2. 农业农村部农业环境重点研究室, 北京 100081;
3. 北京林业大学水土保持学院, 北京 100083
2. Key Laboratory of Agro-Environment, Ministry of Agriculture and Rural Affairs, Beijing 100081, China;
3. School of Soil and Water Conservation, Beijing Forestry University, Beijing 100083, China
土壤系统中,有机质、黏土矿物和铁铝氧化物是吸附固定重金属的主要活性反应相[1-2]。有机质往往通过氢键、离子交换、去质子化、疏水作用力、阴离子吸附等机制结合在矿物表面和层间,形成有机矿物复合体[3]。土壤系统具有复杂性和异质性,有机矿物复合体种类丰富,国内外众多研究结果表明,不同有机矿物复合体表面性质差异大,深刻影响着土壤中重金属的环境化学行为[4-5]。因此,对于有机矿物复合体的结构表征及其吸附固定重金属的机制研究是当前土壤环境科学领域的重要研究热点。然而,许多研究基于实验室单一矿物模拟系统,将其研究成果用于预测实际土壤系统重金属的化学行为时效果不甚理想[6]。这是因为实际系统为多相体系,重金属在有机矿物复合体界面的吸附多数情况并不符合组分相加原则[7],因此分离得到土壤团聚体中的天然有机矿物复合体组分,并研究其结构组成和重金属吸附固定机制显得十分必要。
近20年来,基于同步辐射硬X射线光谱技术,在分子水平上以土壤颗粒整体作为研究单位,研究铜吸附固定机制取得了显著进步[7-10]。但是由于硬X射线能量范围限制,土壤有机碳和黏土矿物的分布特征和微观结构及其与铜的作用机制还鲜有报告。同步辐射扫描透射显微术(Scanning Transmission X-ray Microscopy,STXM)具有纳米级(约30 nm)空间分辨率,可以原位表征有机碳、典型矿物(钙、铁、铝、硅和锰等金属氧化物以及黏土矿物)和重金属在亚微米尺度上的空间分布特征及微观结构[11-12],在非均相土壤及有机矿物复合体对铜的吸附机制研究中提供了独特的研究手段。
本研究以红壤为供试土壤,采用团聚体颗粒密度分离法得到颗粒态有机质和有机矿物复合体两个组分,通过元素分析、X射线衍射分析(X-Ray Diffraction,XRD)对其宏观结构进行表征,并开展铜批量吸附实验,利用STXM技术对吸附样品中铜及相关元素进行微观结构表征,以期得到铜在天然有机矿物复合体中的微区分布以及二者结合的分子机制,有望揭示实际红壤系统中铜的环境化学行为,为土壤污染控制与修复提供理论依据。
1 材料与方法 1.1 供试土壤供试土壤采自湖南祁阳红壤水稻土表层(0~ 20 cm),质地为粉砂质黏壤土,黏粒含量为33.1%。基本理化性质根据文献[13]方法测定,结果见表 1。
|
|
表 1 供试红壤基本理化性质 Table 1 Basic physicochemical properties of the red soil used in the experiment |
首先取10 g过2 mm筛的风干土壤于100 mL离心管中,加入40 mL 1.8 g·L-1的多钨酸钠溶液后静置0.5 h,然后利用超声破碎仪(117 W)在冰水浴超声2.5 min,超声能量为487.5 J·mL-1[14];将超声后的土壤悬液离心分离(3 000 r·min-1,30 min)[15],收集上清液中物质得到颗粒态有机质(Particle organic matter,POM),重复两次,直至上清液中无漂浮物为止。离心管中的沉淀即为有机矿物复合体(Organo-mineral complexes,OMC)。为去除密度组分(POM、OMC)中盐基离子,用去离子水进行多次洗涤,直至洗涤液电导率低于5 μS·cm-1[14],冷冻干燥待测。为了确保密度分离的准确性,实验设置四组平行,并将相同密度组分混合后备用。
