤砷的动力学原理更加接近植物吸收的真实情况。常用DGT装置扩散层的厚度为0.8~0.9 mm,与根际的耗竭层厚度相近。相似的浓度梯度和扩散通量,使得DGT对根系吸收的模拟更为准确。
基于上述动力学原理,DGT对砷植物有效性的研究主要分为两类:1)应用DGT评价和预测不同的土壤-植物体系中砷的植物有效性,以期建立更加普适和简便的砷植物有效性评估方法;2)利用DGT获取土壤-植物体系中砷的土壤动力学参数,研究其土壤动力学过程,为深入解释砷的植物有效性提供证据。DGT对砷植物有效性的研究中,DGT装置被视为植物的类似物或替代物。
第一类研究的设计思路是:将植物作为生物指示物,代表土壤中真实的植物有效性砷含量,同时在未种植植物的平行土壤样品中应用DGT进行砷的测定。比较二者测定结果的相关性,即可判断DGT对砷植物有效性的评价效果。很多研究也同时对比了多种基于平衡分配原理的传统化学提取方法。大量应用实例证明,DGT技术对砷植物有效性的预测取得了较化学提取方法更好的相关性。DGT在多种不同种类的土壤中均能保持良好的预测准确性,有潜力成为较为普适的砷植物有效性评估方法[59-60]。也有研究将DGT测定的有效态比例与经典的多步提取法定义的有效态比例进行对比,二者也可很好地吻合[61]。
第二类研究的方法是,将DGT应用于特定的土壤-植物体系,将测定结果带入DIFS(土壤和沉积物中DGT诱导扩散通量,DGT-induced fluxes in soils and sediments)模型后可计算土壤固相砷的解吸动力学参数,如解吸速率常数和响应时间[58, 62-64],进而分析未被扰动的初始状态下,植物有效态砷被消耗后土壤的再补给能力。在植物生长前后分别利用DGT测定土壤砷的有效态,带入模型计算解吸动力学参数,这类参数的变化程度即可反映植物对砷吸收能力的强弱[63, 65]。对土壤再补给能力的分析能够为砷的环境修复和评价提供依据[58]。DGT测定值与土壤溶液浓度的比值称为R值。这一动力学参数能够反映DGT放置期间溶液中砷离子通量的衰减程度和土壤对衰减的响应。DIFS模型还可计算得到Rdiff,反映主体(bulk)土壤溶液扩散作用对砷消耗的补充。通过Rdiff对CDGT进行校正,就得到了土壤中全部能被有效利用的砷浓度CE[66]。这本质上是根据土壤性质对DGT通量的影响,对直接测得的DGT数据进行的校正,由此进一步消除土壤性质的差异对DGT测定的影响,使DGT对生物有效性预测的普适性更强。由此可见,对植物有效性成因的探讨可反过来帮助植物有效性评价体系的建立,而基于动力学原理的被动采样技术在其中发挥了重要作用。
此外,DGT还以相同的原理应用于评价河流沉积物中砷的植物有效性[67],以及预测砷对植物的毒性效应[68-69]。DET技术由于具有与DGT相似的组成、装置和后续元素分析方式,因此在砷的植物有效性研究中有时用作DGT的补充,用于测定含量较高的元素(如Fe、S)在土壤溶液中的总溶解物浓度[18, 70-71]。其他被动采样技术也有望用于砷的植物有效性研究,但目前这方面的工作尚较少。
3 环境微界面砷分布和运移的高分辨表征 3.1 界面中砷的空间分布被动采样技术已用于研究砷在土壤、沉积物、沉积物-水界面和植物根际的一维和二维空间分布特征。早在1997年,Davison等[72]即利用DGT技术结合质子激发X荧光光谱分析(PIXE)获得了河流表层沉积物-微生物席(microbial mat)-水连续体中砷、锌、铁和锰的亚毫米级(100 μm)一维和二维分布,发现微生物席(5~6 mm厚)在砷等元素循环中的重要调节作用。之后,DGT、DET、Peeper和IPI等被动采样技术在土-水系统中砷的空间分布研究中得到广泛应用。借助PIXE、ICP-MS、高分辨(HR)-ICP-MS、激光剥蚀(LA)-ICP-MS等仪器分析被动采样器固定层/平衡层砷的分布,与测试样品空间坐标对应起来,即得到环境介质或微界面处元素的一维/二维分布,空间分辨率达0.1~5 mm(表 2)。
|
|
表 2 被动采样技术在砷空间分布研究中的应用实例 Table 2 Application of passive sampling techniques in the study on spatial distribution of arsenic |
DGT是砷空间分布研究中应用最广泛的被动采样技术。DGT吸附层在饱和前可持续吸附砷,且砷一旦被吸附即被固定下来,位置不再移动,砷形态亦不易发生转化。普通的吸附层上吸附剂颗粒大小在100 μm以上,该类型吸附层可实现毫米级空间分辨。通过选用颗粒更小(≤10 μm)的吸附剂或改进吸附层制备工艺可得到高分辨吸附层,该类型吸附层上吸附剂颗粒足够小且分布更为均匀,能够实现亚毫米级空间分辨。悬浮颗粒试剂-亚氨基二乙酸盐(SPR-IDA)、沉淀状水铁矿(precipitated ferrihydrite,PF)和沉淀状氧化锆(precipitated zirconia,PZ)即是高分辨吸附层的代表,已用于获取亚毫米砷的空间分布。Stockdale等[73, 84]利用PF-DGT技术结合LA-ICP-MS分析获得了SWI处砷浓度的二维高分辨定量成像,捕捉到了砷的异质性分布特征和沉积物中的“热区”(hotspot)现象。Guan等[32]使用PZ-DGT结合LA-ICP-MS分析获得了SWI处砷的二维分布,发现在上覆水和界面下方8 mm沉积物处均存在砷分布“热区”,且与磷、锑和钼具有共存效应。
