2. 中国农业科学院农业资源与农业区划研究所/农业农村部植物营养与肥料重点实验室, 北京 100081
2. Key Laboratory of Plant Nutrition and Fertilizer, Ministry of Agriculture and Rural Affairs/Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing 100081, China
绿肥在提高土壤肥力、改善土壤质量、防止水土流失、资源高效利用等方面具有重要作用,发展绿肥被认为是建立生态可持续农业的重要策略[1]。在我国南方稻区,应用冬闲田种植绿肥是传统的培肥增产措施,大量研究表明,绿肥-水稻轮作体系能够有效保障水稻稳产、高产,并可在节肥20%~40%的条件下保证作物不减产[2-5]。紫云英、黑麦草和油菜是南方稻田常见的绿肥品种,在一般的土壤条件下,这三种绿肥鲜草产量分别可达到平均22 000~30 000 kg⋅hm–2、30 000~40 000 kg⋅hm–2和20 000~30 000 kg⋅hm–2,其植株体在盛花期的碳氮比(C/N)分别约为12~15、40~50和20~25。不同作物生长特性和植株体组成特征不同,因此会引起对土壤过程不同的影响[6]。连续30年的长期定位试验结果表明,种植翻压紫云英、黑麦草和油菜三种冬绿肥较冬闲田水稻增产18.8%~28.8%,其中紫云英的增产效果最佳[4]。
硝化作用是农田生态系统中氮循环的重要步骤,氨氧化过程是硝化作用中的第一步和限速步骤,其中氨单加氧酶(AMO)是氨氧化过程中的限速酶[7]。AMO的编码基因amoA同时存在于氨氧化细菌(AOB)和氨氧化古菌(AOA)中[8]。乙炔可致AMO失活进而抑制硝化作用,排除乙炔后AOA和AOB会重新生成AMO,并恢复硝化作用,此时的硝化强度被称为恢复硝化强度(Recovered nitrification potential,RNP),这部分硝化作用主要由AOA和AOB作用完成,可以表明AOA和AOB的硝化能力[9]。大量研究表明,农田土壤中,多种因素影响AOA和AOB的数量和群落结构,包括土壤pH[10-12]、施肥制度[13-14]、土壤温度[15-16]等因素。AOA和AOB在硝化作用中的相对贡献一直有争议,一些研究认为AOB在硝化作用中起主要作用[17-18],另有研究则认为AOA在稻田系统,特别是红壤稻田的硝化作用中发挥了重要作用[19-20]。
稻田土壤为AOA和AOB提供了特殊的生境,是农田生态系统中硝化作用及其微生物生态研究的重要模型[21]。稻田土壤的硝化作用在农田氮素利用中有重要意义,在不同的土壤性状和田间管理措施下,AOA和AOB在硝化作用中的贡献有较大差异[22-23]。在绿肥-水稻轮作系统中,种植翻压绿肥带入土壤大量有机质,其中紫云英、黑麦草和油菜三种绿肥翻压后,带入土壤的纯氮量分别可达到约55~65、30~40和40~50 kg⋅hm–2 [4,24]。这些有机氮的分解极可能产生大量铵态氮,作为底物促进AOA和AOB生长,影响土壤氮素循环。有研究表明在红壤稻田中,冬种不同绿肥改变了AOA和AOB的数量及群落结构,且AOA对绿肥的响应更敏感[24],然而冬种绿肥对硝化过程中AOA和AOB相对贡献的影响及其机制尚不明确,并且针对碱性水稻土中硝化作用的研究仍属空白。本文应用我国典型的碱性水稻土紫潮泥,研究绿肥-水稻轮作体系中的硝化作用特征、AOA和AOB的基因丰度及相对贡献,明确绿肥措施下稻田硝化作用规律和作用机制,丰富稻田养分管理技术和理论的科学基础。
1 材料与方法 1.1 试验处理及实施盆栽试验设4个处理,分别为冬闲-双季稻(WF)、紫云英-双季稻(MV)、黑麦草-双季稻(RG)、油菜-双季稻(RP)。每处理5个重复,采用随机区组方式排列盆钵。盆栽用瓷钵直径27 cm,内高27.5 cm,面积0.057 m2,每盆装10 kg干土,2016年10月9日装盆后加水浸透,第二天播种绿肥作物。供试土壤取自湖南南县,为典型的碱性水稻土紫潮泥,试验开始前土壤pH为8.05,有机质和全氮分别为31.76 g⋅kg–1和1.70 g⋅kg–1,有效磷和速效钾分别为19.53 mg⋅kg–1和97.81 mg⋅kg–1。盆栽试验在湖南省土壤肥料研究所网室中进行,网室温度、湿度等环境与室外基本相同。
