2. 中国农业大学资源与环境学院, 农田土壤污染防控与修复北京市重点实验室, 北京 100193;
3. 桓台县农业农村局, 山东桓台 256400
2. Beijing Key Laboratory of Farmland Soil Pollution Prevention and Remediation, College of Resources and Environmental Sciences, China Agricultural University, Beijing 100193, China;
3. Agricultural and Rural Bureau of Huantai County, Huantai, Shandong 256400, China
土壤碳库包括有机碳(Soil organic carbon,SOC)和无机碳(Soil inorganic carbon,SIC)库,全球1 m深土层碳储量分别为1 350和950 Pg C,SIC含量以CaCO3占绝对优势[1]。我国SIC库储量(60 Pg C)是SOC库量(50 Pg C)的1.2倍,主要分布在西北和华北地区[2]。通常认为SIC较为稳定,以往研究土壤碳循环多集中在SOC方面,较少考虑SIC的作用[3-4]。随着土壤碳循环研究的深入,人们发现SIC也可以成为碳源(碳酸盐溶解释放)[4-5]或碳汇(次生性碳酸盐形成)[3,6-7]。然而,以往大多数研究忽略了SIC溶解对土壤CO2的贡献,这可能导致对SOC分解的高估,势必造成SOC激发效应评估的不确定[8-9]。
土壤原有碳库背景值很大,土壤碳库的短期变化相对较小,很难直接通过测定土壤碳含量来定量土壤碳库的短期改变,测定土壤CO2的释放是其中比较有效的间接方法[10]。在种植植物的石灰性土壤上,土壤CO2释放源有三个,即外源根源呼吸以及土壤SOC分解和SIC溶解的释放,三源区分CO2是定量土壤碳平衡和SOC激发效应的前提。三源碳释放体系的存在,给土壤CO2区分研究带来挑战,准确三源划分CO2已成为土壤碳释放和平衡评估研究的难点[11-12]。相对于传统的两源区分,三源区分还缺乏系统研究,在石灰性土壤上的研究更是较少[13-14]。
本研究以华北平原石灰性农田土壤为研究对象,利用13C示踪结合自然丰度法,区分玉米根际土壤CO2的来源。研究在玉米关键生育期(拔节期、抽穗期和灌浆期)进行13CO2脉冲标记,先利用13C示踪技术量化玉米转移到地下各组分(根系、SOC和土壤CO2)的光合碳量,用两源法区分根际土壤释放CO2中源于根系和土壤的比例[15],进而利用秸秆碳、SOC和SIC之间的δ13C差异,借助13C三元混合模型,把土壤总碳的分解拆分为SIC溶解与SOC分解[16-17],以期提高华北石灰性农田土壤碳预算评估的准确性。
1 材料与方法 1.1 玉米种植玉米盆栽在中国农业大学西校区温室进行。供试土壤取自中国农业大学曲周试验站农田(0~20 cm),为始成土,具有粉质壤土结构(砂粒62%,粉粒28%,黏粒10%),相关性质见表 1。土壤风干后,磨碎、挑根,过5 mm筛。每盆(直径20 cm×高度35 cm)装风干土9.5 kg,盆内土层深度约27 cm。按N 0.55、P 0.19、K 0.31 g·kg-1风干土的比例预拌肥料(相当于耕层的田间肥料施用量),作为底肥一次性施入。播种前先将玉米(Zea mays L.,纪元1号)种子放在清水中浸泡12 h,然后再取两粒浅埋入盆内土壤中。幼苗生长至三叶期时,每盆留1株。用称重法控制土壤水分,根据玉米不同生育期对水分的需求特点,分别在苗期(播种后0~23 d)、拔节期(24~53 d)、抽穗期(54~66 d)和灌浆期(67~99 d)四个阶段,调整土壤含水量为田间持水量(0.31 g·g-1)的60%、70%~75%、75%~80%和70%~75%。
|
|
表 1 供试土壤的理化性质 Table 1 Physical and chemical properties of the test soil |
分别在玉米出苗后29 d(拔节期)、57 d(抽穗期)和72 d(灌浆期)进行标记。每次选取3盆玉米,放入透明塑料制成的密闭标记室进行13CO2脉冲标记[15,18](图 1)。(1)标记前,连接CO2吸收装置,让标记过程中根际呼吸产生的13CO2汇聚在盆内;(2)将盆内土壤和隔板间的空气与标记室空气隔开,隔板和PVC柱的接合处用凡士林密封,并在玉米基部与隔板的间隙中涂上凡士林密封;(3)检查密闭性:将气球套在一空心管上,在另一端管口用吸耳球鼓气,根据气球膨胀状态判定密封性好坏,然后将空心管管口堵住;(4)将风扇、玉米植株和13C丰度为98%的Ba13CO3放入标记室内,然后将整个标记室密封;(5)标记于上午09:00点开始,用注射器向装有Ba13CO3的烧杯中注入一定量1 mol·L-1的HCl溶液(根据Ba13CO3计算HCl的用量),然后开始标记。此后每隔一段时间,根据CO2检测仪的读数确定是否加入盐酸,当CO2的浓度低于200×10-6时,向烧杯中注入HCl,维持CO2浓度在360×10-6左右,标记时间持续7 h。