DGT测定的是有效态砷的分布,而DET、Peeper和IPI可测定湿地、水稻田和沉积物间隙水中溶解态砷和其他元素(如铁)的分布。DET、Peeper和IPI可单独使用,也可与DGT或其他被动采样技术联用。目前,已有研究将DGT与DET或Peeper以“背靠背”方式联用研究沉积物及其与水的界面处砷的一维分布特征和活化驱动因素,证实了铁锰氧化物还原溶解对砷迁移的重要贡献[74-75, 82]。
3.2 界面中砷的时空分布土水界面和根际界面是两类典型的环境微界面。土水界面控制了沉积物/土壤与上覆水的砷交换,而根际界面是砷被转运至植物体内的门户,均具有重要的环境意义。土水界面和根际界面不仅具有空间异质性的特点,且容易随着外界环境(如水分管理、施肥、污染物输入、微生物降解、根系活动)快速变化[85-88]。因此,掌握砷在微界面的时空变化是理解砷在环境中迁移转化过程的关键。通过优化被动采样器设计和末端测试方法,DET、DGT和Peeper已被广泛应用于土水界面和植物根际一维或二维的砷空间图谱绘制,空间分辨率可达微米~厘米[30, 32, 70, 89]。然而,DET、DGT和Peeper均仅能一次性使用,在一定程度上阻碍了这些被动采样技术在绘制微界面砷时空变化图谱方面的应用。
为解决这一问题,可分批次在相近的土水界面处设置被动采样器,以此获得土水界面处砷时空变化图谱。Arsic等[30]和Bennett等[70]使用该策略研究了表层水氧化还原环境变化对土水界面处砷空间分布的影响,明确了表层水中氧化还原环境对土水界面处砷行为的显著影响,且砷与铁在土水界面处的时空变化是耦合的。Bennett等[70]同步使用3MFS吸附层DGT测试了As(Ⅲ),进一步提出了在土水界面附近可能存在铁砷解耦合微区,这是由于除了铁还原砷释放,土壤颗粒可能也参与了砷的吸附与解吸附过程。然而,这一策略需要假设土壤是均质的,然而实际土壤具异质性。IPI采样器则可原位重复使用,有望促进土水界面砷时空变化研究。Yuan等[38]成功使用IPI采样器绘制了持续8d的砷空间逐日分布,得到与DGT相类似的结果。
绘制根际界面的砷时空变化图谱较土水界面更加困难,这是由于根际界面处于较深层土壤中,且根际界面环境易随着植物的生长或根系活动而快速变化,这使得使用与土水界面时空图谱绘制相似的策略变得困难。Williams等[77]使用DGT成功绘制了整个试验期间根际的砷扩散通量,与此同时,“背靠背”设置的平板光极则可记录试验期间氧气(O2)和pH的时空变化。在最近的研究中,Yin等[83]在更多的土壤根际中重复了相似的试验。截止目前,根际砷高分辨时空变化的研究仍然是一项空白。由于不需要破坏性采样,IPI采样器的使用有望实现根际砷时空变化图谱的绘制。然而,鉴于根际环境的复杂性,进一步的根际砷行为研究需合理联用多种被动采样、平板光极和微生物测序等技术,从多个视角解析砷行为过程。
4 结论与展望土壤等环境介质中的砷与其中的矿物(如铁锰氧化物)、硫化物、溶解氧、有机质和微生物等组分以及植物根系发生微尺度的复杂相互作用,因而具有高度时空异质性的特点。这些微观作用过程决定了砷在环境介质中的赋存形态和向液相/上覆水迁移活化的能力,进而影响砷的有效性和微界面动力学过程。被动采样技术的发展和应用极大地推动了土壤、沉积物和水环境中砷的原位监测、有效性表征和界面过程机制探讨,展现出了强大的生命力,未来有望取得进一步的突破,以下几个方面值得关注。
(1)被动采样技术可测定土壤和沉积物中有效态或溶解态的砷含量以及植物根际砷的二维分布,未来可进一步发挥被动采样技术的优势研究植物吸收砷的微观动力学机制。
(2)被动采样技术测定砷可实现亚毫米至厘米的空间分辨率以及小时至天级的时间分辨率,同一种技术兼顾空间和时间分辨率是未来发展的趋势。
(3)多种被动采样技术的联用可多方位研究砷的活化过程和归趋,实现更便捷地多技术联用可能需要改进现有装置构型或设计辅助性装置。此外,被动采样技术还可与平面光极和土壤原位酶谱等技术联用,多角度研究砷的生物地球化学过程。
(4)土壤和沉积物中生存有大量的动物区系,被动采样技术有望用于模拟动物吸收砷过程的土壤供给限制,即砷的生物有效性。
(5)被动采样技术尤其适合研究砷的界面过程,目前关于生物吸收金属过程已经有了一些建模尝试,如何进一步利用界面砷时空变化的数据构建砷跨界面运移的模型值得思考。
| [1] |
Chatterjee S, Datta S, Gupta D K. Studies on arsenic and human health[M]//Gupta D K, Chatterjee S. Arsenic contamination in the environment: The issues and solutions. Cham: Springer International Publishing, 2017: 37-66.