1.3 密度组分性质测定土壤各密度组分中C、N元素的百分含量用CN元素分析仪(vario PYRO cube)测定,得到各密度组分的全氮(TN)、全碳(TC)以及碳氮比(C/N);用DCB法提取游离态氧化铁(Fed),酸性草酸铵缓冲液提取无定形氧化铁(Feo),ICP-OES测定溶液中铁离子浓度计算得到各密度组分中游离态和无定形态氧化铁含量,并用其差值(Fed - Feo)代表晶质氧化铁[13];土壤矿物组成用X射线衍射分析(X' Pert3 Powder,PANalytical,Netherlands)方法测定,采用Cu Kα射线,波长λ为0.154 06 nm,工作电压为45 kV,电流为40 mA。数据记录步长为0.02°,2θ范围从5°到90°。
1.4 批量吸附实验称取0.5 g土壤组分样品于50 mL塑料离心管中,以0.01 mol·L-1的NaCl为背景电解质,土液比1:50。加入不同体积的含Cu 500 mg·L-1的CuCl2储备液,使得Cu的初始浓度依次为0.50、1.00、2.50、5.00、10.00、20.00、40.00、80.00、100.00 mg·L-1,用NaCl溶液定容,最终体积为25 mL。于25 ℃下连续振荡24 h,维持溶液pH为5.0 ± 0.05,取出后用高速冷冻离心机在5 000 r·min-1离心15 min,取上清液用ICP-OES(Agilent Technologies 5110)测定Cu的浓度,根据差减法计算土壤各组分铜吸附量。
1.5 同步辐射表征STXM试验主要在加拿大光源SM线站(10ID-1)进行。供试样品经冷冻干燥后利用玛瑙研钵研磨成细粉末[16]。进行扫描前,取1 mg样品溶解于1~2 μL去离子水中振荡以使其悬浮,移液枪吸取少量悬浮液滴至Si3N4窗口(75 nm厚)上并使其风干。整个测量过程均使用25 nm菲涅耳波带板进行,该波带板提供了30 nm的最大空间分辨率,在数据收集期间测试腔中样品保持在充满氦气的1/6大气压下,同步检测传输的X射线以生成图像。采集图像时,在样品同一区域依次扫描并收集C1s、Fe 2p、Cu 2p、Al 1s和Si 1s的堆栈信息,停留时间为1~1.5 ms,像素尺寸为30 nm。
1.6 数据处理采用Langmuir方程和Freundlich方程进行红壤有机矿物复合体吸附铜的等温吸附模拟。其中Langmuir模型方程为:
| $\frac{{{C_e}}}{{{Q_e}}} = \frac{{{C_e}}}{{{Q_{\max }}}} + \frac{1}{{b \cdot {Q_{\max }}}}$ |
Freundlich模型方程为:
| $\lg {Q_e} = \lg {K_F} + \frac{1}{n} \cdot \lg {C_e}$ |
式中,Ce为平衡吸附浓度(mg·L-1),Qe为平衡吸附量(mg·kg-1);Qmax为红壤不同密度组分的最大吸附量(mg·kg-1);b为Langmuir方程吸附常数,用来表征吸附剂对所表征金属的吸附强度;KF、n为Freundlich方程的吸附常数。
STXM图像用Axis2000进行数据处理和分析。为得到所有样品的OD(OD = ln(I0/I)图像,首先对每个样品每个元素的堆栈图像进行叠加,并去除无效噪音图像,然后利用样品(I)的透射光通量和在分析粒子(I0)附近的空白区域测量的入射光通量将图像序列转换为光密度。每个元素的分布图像由其光子能量段的平均能量OD图像与能量边前OD图像差值表示,不同元素的分布特征通过RGB分析进行处理,以显示三种元素在同一位置的微尺度空间分布情况。另选取感兴趣的微域,获取铜及相关元素的近边X射线精细结构(Near-Edge X-ray Absorption Fine Structure,NEXAFS)谱,分析铜及相关元素赋存的分子形态。