绿肥作物于2016年10月10日播种,播种量为紫云英(Astragalus sinicus L.,湘紫1号)37.5 kg⋅hm–2,黑麦草(Lolium multiflorum)15 kg⋅hm–2,油菜(Brassica napus L.,华双5号)7.5 kg⋅hm–2。同时设置冬闲对照,绿肥季所有处理均不施肥。绿肥种植期在网室顶部加盖防雨棚,定期浇水保证含水量在田间持水量的60%左右。2017年3月30日,紫云英和油菜到达盛花期,对各绿肥测产、取样并翻压。紫云英、黑麦草、油菜均原盆翻压,鲜草翻压量分别为33 750、67 500、50 625 kg⋅hm–2,三种绿肥翻压时的植株有机碳含量分别为312 g⋅kg–1、501 g⋅kg–1和391 g⋅kg–1,氮含量分别为24 g⋅kg–1、10 g⋅kg–1和17 g⋅kg–1。翻压24 d后,于4月23日移栽早稻,每盆3蔸,早稻移栽前一天施肥,水稻生长期间定期浇水,保持盆中淹水状态。7月13日收获早稻并移栽晚稻,10月10日收获晚稻。早稻和晚稻施肥量相同,磷钾肥均为田间施肥量的1.5倍,氮肥在田间施肥量60%的基础上加1.5倍施用,施肥量分别为N 135 kg⋅hm–2、P2O5 112.5 kg⋅hm–2、K2O 135 kg⋅hm–2,肥料种类为尿素、过磷酸钙和氯化钾,其中氮肥以基追比1:1的比例,分基肥和追肥两次施用,追肥在水稻分蘖期施用,磷肥和钾肥均做基肥一次性施入。
1.2 土壤样品采集共采集6次样品,分别为绿肥盛花期(S1,3月30日)、早稻移栽前(S2,4月22日,绿肥翻压后23 d)、早稻分蘖期(S3,5月8日)、早稻拔节期(S4,6月6日)、早稻收获期(S5,7月13日)、晚稻收获期(S6,10月10日)。取样时用土钻在盆中取三个点(0~20 cm),混合后取500 g左右鲜土带回实验室4℃冷藏备用。
1.3 测定项目及方法土壤基础理化性状的测定采用《土壤农业化学分析方法》[25]中方法,具体如下:土壤全氮:浓H2SO4消煮,半微量凯氏定氮法测定;土壤有机质:重铬酸钾容量法-外加热法;土壤pH:采用2.5︰1水土比,电位法测定;土壤无机氮:采集的鲜土过2 mm筛,采用2 mol⋅L–1 KCl浸提(土水比为1︰5),连续流动分析仪(AA3,SEAL,德国)测定,同时烘干法测定土壤含水量。
硝化潜势(Nitrification Potential,NP)采用悬浊液培养法测定[17],具体方法为称取土壤鲜样9 g,加入60 mL磷酸缓冲液(含1 mmol·L–1 NH4+,3 mmol·L–1 KH2PO4和7 mmol·L–1 K2HPO4),置于100 mL血清瓶中,振荡24 h,200 r·min–1,30℃震荡24 h。振荡期间4次间隔取样,每次吸取5 mL,所取悬浊液8 000 r·min–1离心10 min。上清液采用连续流动分析仪测定硝态氮和亚硝态氮含量,根据硝态氮和亚硝态氮累积量计算硝化潜势。
恢复硝化强度(Recovered nitrification potential,RNP)的测定参考Ouyang等[17]的方法,具体为称取9 g土壤鲜样,加入60 mL磷酸缓冲液置于血清瓶中,盖上胶塞,将土壤悬浊液暴露于0.025 kPa乙炔中6 h,之后通过真空泵抽气6 min去除乙炔。去除乙炔后,所有血清瓶在盖子松开的条件下200 r·min–1、30℃震荡24 h。乙炔移除大约24 h之后氨单加氧酶(AMO)可重新合成,硝化作用重新进行,硝态氮和亚硝态氮开始积累。乙炔移除后24~48 h之间动态取样4次,每次吸取5 mL,所取悬浊液8 000 r·min–1离心10 min。上清液测定硝态氮和亚硝态氮含量,计算累积速率,所得即为RNP。通过添加特异性细菌抑制剂抑制氨氧化细菌(AOB)的活性,从而计算氨氧化古菌(AOA)在硝化作用中的贡献,RNP与AOA作用的恢复硝化强度(RNPAOA)的差值即为AOB作用的恢复硝化强度(RNPAOB)。具体操作为移除乙炔后,添加卡那霉素(Kanamycin,终浓度800 μg·mL–1)和大观霉素(Spectinomycin,终浓度200 μg·mL–1)作为抑制剂抑制AOB中AMO的合成,其余过程同RNP,计算得到的硝化速率即为RNPAOA,RNP与RNPAOA的差值即为RNPAOB。
1.4 AOA和AOB amoA基因丰度测定使用DNA提取试剂盒FastDNA Spin Kit for Soil(MP Bio,Santa Ana,CA,美国)提取土壤DNA,提取所得DNA用微量紫外分光光度计Nanodrop 2000(Thermo Fisher,Waltham,MA,美国)测定浓度。
AOA和AOB amoA基因丰度应用荧光定量PCR仪(ABI 7500 Thermocycler,Applied Biosystems,California,美国)测定,每样品3反应,同时包含一个未加DNA模板的反应体系(阴性对照)。反应体系为20 μL,包括2×SYBR premixture 9.2 μL,10 μmol·L–1前后引物各0.4 μL,1 μL模板DNA,9 μL ddH2O。AOA-amoA基因采用引物对Arch- amoA26F/Arch-amoA417R,引物序列为GAC TAC ATM TTC TAY ACW GAY TGG GC/GGK GTC ATR TAT GGW GGY AAY GTT GG[26];AOB-amoA基因采用引物对amoA-1F/amoA-2R,引物序列为GGG GTT TCT ACT GGT GGT/CCC CTC KGS AAA GCC TTC TTC[27]。反应条件均为:95℃预变性5 min,95℃变性15 s、60℃退火30 s、72℃延伸40 s,共40个循环。