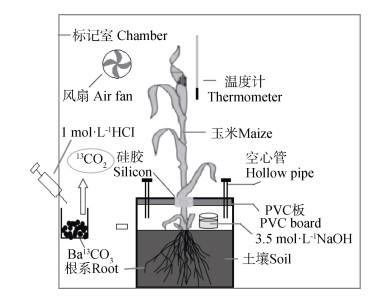
|
图 1 13CO2脉冲标记和土壤CO2吸收装置 Fig. 1 Diagrams of the 13C pluse labeling and soil CO2 absorption devices |
在27 d的示踪期内(从玉米开始标记到破坏性取样),用中性硅酮胶对隔板和PVC盆的接合处进行密封,在茎与隔板的间隙同样涂抹真空绝缘硅树脂(图 1)。每3天更换1次3.5 mol·L-1的NaOH溶液,在27 d示踪期内共进行9次动态取样,定期用空气泵在土壤与隔板间注入一定量无CO2的空气,为玉米地下部提供氧气。以酚酞作指示剂,用稀盐酸滴定NaOH溶液中未参加反应的NaOH,根据稀盐酸和NaOH的体积、浓度,计算土壤CO2释放量。将过量BaCl2溶液加入到土壤CO2的NaOH溶液中,形成BaCO3沉淀,于60 ℃下烘干至恒重,将每次取样的BaCO3沉淀等比例混合,搅拌混匀形成BaCO3沉淀悬浊液,用注射器先吸取适量悬浊液,然后吸取3 mL 2 mol·L-1的H2SO4溶液,化学反应生成CO2,将针头立刻插入到带橡皮塞的5 mL真空采气瓶中,待注射器中生成的CO2达到7 mL时,拔出针头,把CO2注入到采气瓶中,用DELTAplus XP型质谱仪测定CO2-δ13C值。
1.4 植株与土壤的取样和测定经过27 d的示踪期,分别在玉米出苗后56、84和99 d时,破坏性取样,从玉米基部剪断植株,将盆中土壤反复过2 mm筛,剔除根系、研磨,过0.15 mm筛,测定根系的δ13C值。取约20 g土壤置于白色板上,剔除残留细根后加入50 mL的3 mol·L-1的HCl溶液,用于去除土壤中的碳酸盐。充分搅拌并静止2 d后,以3 000 r·min-1的转速离心3 min,将上清液倒掉,重复此过程,洗至上清液中性为止,并将酸化前的上清液倒回烧杯中,在60℃条件下烘干,研磨,过0.15 mm筛,用DELTAplus XP型质谱仪测定SOC-δ13C值。SIC-δ13C值测定:在70℃,于真空系统中将土壤样品与100%的H3PO4反应3 h,用DELTAplus XP型质谱仪分析生成CO2的δ13C值。碳同位素值采用PDB(Peedee Belemnite)标准。
1.5 计算方法1)量化地下部的光合碳输入和两源区分根际CO2释放(13C脉冲标记法)[15,19]。
分别在拔节期、抽穗期和灌浆期,对玉米进行13C脉冲标记后,地上部光合固定的13C转运到地下部各组分,包括根系、根际沉积物和土壤CO2释放,则进入地下各组分的13C量为:
| ${}^{13}{C_{\rm{i}}} = {C_{\rm{i}}} \times \frac{{\left( {{F_{\rm{i}}}{\rm{ - }}{F_{{\rm{NL}}}}} \right)}}{{100}} \times 1000$ | (1) |
式中,13Ci和Ci分别为各组分的13C量(mg·pot-1)和C量(g·pot-1),Fi和FNL为各组分标记和不标记的13C百分含量。
各个时期输入到地下各组分的光合碳量(g·pot-1):
| $ S{C}_{\rm{i}}=\Delta {C}_{地上部}\times \frac{{}^{13}{C}_{\rm{i}}}{{}^{13}{C}_{地上部}}$ | (2) |
式中,ΔC地上部为各生育时期内地上部的生物碳量变化,以地上部作为参照是考虑到地上部的生物碳量可以准确定量。
通过式(2)定量根源呼吸(CRoot),进而土壤CO2(CT)划分为土壤源CO2(CSoil)和根源呼吸:
| ${C_{\rm{T}}} = {C_{{\rm{Root}}}} + {C_{{\rm{Soil}}}}$ | (3) |
| $1 = {F_{{\rm{Root}}}} + {F_{{\rm{Soil}}}}$ | (4) |
式中,FRoot和FSoil分别为根源呼吸和土壤总碳释放占根际释放CO2的比值。
2)根据玉米根碳、SOC与SIC之间的δ13C差异(分别为-14.1‰、-3.4‰和-22.2‰),借助13C三元混合模型,进一步两源划分土壤源CO2(13C自然丰度结合13C脉冲标记法)[16-17]。
| ${F_{{\rm{Soil}}}} = {F_{{\rm{SIC}}}} + {F_{{\rm{SOC}}}}$ | (5) |