2 结果与讨论 2.1 土壤及其密度组分的碳氮组成红壤颗粒密度分离后,土体土壤和各组分的TC和TN含量差异显著(表 2)。OMC的TC和TN含量分别较POM低84.64%和74.2%,表明密度分离后OMC的土壤有机碳和总氮显著下降。土壤C/N通常被认为是土壤氮素矿化能力的标志,低C/N表示有机质分解程度和氮矿化速率高。本实验中POM的C/N是OMC的1.68倍,说明OMC中有机质分解程度和氮矿化程度高。John等[17]研究了不同土壤利用方式下各密度组分的C/N,发现在所有土壤及其所有深度下密度组分的C/N均为POM(12.0~26.5)高于OMC(6.3~13.6)。红壤水稻土POM和OMC碳含量占其TC(BS)的比例分别为22.98%和76.8%,这是因为虽然POM的TC显著高于OMC,而OMC在土体中的含量远远超过POM,二者质量分数分别为95.6%和4.39%,即土体土壤主要由OMC组成。蔡岸冬等[18]通过文献检索研究了农田六大土壤类型中矿物结合态有机碳的特征,指出农田红壤中矿物结合态有机碳含量的中值为74%。因此,红壤土体土壤BS主要由OMC组分构成,同时OMC也是主要碳库,具有较高的有机质分解程度和氮矿化程度。
|
|
表 2 红壤土体土壤及各组分的质量分数及碳氮组成 Table 2 Mass fraction and C、N contents of different density fractions and bulk soil of the red soil |
土壤各组分的矿物组成如图 1所示。XRD结果表明,BS及OMC的矿物组成相似,均以原生矿物石英为主,次生矿物含量较低,且主要为2:1型白云母,OMC中还含少量1:1型多水高岭石。周琴等[19]研究湖南红壤的胶体和非胶体部分,发现有机质含量较低的非胶体部分,其矿物组成以原生矿物石英为主,其次为次生矿物水云母,高岭石含量不高;而有机质含量较高的胶体部分,检测出了三水铝石、针铁矿、赤铁矿等铁铝氧化物矿物。Wiseman和Püttmann[20]也认为无定形或非晶质氧化物对有机碳有强烈的键合作用,且指出Feo在所有土壤上显著相关。王莹等[21]研究红壤水稻土各粒级团聚体内氧化铁的分布特征,也发现无定形铁(Feo)与有机碳含量呈显著正相关。铁形态分析结果表明(表 3),在本研究中TC含量最高的POM中无定形铁含量为OMC组分的1.58倍,较比BS中无定形铁含量也高出0.29倍,这与上述学者研究结果相吻合。XRD和铁形态提取结果表明,通常认为的POM中同样含有较多有机氧化物复合体,而OMC主要矿物类型为黏土矿物。
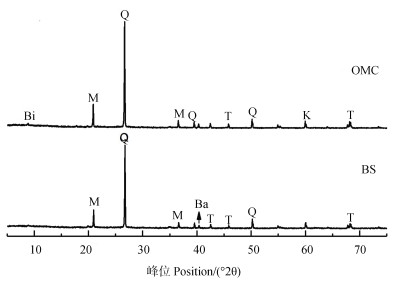
|
注:Q,石英;M,白云母;T,四方锰铁矿;Ba,软水铝石;K,高岭石;Bi,黑云母。Note:Q,Quarts;M,Muscovite;T,Tetragonal manganese ore;Ba,Bauxite;K,Kaolinite;Bi,Biotite. 图 1 红壤原土(BS)和有机矿物复合体(OMC)的XRD图谱 Fig. 1 X-ray diffraction of bulk soil(BS)and organo-mineral complexes(OMC)in the red soil |
|
|
表 3 红壤土体土壤及各组分的铁形态及矿物组成 Table 3 Fe fractions and mineral composition of the bulk soil and fractions of the red soil |
