由土壤病原细菌引起的植物土传病害日益引起人们的重视。常见的土传病原细菌有引起番茄、烟草等茄科作物青枯病的青枯菌(Ralstonia solanacearum)、诱发豆科作物产生冠瘿瘤的根癌农杆菌(Agrobacterium tumefaciens)和导致甘薯茎腐病的达旦提狄克氏菌(Dickeya dadantii)[1]。土壤是植物土传病原细菌生活史循环中的重要生境,当不存在寄主植物时,病原细菌可通过改变细胞状态在土壤或水环境中存活数年之久[2-4];一旦感知到寄主植物信号,便会从土体向根际及寄主根系迁移,进而定殖根表和吸附植物皮层,随后分泌致病因子穿透木质部,进入寄主体内繁殖,最终随着寄主植物的死亡再次返回土壤环境[3,5]。化学农药、抗病品种和生物防治等手段尽管在病害防控中有较好的潜力,但对于土传病害,其应用效果和稳定性通常受土壤条件的影响[6]。由病害发生三要素可知,病原细菌的成功侵染离不开易感寄主和适宜病原菌发挥致病毒性特征的环境因子。在入侵寄主的过程中,土壤中的病原细菌会经受温度、pH、含氧量、营养物质种类和数量等环境因素的骤变、土著微生物空间与资源竞争、原生动物捕食、噬菌体寄生以及寄主植物分泌抗菌物质等生物胁迫。为达到增殖和传播的目的,病原细菌必须在不断变化的环境中时刻协调生存和致病之间的权衡关系。了解土壤病原细菌适应环境变化的机制对于掌握其传播、扩散和侵染规律有重要意义。
目前,人们对病原细菌与寄主植物的互作机制以及对病原细菌生存和致病能力的影响因素已经有大量的研究和总结[7-8],但是对于从土壤存活至根表入侵这个过程中土壤环境因素对病原细菌生存-致病权衡尚缺少系统的梳理和思考。本文首先总结了土传病原细菌的生存-致病权衡规律和典型现象,归纳了土壤非生物和生物因子对病原细菌生存-致病权衡的影响;其次阐述了土传病原细菌在植物根际入侵过程中的生存-致病权衡机制,并提出一些土传病原细菌生存-致病权衡相关的科学问题。呼吁基于生存-致病权衡理论,建立降低环境中病原细菌丰度和毒性的土传病害生态防控策略,为防控其他土传病原体(如病原真菌)、实现绿色农业可持续发展提供理论参考。
1 土壤病原细菌的生存-致病权衡 1.1 病原细菌的生存-致病权衡规律1859年达尔文在《物种起源》中提到歌德的补偿法则:“In order to spend on one side,nature is forced to economise on the other side”——自然在一处开支,就被迫在另一边节省[9]。不同特征间的负相关关系称为权衡(Trade-off)。权衡效应在动植物领域得到广泛的认可,研究人员认为动植物将有限的资源用于繁殖或者生存是造成权衡的原因,因此,权衡效应的核心是资源配置[10]。权衡效应在微生物界同样适用:一种细胞特性分配的资源多,那么其他特性配置的资源相对减少。微生物特别是病原微生物(病原细菌)具备诸多特性,如生物膜形成、代谢、运动性、毒性、DNA修复能力以及对噬菌体、抗生素或其他胁迫的抗性等,而不同特性之间很可能存在负相关关系。微生物本身的细胞资源、膜蛋白结构和遗传信息储存及处理能力是有限性的,很难同时将不同特性最大化,因此在不同环境条件下表现出了一种特性增强而另一种特性减弱的权衡现象[11]。病原细菌和非致病细菌的区别在于病原细菌能否分泌损害寄主健康的毒性因子,比如可堵塞植物输水系统的胞外多糖、降解植物细胞壁的降解酶、破坏植物免疫的Ⅲ型效应蛋白等[12]。除了与植物互作时发挥直接作用的毒性因子外,一些毒性特征的表达在整个植株发病过程中也十分重要,例如趋化性、运动性、铁载体的分泌和生物膜的形成等决定着病原细菌能否成功入侵寄主根际[12]。权衡是微生物适应环境的基本策略[11],生存-致病权衡对土传病原细菌适应不断变化的土壤-植物环境非常重要。根据权衡理论,当生存条件适宜,病原细菌不需要启动高成本的抗胁迫机制,从而能将更多的资源分配给生长、繁殖、毒性因子等高成本的特性,进而提高病原细菌的致病性;当胁迫增强时,病原细菌必须提升其抵御胁迫能力以保证自身的生存,这通常伴有细胞结构改变导致的资源摄入能力降低、毒性基因丢失、毒性基因表达准确度的降低以及有害突变的增加等[11],即生存成本提高,这样分配给生长、繁殖、毒性因子的资源相对减少,即表现为致病能力下降(图 1)。