| ${\delta _{{\rm{C}}{{\rm{O}}_{\rm{2}}}}} = {\delta _{{\rm{Root}}}}{F_{{\rm{Root}}}} + {\delta _{{\rm{SOC}}}}{F_{{\rm{SOC}}}} + {\delta _{{\rm{SIC}}}}{F_{{\rm{SIC}}}}$ | (6) |
式中,FSIC和FSOC分别为SIC源和SOC源CO2占根际CO2释放的比值(未知量),δCO₂、δRoot、δSIC和δSOC分别为根际释放CO2、根系、SIC和SOC的δ13C值(已知量)。
1.6 数据分析采用Excel 2013软件作图,图数据的表达形式为平均值±标准误。方差分析采用SPSS 17.0软件计算。生物量、根系占植株干重的比值、光合13C分配在不同生育期的之间的显著性差异分析比较采用最小显著差异法(Least Significant Difference,LSD;P < 0.05水平)。
2 结果 2.1 不同生育期玉米生物量变化玉米从拔节到灌浆期,根系生物量无显著变化,而地上部和整个植株生物量在抽穗期达到最大,然后维持不变(图 2a)。从拔节期到灌浆期,根系生物量/整个植株生物量的比值显著递减(图 2b)。
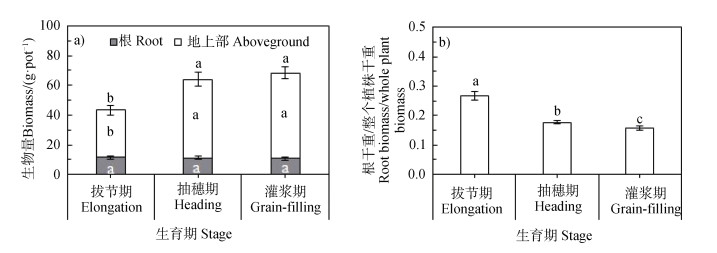
|
注:不同小写字母表示同一组分不同生育期间的显著性差异(P < 0.05),下同。 Note:Different lowercase letters indicate significant difference between growth stages at P < 0.05 level,the same below. 图 2 玉米各生育时期的生物量和根系占植株总重的比值 Fig. 2 Biomass and ratio of root/total in plant weight relative to maize growth stage |
在玉米关键生育期进行13C脉冲标记,地上部保留了大部分的光合13C,并且随着玉米生长,地上部的13C分配比例显著增加,而输入到地下部的13C分配比例显著递减(图 3)。从拔节期到灌浆期,玉米地上部输入到地下部各组分(根系、根际沉积碳和根源呼吸)的光合碳量呈递减趋势,拔节期的各组分碳输入显著高于后两个生育期,后两个生育期之间无显著差异(图 4)。
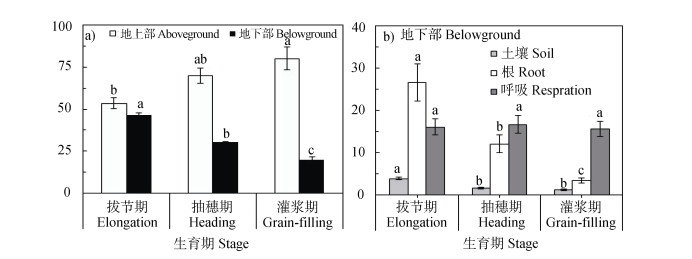
|
图 3 光合固定13C在玉米—土壤系统中各组分中的分配 Fig. 3 Proportion of photosynthesized 13C in each component of the maize-soil system |
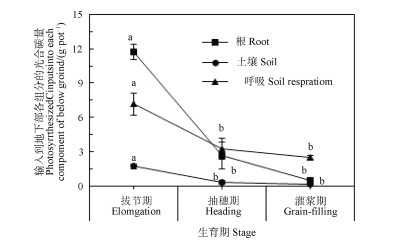
|
图 4 玉米在不同生育期内输入到地下各组分的碳量 Fig. 4 Photosynthesized C inputs into each component of the maize-soil system at various growth stages of maize |
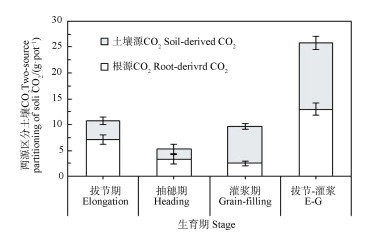
基于13CO2脉冲标记量化的根源呼吸量(图 4),玉米根际的CO2可以划分为根源和土壤源CO2。从玉米拔节期到灌浆期,根源呼吸占土壤CO2的比例呈降低趋势,即从拔节期的66.7%降低至灌浆期的25.8%,在该生长阶段,根源呼吸累计排放量占土壤CO2的比例约为50%(图 5)。
|
注:E-G代表从拔节期到灌浆期,下同。 Note:E-G stands for growth stage from elongation to grain-filling,the same below. 图 5 利用13CO2脉冲标记两源区分玉米根际CO2释放 Fig. 5 Two-source partitioning of CO2 emission from maize rhizosphere with the aid of 13CO2 pulse labeling technique |
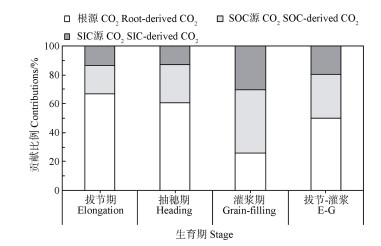
在两源法区分根际CO2的基础上(图 5),借助13C三元混合模型(13C自然丰度法),将土壤源CO2进一步拆分为SOC和SIC源CO2(图 6),发现SOC和SIC源CO2占土壤CO2的贡献比例在灌浆期较高(SIC和SOC源的贡献比例分别为44.1%和30.1%),在前两个生育期较小(SIC和SOC源的平均比例分别为23.1%和13.2%)。从拔节期到灌浆期,SOC和SIC源的累计CO2占根际CO2排放的贡献比例约为30%和20%。