本研究中,POM由于质量分数极低,未能用于等温吸附实验,BS和OMC对Cu(Ⅱ)的吸附等温线如图 2所示。当Cu(Ⅱ)的初始质量浓度小于20 mg·L-1时,BS和OMC可以提供充足的吸附位点,对Cu(Ⅱ)的平衡吸附量随溶液初始浓度的增加而增加;当溶液浓度进一步增大时,吸附位点逐渐饱和,平衡吸附量增加程度放缓,最终在初始Cu(Ⅱ)浓度为100 mg·L-1左右达到吸附平衡。BS与OMC对Cu(Ⅱ)的吸附曲线近似,吸附量相当,说明OMC是红壤吸附重金属Cu(Ⅱ)的主要贡献者。这是因为一方面,OMC与BS在TC、TN、C/N以及Feo在含量上相近(表 2、表 3),而另一方面,主要由于OMC是土体土壤BS主要组分,含量远远超过POM,占土体土壤BS质量比例高达95.6%。McLaren和Crawford [22]同样发现虽然黏土矿物对Cu(Ⅱ)专性吸附能力要比氧化物矿物和有机质小,但由于其含量远高于氧化物矿物和有机质,其作用不可低估。本研究中尽管OMC主要矿物类型则为黏土矿物,但是由于在土体土壤BS质量比例中占据绝对优势,因此是实际系统中红壤吸附重金属Cu(Ⅱ)的主要贡献者。
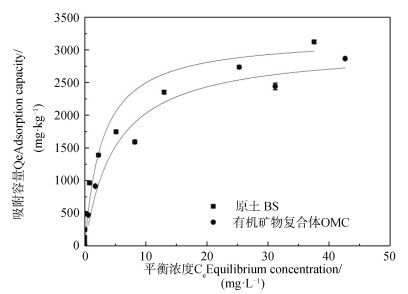
|
图 2 红壤BS和OMC(> 1.8 g·cm-3)吸附Cu2+的等温线 Fig. 2 Cu2+ sorption isotherm of the BS and OMC with density > 1.8 g·cm-3 |
用Langmuir和Freundlich等温吸附方程主要用来描述一定温度下吸附质和吸附剂之间的分配行为。通常土壤中许多吸附过程均能较好地用Freundlich方程来描述,而Langmuir方程由于可以求出最大吸附量Qmax这一具有重要物理意义的常数而广泛被使用。但Langmuir所代表的能量含义为吸附热不随吸附而变化,每个吸附点的能量不变,因此是一种理想吸附,Freundlich则适用于描述不均匀表面的吸附。
利用两种等温吸附模型对实验数据进行拟合,所得拟合参数列于表 4。结果显示,BS与OMC对于铜的吸附行为符合两种等温吸附模型,决定系数R2均接近0.98,其中Freundlich方程R2稍高,这与许多前人研究结果一致[23-24],说明土体土壤BS和OMC中主要为不均一表面的吸附。由Langmuir方程计算出BS与OMC理论最大吸附量Qmax基本相当,分别为3 203.4 mg·kg-1和3 041.5 mg·kg-1。Freundlich方程中的n值通常被认为是土壤对重金属离子吸附作用的强度指标,n越大,表明吸附剂对重金属离子吸附的作用力越大。从表 4可知,BS的n值略高于OMC,说明BS对Cu(Ⅱ)的吸附作用强度比OMC稍大,这很可能还与BS赋存的POM组分相关(表 3),其富含的有机质和铁氧化物是铜的重要吸附相[9]。
|
|
表 4 红壤BS和OMC对Cu2+等温吸附模型拟合参数 Table 4 Fitness of isotherm models of Cu2+ sorption for BS and OMC |
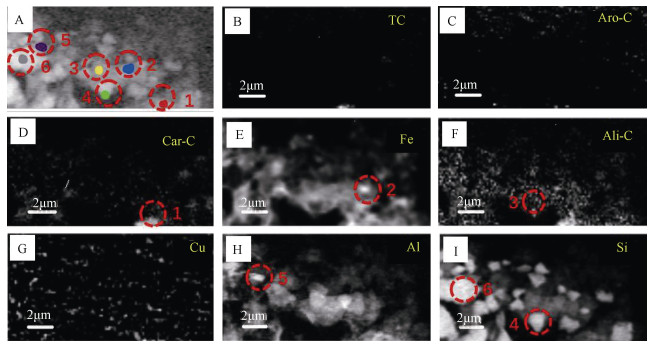
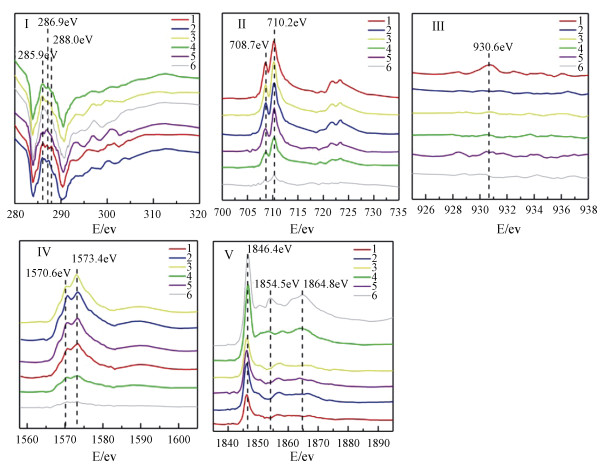
利用STXM技术表征Cu与C、Fe、Al、Si的微区分布特征,结果显示铜在红壤BS和OMC中的分布在亚微米尺度上具有异质性(图 3,图 5)。选取了BS样品中高铁点1、高碳点2以及高铜点3三个微区,三点处有关元素的NEXAFS谱见图 4。碳的K边NEXAFS谱显示点1、2在286 eV(芳香碳)处有一处吸收峰,而点3则在286 eV(芳香碳)及288.1 eV(羧基碳)处均有吸收峰。三点的铁元素NEXAFS图谱则显示,2、3两点具有相似的矿物元素图谱,相对于2、3两点,1点处铁的L3、L 2边NEXAFS谱中708.7 eV(L2边)向高能端偏移(蓝移)而710.2 eV(L3边)向低能端偏移。早前有研究表明铁的2p NEXAFS图谱可以提供铁氧化度的相关信息[25],其中二价铁在708.7 eV有最强峰而三价铁则在710.2 eV具有最强吸收峰[26-27]。