制备荧光定量PCR标准。PCR产物电泳检测后通过割胶回收试剂盒纯化目的条带,对纯化后的PCR产物进行TA克隆,TA克隆后提取质粒,并使用Nanodrop 2000(Thermo Fisher Scientific,Waltham,MA,美国)测定质粒浓度。10倍梯度稀释构建好的各质粒制备标准曲线,选取5个稀释点制备标准曲线。
1.5 统计分析方法试验数据采用SAS8.1软件进行整理和统计分析,方差分析多重比较采用最小显著差异(LSD)法,在P < 0.05水平下检验差异显著性。
2 结果 2.1 不同绿肥处理对水稻产量及氮累积量的影响相比冬闲对照,RG处理降低了早稻产量,但提高了晚稻产量;其他绿肥对早稻和晚稻产量均无显著影响(图 1)。各处理的稻谷氮素累积量同稻谷产量变化趋势相似。MV处理的早稻稻谷氮累积量最高,达到515 mg⋅pot–1,显著高于其他处理;RG处理的晚稻氮素累积量显著高于其他处理(图 1)。
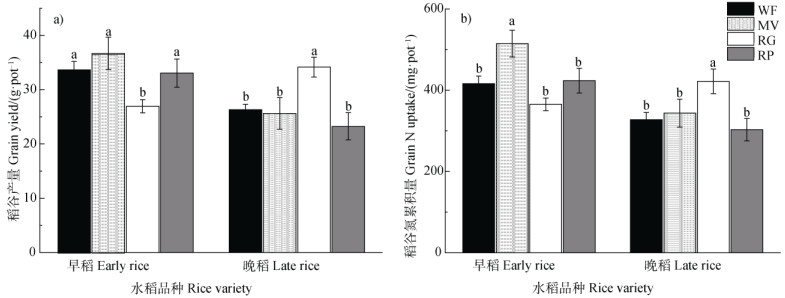
|
注:WF代表冬闲-双季稻,MV代表紫云英-双季稻,RG代表黑麦草-双季稻,RP代表油菜-双季稻;柱状图上的误差线为标准误差,不同小写字母代表同种水稻不同处理间在P < 0.05水平下差异显著。下同。 Note: WF, winter fallow-double cropping rice; MV, milk vetch-double cropping rice; RG, ryegrass-double cropping rice; RP, rape-double cropping rice. Vertical T bars in the histogram indicate SE, different letters indicate significant difference(P < 0.05) between different treatments of the same rice variety. The same below. 图 1 不同绿肥处理下的早稻、晚稻产量(a))及稻谷氮累积量(b)) Fig. 1 Grain yield(a)) and grain N accumulation(b)) of early rice and late rice relative to treatment |
有机质含量早晚稻之间差异不明显,早稻收获期紫云英和黑麦草处理均提高了土壤有机质含量,晚稻收获期各处理间无差异(图 2)。各处理晚稻收获期的全氮含量均低于早稻收获期,早稻收获期各处理全氮含量无显著差异,晚稻收获期冬种紫云英处理相对冬闲增加了土壤全氮含量(图 2)。
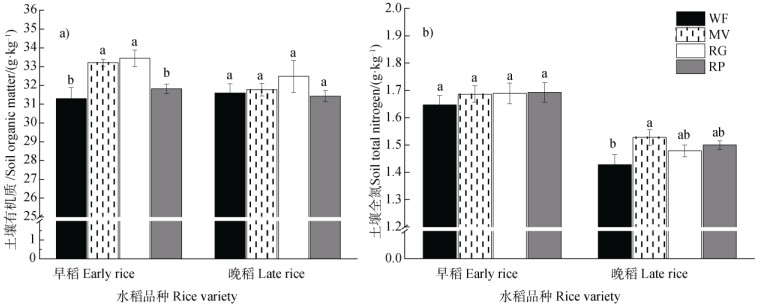
|
图 2 不同绿肥处理下的早稻、晚稻收获期土壤有机质(a))和全氮(b))含量 Fig. 2 Soil organic matter(a)) and total nitrogen(b)) at the harvesting stage of early rice and late rice relative to treatment |
在早稻移栽前、早稻分蘖期和早稻拔节期三个时期,不同绿肥处理均显著增加了土壤铵态氮含量(早稻移栽前RP处理除外),在绿肥盛花期和早稻收获期,各处理间无差异,晚稻收获期RG处理相对冬闲对照显著降低了土壤铵态氮含量(图 3a))。各处理间硝态氮含量的变化趋势与铵态氮相反,除晚稻收获期各处理间无显著差异外,其他时期三种绿肥处理均显著降低了土壤硝态氮含量(图 3b))。各时期土壤pH表现为绿肥盛花期最高,平均8.07,早稻分蘖期最低,平均7.68。绿肥盛花期和晚稻收获期各处理间无显著差异。早稻移栽前、早稻分蘖期和早稻收获期绿肥处理均降低了土壤pH,早稻拔节期仅有油菜处理降低了土壤pH(图 3c))。
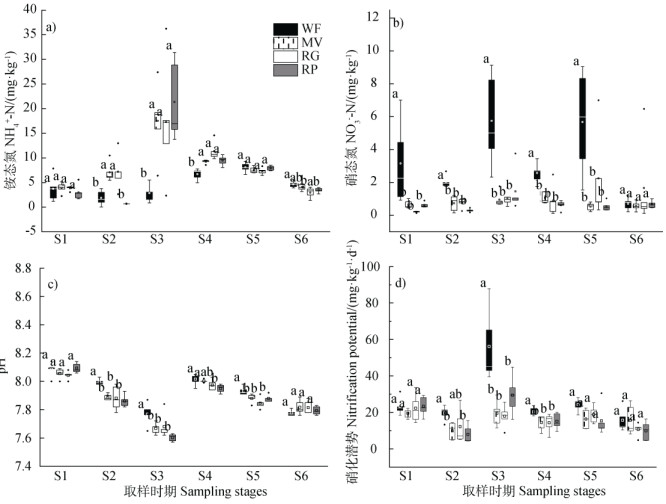
|