(  0) 0) |
| [2] |
Smith A H, Steinmaus C M. Health effects of arsenic and chromium in drinking water:Recent human findings[J]. Annual Review of Public Health, 2009, 30(1): 107-122. DOI:10.1146/annurev.publhealth.031308.100143
(  0) 0) |
| [3] |
Zhao F J, Xie W Y, Wang P. Soil and human health (In Chinese)[J]. Acta Pedologica Sinica, 2020, 57(1): 1-11. [赵方杰, 谢婉滢, 汪鹏. 土壤与人体健康[J]. 土壤学报, 2020, 57(1): 1-11.]
(  0) 0) |
| [4] |
Zhao F J, McGrath S P, Meharg A A. Arsenic as a food chain contaminant:Mechanisms of plant uptake and metabolism and mitigation strategies[J]. Annual Review of Plant Biology, 2010, 61(1): 535-559. DOI:10.1146/annurev-arplant-042809-112152
(  0) 0) |
| [5] |
Zhu Y G, Yoshinaga M, Zhao F J, et al. Earth abides arsenic biotransformations[J]. Annual Review of Earth and Planetary Sciences, 2014, 42(1): 443-467. DOI:10.1146/annurev-earth-060313-054942
(  0) 0) |
| [6] |
Zhu Y G, Xue X M, Kappler A, et al. Linking genes to microbial biogeochemical cycling:Lessons from arsenic[J]. Environmental Science & Technology, 2017, 51(13): 7326-7339.
(  0) 0) |
| [7] |
Xue X M, Zhu Y G. Arsenic biotransformation in soils and its relationship with antibiotic resistance (In Chinese)[J]. Acta Pedologica Sinica, 2019, 56(4): 763-772. [薛喜枚, 朱永官. 土壤中砷的生物转化及砷与抗生素抗性的关联[J]. 土壤学报, 2019, 56(4): 763-772.]
(  0) 0) |
| [8] |
Ardini F, Dan G, Grotti M. Arsenic speciation analysis of environmental samples[J]. Journal of Analytical Atomic Spectrometry, 2020, 35(2): 215-237. DOI:10.1039/C9JA00333A
(  0) 0) |
| [9] |
Meharg A A, Zhao F J. Arsenic and rice . Dordrecht: Springer Netherlands, 2011.
(  0) 0) |
| [10] |
Wang J J, Kerl C F, Hu P J, et al. Thiolated arsenic species observed in rice paddy pore waters[J]. Nature Geoscience, 2020, 13(4): 282-287. DOI:10.1038/s41561-020-0533-1
(  0) 0) |
| [11] |
Planer-Friedrich B, Forberg J, Lohmayer R, et al. Relative abundance of thiolated species of As, Mo, W, and Sb in hot springs of Yellowstone National Park and Iceland[J]. Environmental Science & Technology, 2020, 54(7): 4295-4304.
(  0) 0) |
| [12] |
Smedley P L, Kinniburgh D G. A review of the source, behaviour and distribution of arsenic in natural waters[J]. Applied Geochemistry, 2002, 17(5): 517-568. DOI:10.1016/S0883-2927(02)00018-5
(  0) 0) |
| [13] |
Fan C X. Advances and prospect in sediment-water interface of lakes:A review (In Chinese)[J]. Journal of Lake Sciences, 2019, 31(5): 1191-1218. [范成新. 湖泊沉积物-水界面研究进展与展望[J]. 湖泊科学, 2019, 31(5): 1191-1218.]
(  0) 0) |
| [14] |
He J Z, Zheng Y M, Qu J H. Soil enviromental micro-interfaces and pollution control (In Chinese)[J]. Acta Scientiae Circumstantiae, 2009, 29(1): 21-27. [贺纪正, 郑袁明, 曲久辉. 土壤环境微界面过程与污染控制[J]. 环境科学学报, 2009, 29(1): 21-27.]