因此判断点2、3处以三价铁为主导,而点1即高铁点处则为二、三价铁共存矿物。三个点在铝和硅的K边NEXAFS的吸收峰特征与铝硅酸盐黏土矿物相似[16, 28]。三个点中仅有点3在930.6 eV处具有较为明显的铜吸收峰,同时点3碳的K边NEXAFS谱与点1、2具有明显差异。Manceau和Matynia[29]在研究铜与天然有机质的结合性质时同样发现,在酸性土壤条件下,铜与有机质的稳定螯合物主要是由铜与α位上的羧基和羟基配位体结合而成,因此点3高铜点的铜可能与羧基碳的存在有关。
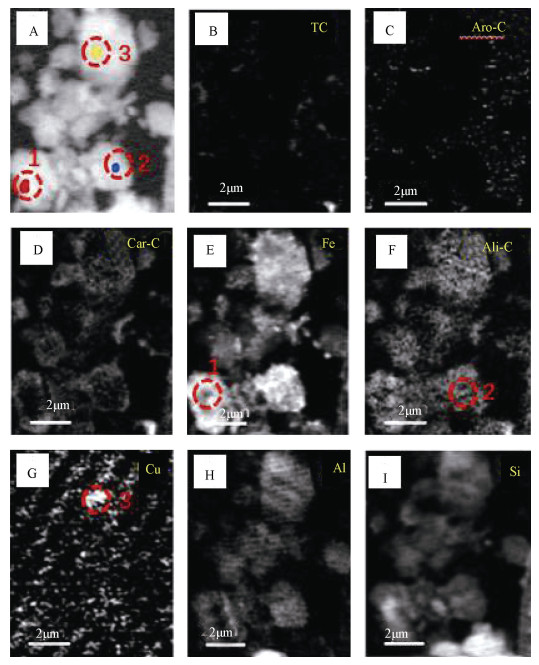
|
注:总碳(TC,从290—292 eV减去280—282 eV的能量区域);芳烃-C(Aro-C,减去280—282 eV和284.5—286 eV的能量区域);脂肪族-C(Ali-C,从287.3—287.8 eV减去284.5—286 eV的能量区域);羧基-C(Car-C,288—289 eV减去284.5—286 eV的能区);Fe(709.7—710.3 eV减去700—705 eV的能区);Cu(931—932 eV减去926.3—927.3 eV的能区);Al(1 566.9—1 567.5 eV减去1 555—1 560 eV的能区);Si(1 847—1 847.6 eV减去1 835—1 840 eV的能区)。下同。Note:The color of the spectra and the color of the region from which they were extracted are the same. Total carbon(TC,the energy region resulting from subtracting 280-282 eV from 290-292 eV);Aromatic-C(Aro-C,the energy region resulting from subtracting 280-282 eV from 284.5-286 eV);Aliphatic-C(Ali-C,the energy region resulting from subtracting 284.5-286 eV from 287.3-287.8 eV);Carboxylic-C(Car-C,the energy region resulting from subtracting 284.5-286 eV from 288-289 eV);Fe(the energy region resulting from subtracting 700-705 eV from 709.7-710.3 eV);Cu(the energy region resulting from subtracting 926.3-927.3 eV from 931-932 eV);Al(the energy region resulting from subtracting 1 555-1 560 eV from 1 566.9-1 567.5 eV);and Si(the energy region resulting from subtracting 1 835-1 840 eV from 1 847-1 847.6 eV). The same below. 图 3 红壤BS的铜及相关元素OD差异(OD280—OD1890)图像(A—I) Fig. 3 Images(A-I)of OD(OD280-OD1890)difference between Cu and related elements in BS |
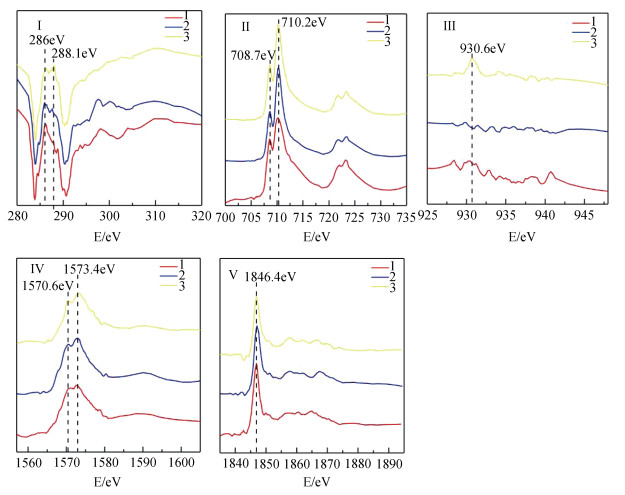
|