注:S1代表绿肥盛花期,S2代表早稻移栽前,S3代表早稻分蘖期,S4代表早稻拔节期,S5代表早稻收获期,S6代表晚稻收获期。不同小写字母表示相同取样时期不同处理间差异显著(P < 0.05)下同。 Note: S1 stands for the full-bloom stage of the green manure crop; S2 for the pre-transplanting stage of early rice; S3 for the tillering stage of early rice; S4 for the jointing stage of early rice; S5 for the post-harvest stage of early rice; S6 for the post-harvest stage of late rice. Different letters indicate significant difference(P < 0.05) between different treatments of the same sampling stage. The same below. 图 3 不同时期土壤铵态氮(a))、硝态氮(b))、土壤pH(c))和硝化潜势(d)) Fig. 3 Seasonal variations of soil NH4+-N(a)), NO3–-N(b)), pH(c))and nitrification potential(d)) relative to treatment |
不同时期硝化潜势变化不明显,并且各时期不同处理间规律相似(图 3d))。绿肥盛花期、早稻收获期和晚稻收获期各处理间无显著差异。早稻移栽前、分蘖期和拔节期,三种绿肥处理均降低了稻田土壤的硝化潜势(早稻移栽前RG处理除外)。其中早稻分蘖期冬闲处理的硝化潜势最高,达到了56.15 mg⋅kg–1⋅d–1(以N计,下同)。
2.3 AOA和AOB在硝化作用中的贡献AOA和AOB在硝化作用中的相对贡献在不同时期有较大差异,大部分样品中AOB在RNP中的贡献率大于50%。在6个时期中,MV处理降低了早稻移栽前和拔节期的RNPAOA,RP处理增加了绿肥盛花期RNPAOA、降低了早稻移栽前和拔节期的RNPAOA,RG处理在早稻拔节期的RNPAOA显著低于冬闲及其他两种绿肥处理。RNPAOB与RNPAOA的变化规律不同,绿肥盛花期MV处理降低了RNPAOB,在早稻移栽前和分蘖期,三种绿肥处理均增加了RNPAOB。在早稻收获期和晚稻收获期,各处理间RNP及AOA和AOB的相对贡献均无差异(图 4)。

|
注:柱状图上的误差线为SE值,不同小写字母代表氨氧化古菌(AOA)和氨氧化细菌(AOB)在恢复硝化强度中的贡献在P < 0.05水平下差异显著,不同大写字母代表恢复硝化强度在P < 0.05水平下差异显著。堆叠柱形图中灰色柱子(RNPAOA)和白色柱子(RNPAOB)分别代表AOA和AOB在恢复硝化强度(RNP)中的贡献,其和即为恢复硝化强度(RNP)。 Note: Vertical T bars in the histogram indicate SD, different lowercase letters indicate significant difference(P < 0.05) in relative contribution of ammonia-oxidizing archaea(AOA) and ammonia-oxidizing bacteria(AOB) to recovered nitrification potential; different capital letters indicate significant difference(P < 0.05) in recovered nitrification potential. The gray(RNPAOA) and white(RNPAOB) bar in the stacked bar diagram indicated the relative contribution of AOA and AOB to recovered nitrification potential(RNP), and the sum of RNPAOA and RNPAOB was RNP. 图 4 不同时期土壤恢复硝化强度及AOA和AOB在恢复硝化强度中的相对贡献 Fig. 4 Seasonal variation of the soil recovered nitrification potential and the relative contribution of AOA and AOB relative to growth stage and treatment |
在各时期不同处理中,AOA amoA基因丰度均高于AOB amoA,各样品中AOA/AOB amoA值为8~1 370,不同绿肥处理整体上提高了AOA和AOB amoA基因丰度。对于AOA,MV处理在绿肥盛花期、早稻移栽前和分蘖期相对于冬闲对照显著提高了AOA amoA基因丰度,RG处理仅在早稻移栽前提高了AOA amoA基因丰度,RP处理在早稻移栽前、分蘖期和拔节期显著提高AOA amoA基因丰度。对于AOB,MV处理提高了绿肥盛花期、早稻和晚稻收获期的AOB amoA基因丰度,RG处理提高了除晚稻收获期外其他时期的AOB amoA基因丰度,RP处理提高了绿肥盛花期和早稻分蘖期的AOB amoA基因丰度(图 5)。