(  0) 0) |
| [15] |
Zhu Y G. Micro-interfacial processes in soil-plant systems and their environmental impacts (In Chinese)[J]. Acta Scientiae Circumstantiae, 2003, 23(2): 205-210. DOI:10.3321/j.issn:0253-2468.2003.02.010 [朱永官. 土壤-植物系统中的微界面过程及其生态环境效应[J]. 环境科学学报, 2003, 23(2): 205-210.]
(  0) 0) |
| [16] |
Wang J, Wang Q Q, Jiang Z M, et al. Transformation and bioavailability of exogenous As in soil as influenced by humic acids and its active components (In Chinese)[J]. Soils, 2018, 50(3): 522-529. [王俊, 王青清, 蒋珍茂, 等. 腐殖酸对外源砷在土壤中形态转化和有效性的影响[J]. 土壤, 2018, 50(3): 522-529.]
(  0) 0) |
| [17] |
Luo J, Wang J F, Yang H Q, et al. Progress in research on in-situ monitoring technology for determining the phosphate contents of sediment pore water (In Chinese)[J]. Earth and Environment, 2014, 42(5): 688-694. [罗婧, 王敬富, 杨海全, 等. 湖泊沉积物孔隙水磷酸盐含量原位监测技术研究进展[J]. 地球与环境, 2014, 42(5): 688-694.]
(  0) 0) |
| [18] |
Fang W, Williams P N, Fang X, et al. Field-scale heterogeneity and geochemical regulation of arsenic, iron, lead, and sulfur bioavailability in paddy soil[J]. Environmental Science & Technology, 2018, 52(21): 12098-12107.
(  0) 0) |
| [19] |
Jin Z F, Ding S M, Sun Q, et al. High resolution spatiotemporal sampling as a tool for comprehensive assessment of zinc mobility and pollution in sediments of a eutrophic lake[J]. Journal of Hazardous Materials, 2019, 364: 182-191. DOI:10.1016/j.jhazmat.2018.09.067
(  0) 0) |
| [20] |
Rathnayake Kankanamge N, Bennett W W, Teasdale P R, et al. Comparing in situ colorimetric DET and DGT techniques with ex situ core slicing and centrifugation for measuring ferrous iron and dissolved sulfide in coastal sediment pore waters[J]. Chemosphere, 2017, 188: 119-129. DOI:10.1016/j.chemosphere.2017.08.144
(  0) 0) |
| [21] |
Fang X, Luo J, Gao Y, et al. Theory and application of diffusive gradients in thin-films in the environment:High-resolution analysis and its applications in soils and sediments (In Chinese)[J]. Journal of Agro-Environment Science, 2017, 36(9): 1693-1702. [房煦, 罗军, 高悦, 等. 梯度扩散薄膜技术(DGT)的理论及其在环境中的应用Ⅱ:土壤与沉积物原位高分辨分析中的方法与应用[J]. 农业环境科学学报, 2017, 36(9): 1693-1702.]
(  0) 0) |
| [22] |
Wei T J, Guan D X, Fang W, et al. Theory and application of diffusive gradients in thin-films(DGT)in the environment Ⅲ:Theoretical basis and application potential in phytoavailability assessment (In Chinese)[J]. Journal of Agro-Environment Science, 2018, 37(5): 841-849. [魏天娇, 管冬兴, 方文, 等. 梯度扩散薄膜技术(DGT)的理论及其在环境中的应用Ⅲ——植物有效性评价的理论基础与应用潜力[J]. 农业环境科学学报, 2018, 37(5): 841-849.]
(  0) 0) |
| [23] |
Ratering S, Schnell S. Localization of iron-reducing activity in paddy soilby profile studies[J]. Biogeochemistry, 2000, 48(3): 341-365. DOI:10.1023/A:1006252315427
(  0) 0) |
| [24] |
Ratering S, Schnell S. Nitrate-dependent iron(Ⅱ)oxidation in paddy soil[J]. Environmental Microbiology, 2001, 3(2): 100-109. DOI:10.1046/j.1462-2920.2001.00163.x
(  0) 0) |
| [25] |
Xie Y, Xie H J, Chen Y M, et al. Comparisons of measurements of contaminant concentration in landfill bottom soils with theoretical solutions (In Chinese)[J]. Journal of Natural Disasters, 2009, 18(5): 62-69. DOI:10.3969/j.issn.1004-4574.2009.05.010 [谢焰, 谢海建, 陈云敏, 等. 填埋场底土污染物浓度实测值和理论解的比较[J]. 自然灾害学报, 2009, 18(5): 62-69.]