图 4 红壤BS的铜及相关元素在选择区域(1—3)的NEXAFS谱(Ⅰ—Ⅴ) Fig. 4 NEXAFS spectra of Cu and related elements in the selected regions(1-3)of the BS of the red soil(Ⅰ-Ⅴ) |
|
图 5 红壤OMC的铜及相关元素OD差异(OD280—OD1890)图像(A—I) Fig. 5 Images(A-I)of OD(OD280-OD1890)differences between Cu and related elements in OMC |
图 5为OMC的STXM-NEXAFS图像,同样选取了高碳点1和点3、高铁点2、高铝硅点4—6的NEXAFS图谱,结果见图 6。图谱中显示仅有点1处在930.6 eV处出现微弱铜信号峰,而点1同时也是高羧基碳点。碳的K边NEXAFS谱显示,点1在285.9 eV、286.9 eV、288.0 eV分别具有小的吸收峰,前期研究表明这三处吸收峰分别代表芳香碳、脂肪碳和羧基碳,即点1为这三种有机碳的赋存微区。点6在铝、铁NEXAFS谱中无信号峰,在Si谱中有类似于石英的吸收峰特征,同时在288.0 eV(羧基碳)处具有吸收峰,应为羧基碳包裹在石英表面。点6处含有羧基碳却未检测到铜的吸收峰,可能是因为羧基碳对铜的吸附与铁铝氧化物具有一定关系,铜在红壤中极有可能被羧基碳与铁铝氧化物结合成的有机矿物复合体所固定,而非由羧基碳单独固定铜。
|
图 6 红壤OMC的铜及相关元素在选择区域(1—6)的NEXAFS谱(Ⅰ—Ⅴ) Fig. 6 NEXAFS spectra of Cu and related elements of the OMC in the selected regions(Ⅰ-Ⅴ) |
红壤颗粒态有机质(POM)和有机矿物复合体(OMC)的碳含量及铁形态、矿物组成差异大,主体土壤BS主要由OMC组成,POM含量少。OMC富含黏土矿物,且以原生矿物石英为主,次生矿物含量较低,主要为2︰1型白云母,含少量1︰1型多水高岭石。BS及OMC对铜吸附等温线类似,等温吸附曲线符合Langmuir方程和Freundlich方程,Langmuir拟合表明BS及OMC理论最大吸附量Qmax基本相当,说明红壤对铜的吸附主要由OMC决定。STXM-NEXAFS分析发现红壤中铜在亚微米尺度上的分布具有异质性,羧基碳对于红壤中铜的固定具有一定作用,铜在红壤中主要被羧基碳与铁铝氧化物结合成的有机矿物复合体所固定。因此,有机矿物复合体对于实际土壤系统中Cu(Ⅱ)的吸附具有重要贡献,主要控制着红壤中铜的形态分布与生物有效性。
| [1] |
Flogeac K, Guillon E, Aplincourt M. Surface complexation of copper(Ⅱ) on soil particles: EPR and XAFS studies[J]. Environmental Science & Technology, 2004, 38(11): 3098-3103.
(  0) 0) |
| [2] |
Tiberg C, Sjöstedt C, Persson I, et al. Phosphate effects on copper(Ⅱ) and lead(Ⅱ) sorption to ferrihydrite[J]. Geochimica et Cosmochimica Acta, 2013, 120: 140-157. DOI:10.1016/j.gca.2013.06.012
(  0) 0) |
| [3] |
Huang P M. Soil mineral-organic matter——microorganism interactions: Fundamentals and impacts[J]. Advances in Agronomy, 2004, 82: 391-472.