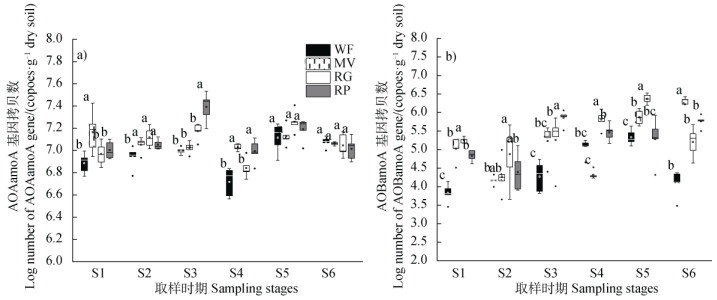
|
图 5 不同时期土壤中氨氧化古菌(a))及氨氧化细菌(b))amoA基因丰度 Fig. 5 Seasonal variations of the abundance AOA amoA(a)) and AOB amoA(b)) genes relative to treatment |
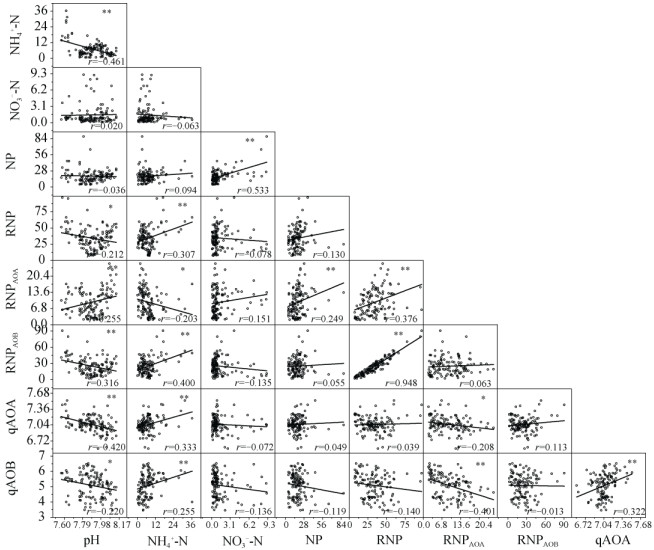
土壤pH、NH4+-N和NO3–-N含量对土壤硝化作用有较强影响,具体表现为土壤pH与RNP、RNPAOB、AOA amoA和AOB amoA基因丰度显著负相关,与RNPAOA显著正相关。土壤NH4+-N含量同pH相反,与RNP、RNPAOB、AOA amoA和AOB amoA基因丰度显著正相关,与RNPAOA显著负相关。土壤NO3–-N含量仅与NP显著正相关。RNPAOA与AOA amoA基因丰度间显著负相关,RNPAOB与AOB amoA基因丰度间无相关关系(图 6)。
|
注:图中r值为皮尔森相关系数,**代表P < 0.01,n=120。NP为硝化潜势,RNP为恢复硝化强度,RNPAOA为AOA作用的硝化强度,RNPAOB为AOB作用的硝化强度,qAOA为AOA amoA基因丰度,qAOB为AOB amoA基因丰度。 Note: In the figure, r stands for Pearson's correlation coefficient, ** for P < 0.01, n=120. NP for Nitrification potential; RNP for Recovered nitrification potential; RNPAOA for Contribution of AOA to RNP; RNPAOB for Contribution of AOB to RNP; qAOA for AOA amoA gene copoes; and qAOB for AOB amoA gene copies. 图 6 土壤性状与硝化特征间相关性 Fig. 6 Correlation analyses between soil properties and nitrification characteristics |
本研究中紫云英和黑麦草分别提高了早稻和晚稻稻谷氮累积量(图 1),且紫云英处理增加了晚稻收获后的土壤全氮含量(图 2),说明冬种绿肥可以持续、有效地改善稻田氮营养状况,为后茬作物提供持久的养分供应。抑制硝化作用有利于土壤氮素更多地以NH4+-N形式留存于土壤中,是减少农田NO3–-N淋洗损失的重要措施,同时水稻是喜铵作物,稻田中NH4+-N的增加可为水稻提供更多有效氮源[28]。有研究表明双季稻-紫云英种植制度有利于提高水稻氮素利用率,改善稻田氮素循环[29]。本研究中3种绿肥处理均增加了NH4+-N含量,降低了NO3–-N含量(图 3),同时在早稻生育期降低了土壤硝化潜势(图 3)。因此,NP的降低和NH4+-N含量的增加,可能是绿肥提高水稻氮素利用率的重要因素。在冬绿肥-水稻轮作体系中,绿肥生长过程根系与土壤的互作以及翻压还田后的腐解过程均会引起土壤性状及微生物活性的变化,继而改变微生物介导的硝化作用。Paungfoo-Lonhienne等[30]的研究表明,在甘蔗田中轮作豆科作物,可抑制硝化微生物的作用,与本研究结果一致。研究表明种植利用绿肥可有效减少农田氮素淋失[31],NP和NO3–-N含量之间显著正相关(图 6),NP的增加会增加NO3–-N淋失的风险,亦验证了冬种绿肥有利于改善稻田氮素循环过程。已有的稻田硝化作用研究多集中于酸性稻田,对碱性水稻土研究较少。与酸性土壤相比,碱性土壤的NP明显较高[32],因此硝化作用引起的碱性稻田氮素损失不容忽略。本研究针对紫潮泥的特性,关注种植绿肥对紫潮泥硝化作用的抑制情况及作用机制,填补了碱性稻田硝化作用研究的空白。