|
图 6 利用13CO2脉冲标记结合自然丰度三源区分玉米根际CO2释放 Fig. 6 Three-source partitioning of CO2 emission from maize rhizosphere with the aid of 13CO2 pulse labeling combined with 13C natural abundance techniques |
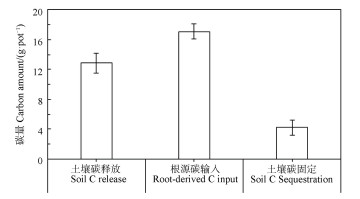
从拔节期到生育期末,玉米生长对土壤转移的光合碳量为17.1 g·pot-1(根系+根际沉积物),土壤本身碳(SOC+SIC)释放碳量为12.9 g·pot-1,因此土壤净固定碳量为4.2 g·pot-1(土壤光合碳输入量减去土壤本身碳释放;图 7)。
|
图 7 玉米从拔节到灌浆期间的土壤碳收支平衡 Fig. 7 Soil C budgeting in maize-planted soil from elongation to grain-filling stages |
区分土壤CO2是量化根源呼吸和土壤本身碳释放的前提,13C/14C同位素可以有效划分CO2的排放来源[10,12]。由于单一13C/14C通常仅可以两源区分土壤CO2,相对于传统的两源法,三源法区分CO2技术挑战较大,一直是研究土壤有机质激发效应的难点。通过同位素三源区分土壤CO2组分,一般通过如下7种途径(表 2):1)稳定同位素溯源软件[20-21],2)附加法[13-14],3)13C源合并和组合法[22],4)13C和18O自然丰度法[23],5)14C标记结合13C自然丰度[11-12,24],6)14C和13C双标记[25-26],以及7)13C标记和自然丰度[16-17]。根据同位素线性混合模型,n个同位素,仅适用于精确区分n+1个源的贡献比例[20],因此,采用13C和14C两种C同位素(14C标记结合13C自然丰度、14C和13C双标记),可以较为精确三源区分土壤CO2释放来源。在以上区分方法中,最精准的是14C和13C双标记方法,它可以克服13C自然丰度方法中同位素分馏效应的干扰,也不需要特定的C3/C4植物和土壤条件[25-26]。然而,14C材料有一定辐射危害,受到高度监管,仅限于室内实验[18]。与14C和13C双标记相比,利用不同13C丰度的材料是一个替代方案,例如本研究采用13C标记玉米和13C自然丰度方法三源区分的根际土壤CO2[16-17]:首先13C标记玉米量化根源呼吸和土壤源CO2比例[15],然后借助13C三元混合模型(13C自然丰度玉米),将土壤源CO2进一步划分为源于SOC和SIC的贡献比例。这个方法中由于土壤碳(SOC和SIC)与13C自然丰度根系之间的δ13C差异太小,13C分馏效应存在一定不确定性[22]。
|
|
表 2 三源区分土壤释放CO2的方法比较 Table 2 Comparison of the methods for partitioning soil CO2 emission by three source |
通过对全球23篇关于SIC释放的文献整合分析,发现SIC溶解对土壤总碳(SIC+SOC)释放的贡献在3%~95%之间,平均值为46%,95%置信区间分布为37%~55%(表 3),与本研究的结果接近,即在整个玉米旺盛生长阶段(从拔节到生育期末),SIC源CO2占土壤源CO2的比例为40%(图 6),这就说明SIC溶解对稳定全球碳库和调节CO2浓度方面非常重要[27],然而,以往研究较少考虑SIC溶解对CO2排放的贡献,这将导致SOC分解的高估,影响SOC激发效应的定量[8-9, 28]。
|
|
表 3 碳酸盐溶解对土壤CO2释放的贡献比较 Table 3 Contribution of carbonate dissolution to soil CO2 emission |
随着我国农业集约化水平不断提高,铵态氮肥和尿素用量也不断增加,通过硝化作用产生H+,影响CO2 -HCO3– -CaCO3的弱酸平衡体系,属于强酸与碳酸盐的直接反应,加剧碳酸盐的溶解[37-39, 50]。因此,今后有必要量化氮肥投入对华北农田SIC溶解的影响,提高区域石灰性农田土壤碳预算的准确性。
性土壤中存在着CO2(气相)-HCO3–(液相)-CaCO3(固相)的三相平衡,SOC分解、根源呼吸与SIC溶解/沉淀平衡之间存在耦合关系,体现在SOC分解和根源呼吸产生的CO2,既可以驱动碳酸盐的溶解与释放,也可以与钙、镁离子结合形成次生性碳酸盐[3-4, 7]:
| $ \text{CaCO}_{3(}固相)+ \text{H}_{2}\text{O} + \text{CO}_{2}(气相)≒2{\text{HCO}_{3}}^{−(}液相)+ \text{Ca}^{2+} $ | (7) |