(  0) 0) |
| [4] |
Zhu J, Pigna M, Cozzolino V, et al. Competitive sorption of copper(Ⅱ), chromium(Ⅲ) and lead(Ⅱ) on ferrihydrite and two organomineral complexes[J]. Geoderma, 2010, 159(3/4): 409-416.
(  0) 0) |
| [5] |
Komy Z R, Shaker A M, Heggy S E M, et al. Kinetic study for copper adsorption onto soil minerals in the absence and presence of humic acid[J]. Chemosphere, 2014, 99: 117-124. DOI:10.1016/j.chemosphere.2013.10.048
(  0) 0) |
| [6] |
Bertsch P M, Seaman J C. Characterization of complex mineral assemblages: Implications for contaminant transport and environmental remediation[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(7): 3350-3357. DOI:10.1073/pnas.96.7.3350
(  0) 0) |
| [7] |
Franzblau R E, Daughney C J, Moreau M, et al. Selenate adsorption to composites of Escherichia coli and iron oxide during the addition, oxidation, and hydrolysis of Fe(Ⅱ)[J]. Chemical Geology, 2014, 383: 180-193. DOI:10.1016/j.chemgeo.2014.06.016
(  0) 0) |
| [8] |
Boudesocque S, Guillon E, Aplincourt M, et al. Sorption of Cu(Ⅱ) onto vineyard soils: Macroscopic and spectroscopic investigations[J]. Journal of Colloid and Interface Science, 2007, 307(1): 40-49. DOI:10.1016/j.jcis.2006.10.080
(  0) 0) |
| [9] |
Strawn D G, Baker L L. Speciation of Cu in a contaminated agricultural soil measured by XAFS, μ-XAFS, and μ-XRF[J]. Environmental Science & Technology, 2008, 42(1): 37-42.
(  0) 0) |
| [10] |
Yang J J, Zhu S H, Zheng C Q, et al. Impact of S fertilizers on pore-water Cu dynamics and transformation in a contaminated paddy soil with various flooding periods[J]. Journal of Hazardous Materials, 2015, 286: 432-439. DOI:10.1016/j.jhazmat.2015.01.035
(  0) 0) |
| [11] |
Stuckey J W, Yang J J, Wang J, et al. Advances in scanning transmission X-ray microscopy for elucidating soil biogeochemical processes at the submicron scale[J]. Journal of Environmental Quality, 2017, 46(6): 1166-1174. DOI:10.2134/jeq2016.10.0399
(  0) 0) |
| [12] |
Wang L, Ying R R, Shi J Q, et al. Advancement in study on adsorption of organic matter on soil minerals and its mechanism (In Chinese)[J]. Acta Pedologica Sinica, 2017, 54(4): 805-818. [王磊, 应蓉蓉, 石佳奇, 等. 土壤矿物对有机质的吸附与固定机制研究进展[J]. 土壤学报, 2017, 54(4): 805-818.]
(  0) 0) |
| [13] |
Lu R K. Analytical methods for soil and agro-chemistry (In Chinese). Beijing: China Agricultural Science and Technology Press, 2000. [鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000.]
(  0) 0) |
| [14] |
Amelung W, Zech W. Minimisation of organic matter disruption during particle-size fractionation of grassland epipedons[J]. Geoderma, 1999, 92(1/2): 73-85.
(  0) 0) |
| [15] |
Mueller C W, Gutsch M, Kothieringer K, et al. Bioavailability and isotopic composition of CO2 released from incubated soil organic matter fractions[J]. Soil Biology & Biochemistry, 2014, 69: 168-178. DOI:10.1016/j.soilbio.2013.11.006
(  0) 0) |
| [16] |
Yang J J, Liu J, Dynes J J, et al. Speciation and distribution of copper in a mining soil using multiple synchrotron-based bulk and microscopic techniques[J]. Environmental Science and Pollution Research, 2014, 21(4): 2943-2954. DOI:10.1007/s11356-013-2214-8
(  0) 0) |
| [17] |
John B, Yamashita T, Ludwig B, et al. Storage of organic carbon in aggregate and density fractions of silty soils under different types of land use[J]. Geoderma, 2005, 128(1/2): 63-79.