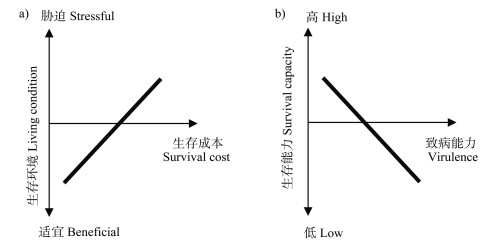
|
图 1 病原菌在不同环境中的生存成本(a))及病原菌的生存与致病的权衡规律(b)) Fig. 1 Survival cost of pathogens in different living environments(a))and the trade-off between survival capacity and virulence of pathogens(b)) |
当遭遇极端环境胁迫时,细菌会进入存活但不可培养状态(viable but non-culturable,VBNC)。在这种状态下,细菌的细胞完整,但呼吸、代谢活性降低,因此VBNC被认为是细菌应对胁迫的一种自我保护机制[13]。进入VBNC状态后,植物病原细菌会丧失致病能力,如青枯菌[14]、根癌土壤杆菌[15]、十字花科黑腐病菌(Xanthomonas campestris pv. campestris[16])、柑橘溃疡病菌(X. axonopodis pv. citri[17])和火疫病病原菌(Erwinia amylovora[18])等。VBNC状态的诱导因素有很多,如不适宜的温度、pH、光照、氧气不足、高盐环境、干燥和重金属胁迫等[19-20]。一旦生存条件适宜或存在诱导物,病原细菌可从VBNC状态复苏并恢复致病能力。据报道,4℃的低温条件可诱导青枯菌进入VBNC状态[14],但当VBNC状态的青枯菌靠近寄主植物根系后能复苏,且能继续侵染植物[21]。火疫病病原菌在硫酸铜的胁迫下进入VBNC状态;将VBNC状态的火疫病病原菌接种至寄主植物后,发现植物发病症状显著低于未受胁迫的病原菌处理;不过复苏后的病原菌的致病性却与正常状态下的病原菌致病力相当[18]。由于检测难度大,VBNC状态的病原菌是农业生产的一大隐患。
1.2.2 表型转换微生物会通过改变表型来增加种群多样性,从而增强对环境的适应性[22]。该现象常见于人类病原菌或病原真菌入侵寄主过程中,通常伴随着毒性的改变[23-24]。在土传病原细菌中,野生型青枯菌在土壤、静止液体和琼脂平板的长期培养[25-30]条件下菌落形态会发生变化,即从黏液态转变为非黏液态(图 2)。这个过程称为青枯菌的表型转换(phenotypic conversion,PC)[31],转换后的青枯菌不能引起宿主发病[23,31-32]。已经探明青枯菌的phcA毒性网络系统调控表型转换,通过群体感应调控青枯菌生存与致病的权衡。在资源充足的情况下,青枯菌种群数量累积,当种群密度达到一定的阈值时启动phcA毒性系统表达,上调一系列毒性因子,如EPS(胞外多糖)、植物细胞壁降解酶、Ⅲ型分泌系统等。但当资源有限时,种群数量降低,phcA受抑制,毒性减弱,但是资源利用能力、运动能力和种群增长速率均增强[33]。有研究表明,在适宜条件下,PC突变体还可转变为野生型菌株,从而恢复其致病能力[34]。但是这种现象在自然环境下尚未见报道。
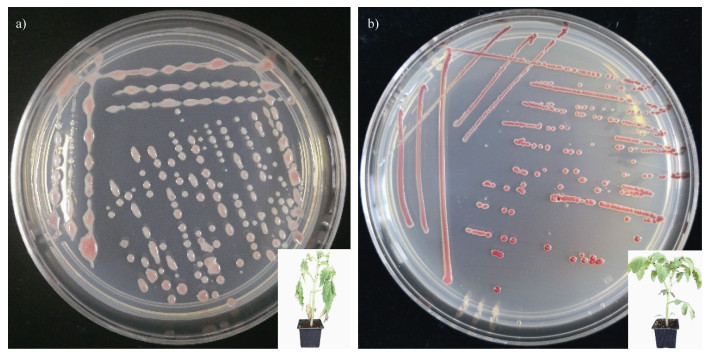
|
图 2 青枯菌的表型转换:a)为黏液型青枯菌,一般致病性强;b)为非黏液型青枯菌,一般丧失致病性 Fig. 2 Phenotype conversion of Ralstonia solanacearum. Mucoid and virulent colonies(a)); Non-mucoid and avirulent ones(b)) |
土壤非生物因子的变化对土壤病原细菌生存与致病能力的影响较大。众所周知,土壤温度、养分浓度、pH、水分和氧气条件等会随着季节、天气或是有无植物而发生瞬时剧烈或漫长微弱的改变。在遭遇非生物胁迫时,微生物将细胞资源优先分配给高成本的胁迫应激保护机制,削减细胞增殖所需资源[35]。对病原细菌而言,对自我保护机制的高投入可能会导致减少甚至停止毒性因子的表达。例如,因环境温度过低而进入VBNC状态时[14],病原细菌对pH、重金属、高温、抗生素等物理和化学胁迫的抗性水平显著提高[36-37]。此时,病原细菌虽然具备致病的潜力,但不能引起寄主发病[38-40]。