本研究中,根源呼吸占根际土壤CO2的贡献比例为50%。玉米生长增加土壤中CO2分压,可能加剧SIC的溶解与释放。此外,根系生长也可以分泌质子和有机酸,促进SIC的溶解[13, 51]。因此,玉米根际效应对SIC溶解的影响是不可忽视的。
3.3 玉米生长对土壤碳平衡的影响尽管作物生长可以产生正根际效应,促进SOC分解和SIC溶解,但引发正根际效应的植物来源的有机碳(根际沉积碳和根系)并未被微生物完全分解释放到大气中,残留部分可以补充因根际效应引起的SOC和SIC损失[52],在本研究中土壤碳收支平衡表现为碳汇。在石灰性土壤,作物生长不仅可以贡献SOC的截存[15, 53-54],还可以贡献SIC的次生形成[44],但由于土壤SIC背景太大和13C检测限(约为10–7 mol)较高,本研究在SIC中没有检测到标记的13C信号[15],今后可以考虑用检测限更加敏感的14C(约为10–13 mol)来解决[55-56]。
4 结论对于石灰性土壤,从玉米拔节期到生育期末,根源呼吸和土壤总碳(SIC+SOC)释放对根际土壤CO2释放的贡献比值为1:1,SIC溶解对土壤源释放CO2的贡献比例约为40%,说明SIC溶解对于土壤碳库稳定和大气CO2浓度调节具有双重作用。若忽视SIC溶解对土壤CO2释放的贡献,有可能导致对SOC分解的高估,进而影响土壤碳平衡的评估。从拔节期到生育期末,玉米生长对地下部的光合碳输入(根系+根际沉积物)超过土壤总碳(SIC+SOC)释放的损失,土壤表现为碳汇。
| [1] |
Lal R. Soil carbon sequestration impacts on global climate change and food security[J]. Science, 2004, 304(5677): 1623-1627. DOI:10.1126/science.1097396
(  0) 0) |
| [2] |
Pan G X. Study on carbon reservoir in soils of China (In Chinese)[J]. Bulletin of Science and Technology, 1999, 15(5): 330-332. DOI:10.3969/j.issn.1001-7119.1999.05.002 [潘根兴. 中国土壤有机碳和无机碳库量研究[J]. 科技通报, 1999, 15(5): 330-332.]
(  0) 0) |
| [3] |
Bughio M A, Wang P L, Meng F Q, et al. Neoformation of pedogenic carbonate and conservation of lithogenic carbonate by farming practices and their contribution to carbon sequestration in soil[J]. Journal of Plant Nutrition and Soil Science, 2017, 180(4): 454-463. DOI:10.1002/jpln.201500650
(  0) 0) |
| [4] |
Zamanian K, Zarebanadkouki M, Kuzyakov Y. Nitrogen fertilization raises CO2 efflux from inorganic carbon: A global assessment[J]. Global Change Biology, 2018, 24(7): 2810-2817. DOI:10.1111/gcb.14148
(  0) 0) |
| [5] |
Liu S S, Zhou L H, Li H, et al. Shrub encroachment decreases soil inorganic carbon stocks in Mongolian grasslands[J]. Journal of Ecology, 2020, 108(2): 678-686. DOI:10.1111/1365-2745.13298
(  0) 0) |
| [6] |
Wang X J, Xu M G, Wang J P, et al. Fertilization enhancing carbon sequestration as carbonate in arid cropland: Assessments of long-term experiments in Northern China[J]. Plant and Soil, 2014, 380(1/2): 89-100.