(  0) 0) |
| [18] |
Cai A D, Xu X R, Zhang X B, et al. Capacity and characteristics of mineral associated soil organic carbon under various land uses (In Chinese)[J]. Scientia Agricultura Sinica, 2014, 47(21): 4291-4299. DOI:10.3864/j.issn.0578-1752.2014.21.014 [蔡岸冬, 徐香茹, 张旭博, 等. 不同利用方式下土壤矿物结合态有机碳特征与容量分析[J]. 中国农业科学, 2014, 47(21): 4291-4299.]
(  0) 0) |
| [19] |
Zhou Q, Jiang J, Xu R K. Adsorption of Cu(Ⅱ), Pb(Ⅱ) and Cd(Ⅱ) on colloidal and non-colloidal particles in two red soils (In Chinese)[J]. Acta Pedologica Sinica, 2018, 55(1): 131-138. [周琴, 姜军, 徐仁扣. Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)在红壤胶体和非胶体颗粒上吸附的比较[J]. 土壤学报, 2018, 55(1): 131-138.]
(  0) 0) |
| [20] |
Wiseman C L S, Püttmann W. Interactions between mineral phases in the preservation of soil organic matter[J]. Geoderma, 2006, 134(1/2): 109-118.
(  0) 0) |
| [21] |
Wang Y, Yao S H, Li H X, et al. Relationship between distribution patterns of iron oxidates and soil organic matter in aggregates of paddy soil in a long-term fertilization (In Chinese)[J]. Soils, 2013, 45(4): 666-672. DOI:10.3969/j.issn.1674-5906.2013.04.020 [王莹, 尧水红, 李辉信, 等. 长期施肥稻田土壤团聚体内氧化铁分布特征及其与有机碳的关系[J]. 土壤, 2013, 45(4): 666-672.]
(  0) 0) |
| [22] |
McLaren R G, Crawford D V. Studies on soil copper Ⅱ. The specific adsorption of copper by soils[J]. Journal of Soil Science, 1973, 24(4): 443-452. DOI:10.1111/j.1365-2389.1973.tb02311.x
(  0) 0) |
| [23] |
Wang W J, Shao Z Z, He Q. Adsorption affinity of red soil annual clay to Co, Cu, Pb and Zn for different red soil clays (In Chinese)[J]. Acta Pedologica Sinica, 1995, 32(2): 167-178. DOI:10.3321/j.issn:0564-3929.1995.02.002 [王维君, 邵宗曾, 何群. 红壤粘粒对Co、Cu、Pb和Zn吸附亲和力的研究[J]. 土壤学报, 1995, 32(2): 167-178.]
(  0) 0) |
| [24] |
Wu M L. Study on the specific adsorption of copper ion by soils and its characteristics (In Chinese)[J]. Acta Pedologica Sinica, 1989, 26(1): 31-41. DOI:10.3321/j.issn:0564-3929.1989.01.012 [武玫玲. 土壤对铜离子的专性吸附及其特征的研究[J]. 土壤学报, 1989, 26(1): 31-41.]
(  0) 0) |
| [25] |
van Aken P A, Liebscher B. Quantification of ferrous/ferric ratios in minerals: New evaluation schemes of Fe L23 electron energy-loss near-edge spectra[J]. Physics and Chemistry of Minerals, 2002, 29(3): 188-200. DOI:10.1007/s00269-001-0222-6
(  0) 0) |
| [26] |
Dynes J J, Tyliszczak T, Araki T, et al. Speciation and quantitative mapping of metal species in microbial biofilms using scanning transmission X-ray microscopy[J]. Environmental Science & Technology, 2006, 40(5): 1556-1565.
(  0) 0) |
| [27] |
Hitchcock A P, Dynes J J, Lawrence J R, et al. Soft X-ray spectromicroscopy of nickel sorption in a natural river biofilm[J]. Geobiology, 2009, 7(4): 432-453. DOI:10.1111/j.1472-4669.2009.00211.x
(  0) 0) |
| [28] |
Liu J N, Yang J J, Zeng X B, et al. Fe(Ⅲ)-induced sequestration of citric acid on kaolinite surface probed by STXM-NEXAFS spectroscopy[J]. Acta Chimica Sinica, 2017, 75(6): 617. DOI:10.6023/A17030082
(  0) 0) |
| [29] |
Manceau A, Matynia A. The nature of Cu bonding to natural organic matter[J]. Geochimica et Cosmochimica Acta, 2010, 74(9): 2556-2580. DOI:10.1016/j.gca.2010.01.027
(  0) 0) |
 2021, Vol. 58
2021, Vol. 58