(  0) 0) |
| [26] |
Gustave W, Yuan Z F, Sekar R, et al. Arsenic mitigation in paddy soils by using microbial fuel cells[J]. Environmental Pollution, 2018, 238: 647-655. DOI:10.1016/j.envpol.2018.03.085
(  0) 0) |
| [27] |
Seeberg-Elverfeldt J, Schlüter M, Feseker T, et al. Rhizon sampling of porewaters near the sediment-water interface of aquatic systems[J]. Limnology and Oceanography:Methods, 2005, 3(8): 361-371. DOI:10.4319/lom.2005.3.361
(  0) 0) |
| [28] |
Xu X W, Chen C, Wang P, et al. Control of arsenic mobilization in paddy soils by manganese and iron oxides[J]. Environmental Pollution, 2017, 231: 37-47. DOI:10.1016/j.envpol.2017.07.084
(  0) 0) |
| [29] |
Song J, Luo Y M, Zhao Q G, et al. Nutrient release at sediment-water interface I. Application of Rhizon-SMS in study of nitrogen release from sediments (In Chinese)[J]. Acta Pedologica Sinica, 2000, 37(4): 515-20. DOI:10.3321/j.issn:0564-3929.2000.04.011 [宋静, 骆永明, 赵其国, 等. 沉积物-水界面营养盐释放研究I.根际土壤溶液采样器在底泥氮释放研究中的应用[J]. 土壤学报, 2000, 37(4): 515-20.]
(  0) 0) |
| [30] |
Arsic M, Teasdale P R, Welsh D T, et al. Diffusive gradients in thin films(DGT)reveals antimony and arsenic mobility differences in a contaminated wetland sediment during an oxic-anoxic transition[J]. Environmental Science & Technology, 2018, 52(3): 1118-1127.
(  0) 0) |
| [31] |
Davison W, Zhang H. In situ speciation measurements of trace components in natural waters using thin-film gels[J]. Nature, 1994, 367(6463): 546-548. DOI:10.1038/367546a0
(  0) 0) |
| [32] |
Guan D X, Williams P N, Luo J, et al. Novel precipitated zirconia-based DGT technique for high-resolution imaging of oxyanions in waters and sediments[J]. Environmental Science & Technology, 2015, 49(6): 3653-3661.
(  0) 0) |
| [33] |
Santner J, Larsen M, Kreuzeder A, et al. Two decades of chemical imaging of solutes in sediments and soils——a review[J]. Analytica Chimica Acta, 2015, 878: 9-42. DOI:10.1016/j.aca.2015.02.006
(  0) 0) |
| [34] |
Barrett P M, Hull E A, Burkart K, et al. Contrasting arsenic cycling in strongly and weakly stratified contaminated lakes:Evidence for temperature control on sediment-water arsenic fluxes[J]. Limnology and Oceanography, 2019, 64(3): 1333-1346. DOI:10.1002/lno.11119
(  0) 0) |
| [35] |
Martin A J, Pedersen T F. Seasonal and interannual mobility of arsenic in a lake impacted by metal mining[J]. Environmental Science & Technology, 2002, 36(7): 1516-1523.
(  0) 0) |
| [36] |
Teasdale P R, Batley G E, Apte S C, et al. Pore water sampling with sediment peepers[J]. TRAC Trends in Analytical Chemistry, 1995, 14(6): 250-256. DOI:10.1016/0165-9936(95)91617-2
(  0) 0) |
| [37] |
Yuan Z F, Gustave W, Sekar R, et al. Simultaneous measurement of aqueous redox sensitive elements and their species across soil-water interface[OL]. EarthArXiv, 2019, DOI: 10.31223/osf.io/gjad4.
(  0) 0) |
| [38] |
Yuan Z F, Gustave W, Bridge J, et al. Tracing the dynamic changes of element profiles by novel soil porewater samplers with ultralow disturbance to soil-water interface[J]. Environmental Science & Technology, 2019, 53(9): 5124-5132.