(  0) 0) |
| [7] |
Dong X L, Singh B P, Li G T, et al. Biochar increased field soil inorganic carbon content five years after application[J]. Soil and Tillage Research, 2019, 186: 36-41. DOI:10.1016/j.still.2018.09.013
(  0) 0) |
| [8] |
Chen L, Liu L, Qin S, et al. Regulation of priming effect by soil organic matter stability over a broad geographic scale[J]. Nature Communications, 10(1): 5112. DOI:10.1038/s41467-019-13119-z
(  0) 0) |
| [9] |
Fang Y Y, Singh B P, Collins D, et al. Nutrient stoichiometry and labile carbon content of organic amendments control microbial biomass and carbon-use efficiency in a poorly structured sodic-subsoil[J]. Biology and Fertility of Soils, 2020, 56(2): 219-233. DOI:10.1007/s00374-019-01413-3
(  0) 0) |
| [10] |
Kuzyakov Y. Review: Factors affecting rhizosphere priming effects[J]. Journal of Plant Nutrition and Soil Science, 2002, 165(4): 382-396. DOI:10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-#
(  0) 0) |
| [11] |
Tian J, Pausch J, Yu G R, et al. Aggregate size and glucose level affect priming sources: A three-source-partitioning study[J]. Soil Biology and Biochemistry, 2016, 97: 199-210. DOI:10.1016/j.soilbio.2016.03.013
(  0) 0) |
| [12] |
Cui J, Ge T, Kuzyakov Y, et al. Interactions between biochar and litter priming: A three-source 14C and δ13C partitioning study[J]. Soil Biology and Biochemistry, 2017, 104: 49-58. DOI:10.1016/j.soilbio.2016.10.014
(  0) 0) |
| [13] |
Tamir G, Shenker M, Heller H, et al. Can soil carbonate dissolution lead to overestimation of soil respiration?[J]. Soil Science Society of America Journal, 2011, 75(4): 1414-1422. DOI:10.2136/sssaj2010.0396
(  0) 0) |
| [14] |
Fang Y Y, Singh B P, Collins D, et al. Nutrient stoichiometry and labile carbon content of organic amendments control microbial biomass and carbon-use efficiency in a poorly structured sodic-subsoil[J]. Biology and Fertility of Soils, 2020, 56(2): 219-233. DOI:10.1007/s00374-019-01413-3
(  0) 0) |
| [15] |
Sun Z A, Wu S X, Zhang Y W, et al. Effects of nitrogen fertilization on pot-grown wheat photosynthate partitioning within intensively farmed soil determined by 13C pulse-labeling[J]. Journal of Plant Nutrition and Soil Science, 2019, 182(6): 896-907. DOI:10.1002/jpln.201800603
(  0) 0) |
| [16] |
Whitman T, Lehmann J. A dual-isotope approach to allow conclusive partitioning between three sources[J]. Nature Communications, 2015, 6: 8708. DOI:10.1038/ncomms9708
(  0) 0) |
| [17] |
Kerré B, Hernandez-Soriano M C, Smolders E. Partitioning of carbon sources among functional pools to investigate short-term priming effects of biochar in soil: A 13C study[J]. Science of the Total Environment, 2016, 547: 30-38. DOI:10.1016/j.scitotenv.2015.12.107
(  0) 0) |
| [18] |
Sun Z, Chen Q, Han X, et al. Allocation of photosynthesized carbon in an intensively farmed winter wheat-soil system as revealed by 14CO2 pulse labelling[J]. Scientific Reports, 8(1): 3160. DOI:10.1038/s41598-018-21547-y
(  0) 0) |
| [19] |
Hafner S, Unteregelsbacher S, Seeber E, et al. Effect of grazing on carbon stocks and assimilate partitioning in a Tibetan montane pasture revealed by 13CO2 pulse labeling[J]. Global Change Biology, 2012, 18(2): 528-538. DOI:10.1111/j.1365-2486.2011.02557.x
(  0) 0) |
| [20] |
Phillips D L, Gregg J W. Source partitioning using stable isotopes: Coping with too many sources[J]. Oecologia, 2003, 136(2): 261-269. DOI:10.1007/s00442-003-1218-3
(  0) 0) |
| [21] |
Plestenjak G, Eler K, Vodnik D, et al. Sources of soil CO2 in calcareous grassland with woody plant encroachment[J]. Journal of Soils and Sediments, 2012, 12(9): 1327-1338. DOI:10.1007/s11368-012-0564-3
(  0) 0) |
| [22] |
Kuzyakov Y, Bol R. Three sources of CO2 efflux from soil partitioned by 13C natural abundance in an incubation study[J]. Rapid Communications in Mass Spectrometry, 2005, 19(11): 1417-1423. DOI:10.1002/rcm.1938
(  0) 0) |
| [23] |
Lin G H, Ehleringer J R, Rygiewicz P T, et al. Elevated CO2 and temperature impacts on different components of soil CO2 efflux in Douglas-fir terracosms[J]. Global Change Biology, 1999, 5(2): 157-168. DOI:10.1046/j.1365-2486.1999.00211.x