3.2 绿肥调控对AOA和AOB及其在硝化作用中贡献的影响AOA和AOB对硝化作用的贡献针对不同氮源和土壤性状有较大差异[33]。土壤理化性状对硝化微生物的活性有重要影响[23],冬种绿肥通过改变土壤性状影响土壤硝化潜势及AOA和AOB的作用。本研究中土壤pH、NH4+-N和NO3–-N含量的变化均与土壤硝化作用显著相关(图 6),说明环境条件和底物的改变,是引起硝化作用差异的可能原因。土壤pH是影响硝化作用的重要因素[10-11],冬种绿肥降低了碱性土壤的pH,可能是其改善土壤养分性状并降低硝化作用的原因之一。研究表明化学氮肥的投入能够增加AOB的数量,并且AOB的群落结构对化肥氮的响应更敏感[18,34]。而有机物的投入对AOA有更大影响,AOA的代谢途径更多样,可应用更多类型的碳源[35-36]。AOA和AOB之间蛋白质合成机制的差异可能是引起两者在不同氮源中贡献不一致的内在原因。本研究中AOB在硝化作用中所占比例在各时期和各处理中均大于50%(早稻移栽前冬闲处理除外)(图 4),说明在碱性紫潮泥中,相对于AOA,AOB在硝化作用中占主导地位,其中早稻生育期间(分蘖期和拔节期)AOB占比最高(图 4)。有研究表明,温度对AOA和AOB在硝化作用中各自贡献的影响不同,AOA和AOB作用的最优温度有显著差异[37]。不同取样时期的土壤温度和环境温度差异很大,可很好地解释AOA和AOB在恢复硝化强度中相对贡献的季节变异。
绿肥处理在不同取样时期对AOA和AOB在硝化作用中相对贡献的影响不一致,冬种绿肥降低了绿肥盛花期和晚稻收获期、增加了早稻生育期间AOB在RNP中的相对贡献(图 4),说明绿肥对AOA和AOB的调控特征随土壤和环境条件的变化而有所差异。对于氨氧化微生物的种群数量,绿肥处理在多数时期均提高了AOA和AOB amoA基因丰度(图 5),说明绿肥作用可促进氨氧化微生物的整体数量,与以往的研究结果[24]相似。然而,amoA基因丰度的增加也许并不能完全代表氨氧化微生物硝化能力的增强。在氨氧化微生物的研究中,RNP是基于AMO的重新生成的,而不是amoA基因的增加,因此amoA基因丰度同RNP及AOA和AOB的相对贡献之间无直接联系[9]。在今后的研究中,可通过对RNA水平的基因活性及表达的研究加以证实,也是加强绿肥对硝化作用调控机制研究的重要方向。
4 结论在碱性紫潮泥中,冬种三种绿肥均显著降低了早稻移栽前、分蘖期和拔节期的硝化潜势,同时增加了多数取样时期的铵态氮含量、降低了硝态氮含量,硝态氮含量同硝化潜势间显著正相关,说明绿肥可降低稻田土壤中的硝态氮淋失风险。不同绿肥处理在早稻分蘖期提高了氨氧化细菌作用的硝化强度(RNPAOB),在早稻移栽前和拔节期降低了氨氧化古菌作用的硝化强度(RNPAOA)。绿肥处理提高了氨氧化细菌和氨氧化古菌的amoA基因丰度。通过分析绿肥处理对氨氧化细菌和氨氧化古菌的调控特征,可为水稻-绿肥轮作体系中的稻田氮素管理提供理论依据。
| [1] |
Cao W D, Bao X G, Xu C X, et al. Reviews and prospects on science and technology of green manure in China (In Chinese)[J]. Journal of Plant Nutrition and Fertilizer, 2017, 23(6): 1450-1461. [曹卫东, 包兴国, 徐昌旭, 等. 中国绿肥科研60年回顾与未来展望[J]. 植物营养与肥料学报, 2017, 23(6): 1450-1461.]
(  0) 0) |
| [2] |
Xie Z, Tu S, Shah F, et al. Substitution of fertilizer-N by green manure improves the sustainability of yield in double-rice cropping system in South China[J]. Field Crops Research, 2016, 188: 142-149. DOI:10.1016/j.fcr.2016.01.006
(  0) 0) |
| [3] |
Yang L, Bai J, Zeng N, et al. Diazotroph abundance and community structure are reshaped by straw return and mineral fertilizer in rice-rice-green manure rotation[J]. Applied Soil Ecology, 2019, 136: 11-20. DOI:10.1016/j.apsoil.2018.12.015
(  0) 0) |
| [4] |
Gao J S, Xu M G, Dong C H, et al. Effects of long-term rice-rice-green manure cropping rotation on rice yield and soil fertility (In Chinese)[J]. Acta Agronomica Sinica, 2013, 39(2): 343-349. [高菊生, 徐明岗, 董春华, 等. 长期稻-稻-绿肥轮作对水稻产量及土壤肥力的影响[J]. 作物学报, 2013, 39(2): 343-349.]