(  0) 0) |
| [39] |
Chen C E, Zhang H, Ying G G, et al. Passive sampling:A cost-effective method for understanding antibiotic fate, behaviour and impact[J]. Environment International, 2015, 85: 284-291. DOI:10.1016/j.envint.2015.10.001
(  0) 0) |
| [40] |
Xu D, Wu W, Ding S M, et al. A high-resolution dialysis technique for rapid determination of dissolved reactive phosphate and ferrous iron in pore water of sediments[J]. Science of the Total Environment, 2012, 421-422: 245-252. DOI:10.1016/j.scitotenv.2012.01.062
(  0) 0) |
| [41] |
Sun Q, Chen J, Zhang H, et al. Improved diffusive gradients in thin films(DGT)measurement of total dissolved inorganic arsenic in waters and soils using a hydrous zirconium oxide binding layer[J]. Analytical Chemistry, 2014, 86(6): 3060-3067. DOI:10.1021/ac404025e
(  0) 0) |
| [42] |
Luo J, Zhang H, Santner J, et al. Performance characteristics of diffusive gradients in thin films equipped with a binding gel layer containing precipitated ferrihydrite for measuring arsenic(V), selenium(VI), vanadium(V), and antimony(V)[J]. Analytical Chemistry, 2010, 82(21): 8903-8909. DOI:10.1021/ac101676w
(  0) 0) |
| [43] |
Bennett W W, Teasdale P R, Panther J G, et al. New diffusive gradients in a thin film technique for measuring inorganic arsenic and selenium(Ⅳ)using a titanium dioxide based adsorbent[J]. Analytical Chemistry, 2010, 82(17): 7401-7407. DOI:10.1021/ac101543p
(  0) 0) |
| [44] |
Huynh T, Zhang H, Noller B. Evaluation and application of the diffusive gradients in thin films technique using a mixed-binding gel layer for measuring inorganic arsenic and metals in mining impacted water and soil[J]. Analytical Chemistry, 2012, 84(22): 9988-9995. DOI:10.1021/ac302430b
(  0) 0) |
| [45] |
Xu L, Sun Q, Ding S M, et al. Simultaneous measurements of arsenic and sulfide using diffusive gradients in thin films technique(DGT)[J]. Environmental Geochemistry and Health, 2018, 40(5): 1919-1929. DOI:10.1007/s10653-017-9968-8
(  0) 0) |
| [46] |
Österlund H, Faarinen M, Ingri J, et al. Contribution of organic arsenic species to total arsenic measurements using ferrihydrite-backed diffusive gradients in thin films(DGT)[J]. Environmental Chemistry, 2012, 9(1): 55-62. DOI:10.1071/EN11057
(  0) 0) |
| [47] |
Smolíková V, Pelcová P, Ridošková A, et al. Development and evaluation of the iron oxide-hydroxide based resin gel for the diffusive gradient in thin films technique[J]. Analytica Chimica Acta, 2020, 1102: 36-45. DOI:10.1016/j.aca.2019.12.042
(  0) 0) |
| [48] |
Panther J G, Stillwell K P, Powell K J, et al. Perfluorosulfonated ionomer-modified diffusive gradients in thin films:Tool for inorganic arsenic speciation analysis[J]. Analytical Chemistry, 2008, 80(24): 9806-9811. DOI:10.1021/ac801678u
(  0) 0) |
| [49] |
Bennett W W, Teasdale P R, Panther J G, et al. Speciation of dissolved inorganic arsenic by diffusive gradients in thin films:Selective binding of AsⅢ by 3-mercaptopropyl-functionalized silica gel[J]. Analytical Chemistry, 2011, 83(21): 8293-8299. DOI:10.1021/ac202119t
(  0) 0) |
| [50] |
Rolisola A M C M, Suárez C A, Menegário A A, et al. Speciation analysis of inorganic arsenic in river water by Amberlite IRA 910 resin immobilized in a polyacrylamide gel as a selective binding agent for As(Ⅴ)in diffusive gradient thin film technique[J]. Analyst, 2014, 139(17): 4373-4380. DOI:10.1039/C4AN00555D
(  0) 0) |
| [51] |
Huynh T, Harris H H, Zhang H, et al. Measurement of labile arsenic speciation in water and soil using diffusive gradients in thin films(DGT)and X-ray absorption near edge spectroscopy(XANES)[J]. Environmental Chemistry, 2015, 12(2): 102-111. DOI:10.1071/EN14047
(  0) 0) |
| [52] |
Davison W, Grime G W, Morgan J A W, et al. Distribution of dissolved iron in sediment pore waters at submillimetre resolution[J]. Nature, 1991, 352(6333): 323-325. DOI:10.1038/352323a0
(  0) 0) |
| [53] |
Lehto N J, Davison W, Zhang H. The use of ultra-thin diffusive gradients in thin-films(DGT)devices for the analysis of trace metal dynamics in soils and sediments:A measurement and modelling approach[J]. Environmental Chemistry, 2012, 9(4): 415-423. DOI:10.1071/EN12036
(  0) 0) |
| [54] |
Ciffroy P, Nia Y, Garnier J M. Probabilistic multicompartmental model for interpreting DGT kinetics in sediments[J]. Environmental Science & Technology, 2011, 45(22): 9558-9565.
(  0) 0) |
| [55] |
Guan D X. Diffusive gradients in thin-films(DGT):An effective and simple tool for assessing contaminant bioavailability in waters, soils and sediments . Amsterdam: Elsevier, 2019: 111-124.
(  0) 0) |
| [56] |
Redman A D, Macalady D L, Ahmann D. Natural organic matter affects arsenic speciation and sorption onto hematite[J]. Environmental Science & Technology, 2002, 36(13): 2889-2896.
(  0) 0) |
| [57] |
Kuzyakov Y, Razavi B S. Rhizosphere size and shape:Temporal dynamics and spatial stationarity[J]. Soil Biology and Biochemistry, 2019, 135: 343-360. DOI:10.1016/j.soilbio.2019.05.011
(  0) 0) |
| [58] |
Fitz W J, Wenzel W W, Zhang H, et al. Rhizosphere characteristics of the arsenic hyperaccumulator Pteris vittata L. and monitoring of phytoremoval efficiency[J]. Environmental Science & Technology, 2003, 37(21): 5008-5014.