(  0) 0) |
| [24] |
Blagodatskaya E, Yuyukina T, Blagodatsky S, et al. Three-source-partitioning of microbial biomass and of CO2 efflux from soil to evaluate mechanisms of priming effects[J]. Soil Biology and Biochemistry, 2011, 43(4): 778-786. DOI:10.1016/j.soilbio.2010.12.011
(  0) 0) |
| [25] |
Shahbaz M, Kumar A, Kuzyakov Y, et al. Priming effects induced by glucose and decaying plant residues on SOM decomposition: A three-source 13C/14C partitioning study[J]. Soil Biology and Biochemistry, 2018, 121: 138-146. DOI:10.1016/j.soilbio.2018.03.004
(  0) 0) |
| [26] |
Shahbaz M, Kumar A, Kuzyakov Y, et al. Interactive priming effect of labile carbon and crop residues on SOM depends on residue decomposition stage: Three-source partitioning to evaluate mechanisms[J]. Soil Biology and Biochemistry, 2018, 126: 179-190. DOI:10.1016/j.soilbio.2018.08.023
(  0) 0) |
| [27] |
Zamanian K, Kuzyakov Y. Contribution of soil inorganic carbon to atmospheric CO2: More important than previously thought[J]. Global Change Biology, 2019, 25(1): e1-e3. DOI:10.1111/gcb.14463
(  0) 0) |
| [28] |
Meng F Q, Dungait J A J, Xu X L, et al. Coupled incorporation of maize(Zea mays L.)straw with nitrogen fertilizer increased soil organic carbon in Fluvic Cambisol[J]. Geoderma, 2017, 304: 19-27. DOI:10.1016/j.geoderma.2016.09.010
(  0) 0) |
| [29] |
Stevenson B A, Verburg P S J. Effluxed CO2-13C from sterilized and unsterilized treatments of a calcareous soil[J]. Soil Biology and Biochemistry, 2006, 38(7): 1727-1733. DOI:10.1016/j.soilbio.2005.11.028
(  0) 0) |
| [30] |
Bertrand I, Delfosse O, Mary B. Carbon and nitrogen mineralization in acidic, limed and calcareous agricultural soils: Apparent and actual effects[J]. Soil Biology and Biochemistry, 2007, 39(1): 276-288. DOI:10.1016/j.soilbio.2006.07.016
(  0) 0) |
| [31] |
Biasi C, Lind S E, Pekkarinen N M, et al. Direct experimental evidence for the contribution of lime to CO2 release from managed peat soil[J]. Soil Biology and Biochemistry, 2008, 40(10): 2660-2669. DOI:10.1016/j.soilbio.2008.07.011
(  0) 0) |
| [32] |
Inglima I, Alberti G, Bertolini T, et al. Precipitation pulses enhance respiration of Mediterranean ecosystems: The balance between organic and inorganic components of increased soil CO2 efflux[J]. Global Change Biology, 2009, 15(5): 1289-1301. DOI:10.1111/j.1365-2486.2008.01793.x
(  0) 0) |
| [33] |
Ramnarine R, Wagner-Riddle C, Dunfield K E, et al. Contributions of carbonates to soil CO2 emissions[J]. Canadian Journal of Soil Science, 2012, 92(4): 599-607. DOI:10.4141/cjss2011-025
(  0) 0) |
| [34] |
Chevallier T, Cournac L, Hamdi S, et al. Temperature dependence of CO2 emissions rates and isotopic signature from a calcareous soil[J]. Journal of Arid Environments, 2016, 135: 132-139. DOI:10.1016/j.jaridenv.2016.08.002
(  0) 0) |
| [35] |
Lardner T, George S, Tibbett M. Interacting controls on innate sources of CO2 efflux from a calcareous arid zone soil under experimental acidification and wetting[J]. Journal of Arid Environments, 2015, 122: 117-123. DOI:10.1016/j.jaridenv.2015.07.001
(  0) 0) |
| [36] |
董燕婕. 塿土剖面不同碳库贮量及释放特性研究[D]. 陕西杨凌: 西北农林科技大学, 2013. Dong Y J. Carbon stock and stabilization in Lou soil[D]. Yangling, Shaanxi: Northwest Agricultural and Forestry University, 2013. (  0) 0) |
| [37] |
于伟家. 施用氮肥对石灰性土壤碳释放及其来源研究[D]. 陕西杨凌: 西北农林科技大学, 2018. Yu W J. Effects of application of nitrogen fertilizer on carbon emissions and their sources from calcareous soils[D]. Yangling, Shaanxi: Northwest Agricultural and Forestry University,, 2018. (  0) 0) |
| [38] |
Meng Y, Cai M, Shi Q Y, et al. Effects of nitrogen fertilizer application on carbon dioxide emission from calcareous soil (In Chinese)[J]. Chinese Journal of Soil Science, 2015, 46(4): 948-954. [孟延, 蔡苗, 师倩云, 等. 氮肥用量对石灰性土壤二氧化碳释放的影响[J]. 土壤通报, 2015, 46(4): 948-954.]
(  0) 0) |
| [39] |
Meng Y, Li X S, Hao P Q, et al. Effect of different N fertilizer applications on CO2 emissions from Lou soil in Central Shaanxi (In Chinese)[J]. Journal of Agro-Environment Science, 2017, 36(9): 1901-1907. [孟延, 李雪松, 郝平琦, 等. 施用不同种类氮肥对陕西关中地区塿土碳释放的影响[J]. 农业环境科学学报, 2017, 36(9): 1901-1907.]