(  0) 0) |
| [5] |
Wan S X, Zhu H B, Tang S, et al. Effects of Astragalus sinicus manure and fertilizer combined application on biological properties of soil in Anhui double cropping rice areas along the Yangtze River (In Chinese)[J]. Plant Nutrition and Fertilizer Science, 2015, 21(2): 387-395. [万水霞, 朱宏斌, 唐杉, 等. 紫云英与化肥配施对安徽沿江双季稻区土壤生物学特性的影响[J]. 植物营养与肥料学报, 2015, 21(2): 387-395.]
(  0) 0) |
| [6] |
Zhang M F, Tian F, Wang X X, et al. Effects of different green manure varieties on enzyme activities and fertility of tobacco-planting soils (In Chinese)[J]. Soils, 2017, 49(5): 903-908. [张明发, 田峰, 王兴祥, 等. 翻压不同绿肥品种对植烟土壤肥力及酶活性的影响[J]. 土壤, 2017, 49(5): 903-908.]
(  0) 0) |
| [7] |
He J Z, Zhang L M. Advances in ammonia-oxidizing microorganisms and global nitrogen cycle (In Chinese)[J]. Acta Ecologica Sinica, 2009, 29(1): 406-415. DOI:10.3321/j.issn:1000-0933.2009.01.049 [贺纪正, 张丽梅. 氨氧化微生物生态学与氮循环研究进展[J]. 生态学报, 2009, 29(1): 406-415.]
(  0) 0) |
| [8] |
Kowalchuk G A, Stephen J R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology[J]. Annual Review of Microbiology, 2001, 55: 485-529. DOI:10.1146/annurev.micro.55.1.485
(  0) 0) |
| [9] |
Taylor A E, Zeglin L H, Dooley S, et al. Evidence for different contributions of Archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils[J]. Applied and Environmental Microbiology, 2010, 76(23): 7691-7698. DOI:10.1128/AEM.01324-10
(  0) 0) |
| [10] |
Gubry-Rangin C, Novotnik B, Mandic-Mulec I, et al. Temperature responses of soil ammonia-oxidising archaea depend on pH[J]. Soil Biology & Biochemistry, 2017, 106: 61-68.
(  0) 0) |
| [11] |
Wang Z, Meng Y, Zhu-Barker X, et al. Responses of nitrification and ammonia oxidizers to a range of background and adjusted pH in purple soils[J]. Geoderma, 2019, 334: 9-14. DOI:10.1016/j.geoderma.2018.07.038
(  0) 0) |
| [12] |
Cao Y Q, Yan X J, Luo H Y, et al. Nitrification activity and microbial community structure in purple soils with different pH (In Chinese)[J]. Acta Pedologica Sinica, 2018, 55(1): 194-202. [曹彦强, 闫小娟, 罗红燕, 等. 不同酸碱性紫色土的硝化活性及微生物群落组成[J]. 土壤学报, 2018, 55(1): 194-202.]
(  0) 0) |
| [13] |
Wang P P, Duan Y H, Xu M G, et al. Nitrification potential in fluvo-aquic soils different in fertility and its influencing factors (In Chinese)[J]. Acta Pedologica Sinica, 2019, 56(1): 124-134. [王萍萍, 段英华, 徐明岗, 等. 不同肥力潮土硝化潜势及其影响因素[J]. 土壤学报, 2019, 56(1): 124-134.]
(  0) 0) |
| [14] |
Kong Y L, Ling N, Xue C, et al. Long-term fertilization regimes change soil nitrification potential by impacting active autotrophic ammonia oxidizers and nitrite oxidizers as assessed by DNA stable isotope probing[J]. Environmental Microbiology, 2019, 21(4): 1224-1240. DOI:10.1111/1462-2920.14553
(  0) 0) |
| [15] |
Zhang Q, Li Y, He Y, et al. Elevated temperature increased nitrification activity by stimulating AOB growth and activity in an acidic paddy soil[J]. Plant and Soil, 2019, 445(1/2): 71-83.
(  0) 0) |
| [16] |
Xia W W, Zhang C X, Zeng X W, et al. Autotrophic growth of nitrifying community in an agricultural soil[J]. The ISME Journal, 2011, 5(7): 1226-1236. DOI:10.1038/ismej.2011.5
(  0) 0) |
| [17] |
Ouyang Y, Norton J M, Stark J M, et al. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil[J]. Soil Biology & Biochemistry, 2016, 96: 4-15.
(  0) 0) |
| [18] |
Wu Y C, Lu L, Wang B Z, et al. Long-term field fertilization significantly alters community structure of ammonia-oxidizing bacteria rather than archaea in a paddy soil[J]. Soil Science Society of America Journal, 2011, 75(4): 1431-1439. DOI:10.2136/sssaj2010.0434
(  0) 0) |
| [19] |
Shen J P, Zhang L M, Di H J, et al. A review of ammonia-oxidizing bacteria and archaea in Chinese soils[J]. Frontiers in Microbiology, 2012, 3: 296.