(  0) 0) |
| [59] |
Dai Y C, Nasir M, Zhang Y L, et al. Comparison of DGT with traditional extraction methods for assessing arsenic bioavailability to Brassica chinensis in different soils[J]. Chemosphere, 2018, 191: 183-189. DOI:10.1016/j.chemosphere.2017.10.035
(  0) 0) |
| [60] |
Wang J J, Bai L Y, Zeng X B, et al. Assessment of arsenic availability in soils using the diffusive gradients in thin films(DGT)technique-a comparison study of DGT and classic extraction methods[J]. Environmental Science:Processes & Impacts, 2014, 16(10): 2355-2361.
(  0) 0) |
| [61] |
Liang S, Guan D X, Ren J H, et al. Effect of aging on arsenic and lead fractionation and availability in soils:Coupling sequential extractions with diffusive gradients in thin-films technique[J]. Journal of Hazardous Materials, 2014, 273: 272-279. DOI:10.1016/j.jhazmat.2014.03.024
(  0) 0) |
| [62] |
Cattani I, Capri E, Boccelli R, et al. Assessment of arsenic availability to roots in contaminated Tuscany soils by a diffusion gradient in thin films(DGT)method and uptake by Pteris vittata and Agrostis capillaris[J]. European Journal of Soil Science, 2009, 60(4): 539-548. DOI:10.1111/j.1365-2389.2009.01144.x
(  0) 0) |
| [63] |
Xu J Y, Li H B, Liang S, et al. Arsenic enhanced plant growth and altered rhizosphere characteristics of hyperaccumulator Pteris vittata[J]. Environmental Pollution, 2014, 194: 105-111. DOI:10.1016/j.envpol.2014.07.017
(  0) 0) |
| [64] |
Zhang L P, Sun Q, Ding S M, et al. Characterization of arsenic availability in dry and flooded soils using sequential extraction and diffusive gradients in thin films(DGT)techniques[J]. Environmental Science and Pollution Research, 2017, 24(18): 15727-15734. DOI:10.1007/s11356-017-9190-3
(  0) 0) |
| [65] |
Senila M, Tanaselia C, Rimba E. Investigations on arsenic mobility changes in rizosphere of two ferns species using DGT technique[J]. Carpathian Journal of Earth and Environmental Sciences, 2013, 8(3): 145-154.
(  0) 0) |
| [66] |
Zhang H, Zhao F J, Sun B, et al. A new method to measure effective soil solution concentration predicts copper availability to plants[J]. Environmental Science & Technology, 2001, 35(12): 2602-2607.
(  0) 0) |
| [67] |
Song Z X, Shan B Q, Tang W Z. Evaluating the diffusive gradients in thin films technique for the prediction of metal bioaccumulation in plants grown in river sediments[J]. Journal of Hazardous Materials, 2018, 344: 360-368. DOI:10.1016/j.jhazmat.2017.10.049
(  0) 0) |
| [68] |
An J, Jeong B, Jeong S, et al. Diffusive gradients in thin films technique coupled to X-ray fluorescence spectrometry for the determination of bioavailable arsenic concentrations in soil[J]. Spectrochimica Acta Part B:Atomic Spectroscopy, 2020, 164: 105752. DOI:10.1016/j.sab.2019.105752
(  0) 0) |
| [69] |
Mojsilovic O, McLaren R G, Condron L M. Modelling arsenic toxicity in wheat:Simultaneous application of diffusive gradients in thin films to arsenic and phosphorus in soil[J]. Environmental Pollution, 2011, 159(10): 2996-3002. DOI:10.1016/j.envpol.2011.04.022
(  0) 0) |
| [70] |
Bennett W W, Teasdale P R, Panther J G, et al. Investigating arsenic speciation and mobilization in sediments with DGT and DET:A mesocosm evaluation of oxic-anoxic transitions[J]. Environmental Science & Technology, 2012, 46(7): 3981-3989.
(  0) 0) |
| [71] |
Bennett W W, Teasdale P R, Welsh D T, et al. Inorganic arsenic and iron(Ⅱ)distributions in sediment porewaters investigated by a combined DGT-colourimetric DET technique[J]. Environmental Chemistry, 2012, 9(1): 31-40. DOI:10.1071/EN11074
(  0) 0) |
| [72] |
Davison W, Fones G R, Grime G W. Dissolved metals in surface sediment and a microbial mat at 100-μm resolution[J]. Nature, 1997, 387(6636): 885-888. DOI:10.1038/43147
(  0) 0) |
| [73] |
Stockdale A, Davison W, Zhang H. High-resolution two-dimensional quantitative analysis of phosphorus, vanadium and arsenic, and qualitative analysis of sulfide, in a freshwater sediment[J]. Environmental Chemistry, 2008, 5(2): 143-149. DOI:10.1071/EN07096
(  0) 0) |
| [74] |
Gillan D C, Baeyens W, Bechara R, et al. Links between bacterial communities in marine sediments and trace metal geochemistry as measured by in situ DET/DGT approaches[J]. Marine Pollution Bulletin, 2012, 64(2): 353-362. DOI:10.1016/j.marpolbul.2011.11.001
(  0) 0) |
| [75] |
Pradit S, Gao Y, Faiboon A, et al. Application of DET(diffusive equilibrium in thin films)and DGT(diffusive gradients in thin films)techniques in the study of the mobility of sediment-bound metals in the outer section of Songkhla Lake, Southern Thailand[J]. Environmental Monitoring and Assessment, 2013, 185(5): 4207-4220. DOI:10.1007/s10661-012-2862-z
(  0) 0) |
| [76] |
Kreuzeder A, Santner J, Prohaska T, et al. Gel for simultaneous chemical imaging of anionic and cationic solutes using diffusive gradients in thin films[J]. Analytical Chemistry, 2013, 85(24): 12028-12036. DOI:10.1021/ac403050f
(  0) 0) |
| [77] |
Williams P N, Santner J, Larsen M, et al. Localized flux maxima of arsenic, lead, and iron around root apices in flooded lowland rice[J]. Environmental Science & Technology, 2014, 48(15): 8498-8506.