(  0) 0) |
| [40] |
Čater M, Ogrinc N. Soil respiration rates and in natural beech forest(Fagus sylvatica L.)in relation to stand structure[J]. Isotopes in Environmental and Health Studies, 2011, 47(2): 221-237. DOI:10.1080/10256016.2011.578214
(  0) 0) |
| [41] |
Dumale Jr W, Miyazaki T, Hirai K, et al. SOC turnover and lime-CO2 evolution during liming of an acid andisol and ultisol[J]. Open Journal of Soil Science, 2011, 1(2): 49-53. DOI:10.4236/ojss.2011.12007
(  0) 0) |
| [42] |
Buysse P, Goffin S, Carnol M, et al. Short-term temperature impact on soil heterotrophic respiration in limed agricultural soil samples[J]. Biogeochemistry, 2013, 112: 441-455. DOI:10.1007/s10533-012-9739-7
(  0) 0) |
| [43] |
Schindlbacher A, Borken W, Djukic I, et al. Contribution of carbonate weathering to the CO2 efflux from temperate forest soils[J]. Biogeochemistry, 2015, 124: 273-290. DOI:10.1007/s10533-015-0097-0
(  0) 0) |
| [44] |
Zhang C H, Sun Y Y, Tang G Y, et al. Contributions of carbonates to carbon dioxide release from a calcareous soil in response to experimental warming[J]. Soil Science, 2019, 184(2): 52-59. DOI:10.1097/SS.0000000000000251
(  0) 0) |
| [45] |
Cardinael R, Chevallier T, Guenet B, et al. Organic carbon decomposition rates with depth and contribution of inorganic carbon to CO2 emissions under a Mediterranean agroforestry system[J]. European Journal of Soil Science, 2019. DOI:10.1111/ejss.12908
(  0) 0) |
| [46] |
Fang Y Y, Singh B P, Collins D, et al. Nutrient stoichiometry and labile carbon content of organic amendments control microbial biomass and carbon-use efficiency in a poorly structured sodic-subsoil[J]. Biology and Fertility of Soils, 2020, 56(2): 219-233. DOI:10.1007/s00374-019-01413-3
(  0) 0) |
| [47] |
Ma J, Li Y M, Liu R. The abiotic contribution to total CO2 flux for soils in arid zone[J]. Biogeosciences Discussions, 2015, 12(14): 11217-11244.
(  0) 0) |
| [48] |
Ma J, Liu R, Li Y. Abiotic contribution to total soil CO2 flux across a broad range of land-cover types in a desert region[J]. Journal of Arid Land, 2017, 9(1): 13-26. DOI:10.1007/s40333-016-0061-4
(  0) 0) |
| [49] |
Wang Z Y, Xie J B, Wang Y G, et al. Biotic and abiotic contribution to diurnal soil CO2 fluxes from saline/alkaline soils[J]. Scientific Reports, 10: 5396. DOI:10.1038/s41598-020-62209-2
(  0) 0) |
| [50] |
Raza S, Miao N, Wang P Z, et al. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands[J]. Global Change Biology, 2020, 26(6): 3738-3751. DOI:10.1111/gcb.15101
(  0) 0) |
| [51] |
Tamir G, Shenker M, Heller H, et al. Dissolution and re-crystallization processes of active calcium carbonate in soil developed on tufa[J]. Soil Science Society of America Journal, 2012, 76(5): 1606-1613. DOI:10.2136/sssaj2012.0041
(  0) 0) |
| [52] |
Sun Y, Xu X L, Kuzyakov Y. Mechanisms of rhizosphere priming effects and their ecological significance (In Chinese)[J]. Chinese Journal of Plant Ecology, 2014, 38(1): 62-75. [孙悦, 徐兴良, Kuzyakov Y. 根际激发效应的发生机制及其生态重要性[J]. 植物生态学报, 2014, 38(1): 62-75.]
(  0) 0) |
| [53] |
An T T, Schaeffer S, Li S Y, et al. Carbon fluxes from plants to soil and dynamics of microbial immobilization under plastic film mulching and fertilizer application using 13C pulse-labeling[J]. Soil Biology and Biochemistry, 2015, 80: 53-61. DOI:10.1016/j.soilbio.2014.09.024
(  0) 0) |
| [54] |
Ge T D, Liu C, Yuan H Z, et al. Tracking the photosynthesized carbon input into soil organic carbon pools in a rice soil fertilized with nitrogen[J]. Plant and Soil, 2015, 392(1/2): 17-25.
(  0) 0) |
| [55] |
Gocke M, Pustovoytov K, Kuzyakov Y. Pedogenic carbonate recrystallization assessed by isotopic labeling: A comparison of 13C and 14C tracers[J]. Journal of Plant Nutrition and Soil Science, 2011, 174(5): 809-817. DOI:10.1002/jpln.200900341
(  0) 0) |
| [56] |
Kuzyakov Y, Domanski G. Carbon input by plants into the soil. Review[J]. Journal of Plant Nutrition and Soil Science, 2000, 163(4): 421-431. DOI:10.1002/1522-2624(200008)163:4<421::AID-JPLN421>3.0.CO;2-R
(  0) 0) |
 2021, Vol. 58
2021, Vol. 58