(  0) 0) |
| [20] |
Leininger S, Urich T, Schloter M, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils[J]. Nature, 2006, 442(7104): 806-809. DOI:10.1038/nature04983
(  0) 0) |
| [21] |
Huang L Q, Dong H L, Wang S, et al. Diversity and abundance of ammonia-oxidizing archaea and bacteria in diverse Chinese paddy soils[J]. Geomicrobiology Journal, 2014, 31(1): 12-22. DOI:10.1080/01490451.2013.797523
(  0) 0) |
| [22] |
Wang B Z, Zhao J, Guo Z Y, et al. Differential contributions of ammonia oxidizers and nitrite oxidizers to nitrification in four paddy soils[J]. The ISME Journal, 2015, 9(5): 1062-1075. DOI:10.1038/ismej.2014.194
(  0) 0) |
| [23] |
Wang J, Wang W D, Gu J D. Community structure and abundance of ammonia-oxidizing archaea and bacteria after conversion from soybean to rice paddy in albic soils of Northeast China[J]. Applied Microbiology and Biotechnology, 2014, 98(6): 2765-2778. DOI:10.1007/s00253-013-5213-2
(  0) 0) |
| [24] |
Gao S, Chang D N, Zou C, et al. Archaea are the predominant and responsive ammonia oxidizing prokaryotes in a red paddy soil receiving green manures[J]. European Journal of Soil Biology, 2018, 88: 27-35. DOI:10.1016/j.ejsobi.2018.05.008
(  0) 0) |
| [25] |
Lu R K. Analytical methods for soil and agro-chemistry (In Chinese). Beijing: China Agricultural Science and Technology Press, 2000. [鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科学技术出版社, 2000.]
(  0) 0) |
| [26] |
Park S J, Park B J, Rhee S K. Comparative analysis of archaeal 16S rRNA and amoA genes to estimate the abundance and diversity of ammonia-oxidizing archaea in marine sediments[J]. Extremophiles, 2008, 12(4): 605-615. DOI:10.1007/s00792-008-0165-7
(  0) 0) |
| [27] |
Rotthauwe J H, Witzel K P, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations[J]. Applied and Environmental Microbiology, 1997, 63(12): 4704-4712. DOI:10.1128/aem.63.12.4704-4712.1997
(  0) 0) |
| [28] |
Qian X Q, Shen Q R, Xu G H, et al. Nitrogen form effects on yield and nitrogen uptake of rice crop grown in aerobic soil[J]. Journal of Plant Nutrition, 2004, 27(6): 1061-1076. DOI:10.1081/PLN-120037536
(  0) 0) |
| [29] |
Zhang L J, Yang B J, Huang G Q, et al. Effects of green manure rotation on rice growth dynamics and nitrogen uptake and utilization (In Chinese)[J]. Agricultural Science & Technology, 2015, 16(5): 962-967. [张立进, 杨滨娟, 黄国勤, 等. 绿肥轮作对早稻生长动态及氮素吸收利用的影响[J]. 农业科学与技术(英文版), 2015, 16(5): 962-967.]
(  0) 0) |
| [30] |
Paungfoo-Lonhienne C, Wang W, Yeoh Y K, et al. Legume crop rotation suppressed nitrifying microbial community in a sugarcane cropping soil[J]. Scientific Reports, 2017, 7(1): 16707. DOI:10.1038/s41598-017-17080-z
(  0) 0) |
| [31] |
Valkama E, Lemola R, Känkänen H, et al. Meta-analysis of the effects of undersown catch crops on nitrogen leaching loss and grain yields in the Nordic countries[J]. Agriculture, Ecosystems & Environment, 2015, 203: 93-101.
(  0) 0) |
| [32] |
Zhou Z F, Wang M X, Liu W L, et al. A comparative study of ammonia-oxidizing archaea and bacteria in acidic and alkaline purple soils[J]. Annals of Microbiology, 2016, 66(2): 615-623. DOI:10.1007/s13213-015-1143-9
(  0) 0) |
| [33] |
Meinhardt K A, Stopnisek N, Pannu M W, et al. Ammonia-oxidizing bacteria are the primary N2O producers in an ammonia-oxidizing archaea dominated alkaline agricultural soil[J]. Environmental Microbiology, 2018, 20(6): 2195-2206. DOI:10.1111/1462-2920.14246
(  0) 0) |
| [34] |
Zabaloy M C, Allegrini M, Tebbe D A, et al. Nitrifying bacteria and archaea withstanding glyphosate in fertilized soil microcosms[J]. Applied Soil Ecology, 2017, 117: 88-95.
(  0) 0) |
| [35] |
Walker C B, de la Torre J R, Klotz M G, et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(19): 8818-8823. DOI:10.1073/pnas.0913533107
(  0) 0) |
| [36] |
Tourna M, Stieglmeier M, Spang A, et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(20): 8420-8425. DOI:10.1073/pnas.1013488108
(  0) 0) |
| [37] |
Taylor A E, Giguere A T, Zoebelein C M, et al. Modeling of soil nitrification responses to temperature reveals thermodynamic differences between ammonia-oxidizing activity of archaea and bacteria[J]. The ISME Journal, 2017, 11(4): 896-908. DOI:10.1038/ismej.2016.179
(  0) 0) |
 2022, Vol. 59
2022, Vol. 59