(  0) 0) |
| [78] |
Garnier J M, Garnier J, Jézéquel D, et al. Using DET and DGT probes(ferrihydrite and titanium dioxide)to investigate arsenic concentrations in soil porewater of an arsenic-contaminated paddy field in Bangladesh[J]. Science of the Total Environment, 2015, 536: 306-315. DOI:10.1016/j.scitotenv.2015.07.065
(  0) 0) |
| [79] |
Chen X, Sun Q, Ding S M, et al. Mobile arsenic distribution and release kinetics in sediment profiles under varying pH conditions[J]. Water, Air, & Soil Pollution, 2017, 228(11): 413.
(  0) 0) |
| [80] |
Gao L, Gao B, Xu D Y, et al. Assessing remobilization characteristics of arsenic(As)in tributary sediment cores in the largest reservoir, China[J]. Ecotoxicology and Environmental Safety, 2017, 140: 48-54. DOI:10.1016/j.ecoenv.2017.01.050
(  0) 0) |
| [81] |
Gao L, Gao B, Peng W Q, et al. Assessing potential release tendency of As, Mo and W in the tributary sediments of the Three Gorges Reservoir, China[J]. Ecotoxicology and Environmental Safety, 2018, 147: 342-348. DOI:10.1016/j.ecoenv.2017.08.036
(  0) 0) |
| [82] |
Ma X, Li C, Yang L Y, et al. Evaluating the mobility and labile of As and Sb using diffusive gradients in thin-films(DGT)in the sediments of Nansi Lake, China[J]. Science of the Total Environment, 2020, 713: 136569. DOI:10.1016/j.scitotenv.2020.136569
(  0) 0) |
| [83] |
Yin D X, Fang W, Guan D X, et al. Localized intensification of arsenic release within the emergent rice rhizosphere[J]. Environmental Science & Technology, 2020, 54(6): 3138-3147.
(  0) 0) |
| [84] |
Stockdale A, Davison W, Zhang H. 2D simultaneous measurement of the oxyanions of P, V, As, Mo, Sb, W and U[J]. Journal of Environmental Monitoring, 2010, 12(4): 981-984. DOI:10.1039/b925627j
(  0) 0) |
| [85] |
Chen Z, Zhu Y G, Liu W J, et al. Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice(Oryza sativa)roots[J]. New Phytologist, 2005, 165(1): 91-97.
(  0) 0) |
| [86] |
Kalbitz K, Wennrich R. Mobilization of heavy metals and arsenic in polluted wetland soils and its dependence on dissolved organic matter[J]. Science of the Total Environment, 1998, 209(1): 27-39. DOI:10.1016/S0048-9697(97)00302-1
(  0) 0) |
| [87] |
Leermakers M, Gao Y, Gabelle C, et al. Determination of high resolution pore water profiles of trace metals in sediments of the Rupel River(Belgium)using DET(diffusive equilibrium in thin films)and DGT(diffusive gradients in thin films)techniques[J]. Water Air and Soil Pollution, 2005, 166(1/2/34): 265-286.
(  0) 0) |
| [88] |
Zhang G L, Bai J H, Zhao Q Q, et al. Heavy metals pollution in soil profiles from seasonal-flooding riparian wetlands in a Chinese delta:Levels, distributions and toxic risks[J]. Physics and Chemistry of the Earth, Parts A/B/C, 2017, 97: 54-61. DOI:10.1016/j.pce.2016.11.004
(  0) 0) |
| [89] |
Liu Q, Xie W J, You J E, et al. Environmentally chemical behaviors of heavy metals in wetland sediments:A review (In Chinese)[J]. Soils, 2013, 45(1): 8-16. [刘庆, 谢文军, 游俊娥, 等. 湿地沉积物重金属环境化学行为研究进展[J]. 土壤, 2013, 45(1): 8-16.]
(  0) 0) |
 2021, Vol. 58
2021, Vol. 58