养分资源匮乏也是迫使病原菌权衡生存和毒性表达的常见环境胁迫因素。携带和表达毒性因子成本高昂,当营养资源匮乏时,病原细菌可能启动寡营养物质运输系统,以增加在群落中的优势,比如无毒性的PC型在贫营养条件下较有毒野生型的青枯菌生长更快,对铁离子的利用效率更高[33]。病原细菌也能通过丢失致病基因来增加在贫营养条件下的竞争力[41]。若养分资源极度匮乏不足以支持稳定生长时,大多数微生物会停止分裂而进入生长停滞阶段[42]。环境胁迫过强可能会破坏遗传信息的稳定,从而导致毒性基因难以表达。例如氧化、酸性和高温胁迫条件会影响DNA结构的稳定性,降低达旦提狄克氏菌的主要毒性基因——果胶裂解酶基因的表达[43-44]。
2.2 生物因素胁迫病原细菌在土壤中应对寄生、竞争和捕食等生物胁迫提升生存能力的同时,可能会被迫调整自身的致病能力。例如,细胞表面鞭毛是常见的噬菌体受体[45],同时也是病原菌的重要致病因子[46]。面对噬菌体裂解侵染胁迫,微生物会通过修饰、丢弃、改变其细胞表面的噬菌体受体,提高对噬菌体的抗性,但鞭毛致弱导致运动能力降低,进而降低其致病能力[47]。此外,一些病原菌的噬菌体受体与吸收营养物质有关,所以通过破坏受体而获得噬菌体抗性的突变体会降低其营养吸收,从而减弱其竞争能力[48-49]。同营养级内的其他微生物不仅可通过争夺营养和空间间接影响病原菌的致病能力,还能产生抗生素类物质直接影响病原菌的生存。病原细菌的一些抗性一般是通过染色体突变获得,并涉及关键基因的修饰,以及质粒复制和质粒基因的表达等影响其代谢水平,最终导致抗性菌株生长速率下降、竞争能力减弱[50-51];另一些抗性是通过改变细胞膜渗透性获得,如大肠杆菌孔蛋白通道能同时渗透营养和抗生素,降低抗生素渗透速率、提高抗生素抗性的同时,其资源摄取速率也随之降低,导致生长减缓[52]。此外,土壤中的有益菌还能通过分泌的挥发性有机物(volatile organic compounds,VOCs)抑制青枯菌运动能力、根际定殖、生物膜形成和氧化胁迫应答能力[53],这可能是由于VOCs胁迫导致青枯菌的生存成本提高而毒性因子减弱。为应对原生动物等捕食胁迫,病原菌会增强抗捕食特征,这可能会导致致病相关的特征减弱,如运动能力和资源利用能力下降,从而导致致病能力被削弱[54]。
3 土传病原菌根际入侵过程中的生存-致病权衡土传病原菌侵染植物的第一步是感知寄主并从土体移动至根际,同时要与根际土著微生物竞争资源和空间,才能在根际成功定殖[55]。当根际种群达到一定数量后,病原菌启动毒性基因的表达,从而入侵植物根系[56]。所以在入侵植物体内之前,病原菌就需要启动一些帮助其成功侵染寄主植物的毒性因子以及应对一系列的生存胁迫。
3.1 从土体向根际迁移过程的生存-致病权衡在土壤中,低温、干燥[57]和养分贫瘠的土壤环境会淘汰大量抗胁迫能力低下的病原菌个体,VBNC、饥饿细胞、转换成抗胁迫表型和形成生物膜的细胞可以存活下来[29]。一旦感知到根系分泌物等植物信号时,病原菌利用趋化性向寄主植物根际的方向迁移[58]。趋化作用通常具有寄主特异性,例如青枯菌能被几种特定的氨基酸和有机酸寄主植物信号吸引,而水稻等寄主植物的根系分泌物不能引起青枯菌的定向迁移[58]。趋化作用和运动能力密不可分,微生物靠鞭毛或菌毛运动,青枯菌在鞭毛驱动下向根际移动并通过根系进入植物维管束系统[47]。青枯菌的fliC突变体缺少鞭毛细丝,flhDC突变体不能激活鞭毛基因的表达,这两种突变体完全不能移动也不能朝植物根系运动,所以在土壤中它们的致病能力较野生型青枯菌显著降低[47,58]。病原细菌对胁迫抗性的提高可能会以牺牲驱化运动能力为代价。细菌普遍会通过形成生物膜来提高重金属或抗生素等胁迫的抗性[59-60],对青枯菌趋氧性的研究表明,丧失趋氧能力的突变体青枯菌能形成更厚的生物膜[61],而丧失趋氧性的青枯菌几乎无运动能力[27]。因此,致弱土壤中的病原菌可通过添加可降低病原菌运动能力的生物胁迫或非生物胁迫,比如已有研究发现,根际促生菌——解淀粉芽孢杆菌产生的易挥发性有机物VOCs胁迫可使青枯菌的fliC基因表达下调,降低青枯菌向根际迁移和定殖的能力[53]。
3.2 根际定殖与增殖过程的生存-致病权衡到达寄主植物根际后,病原菌需要与土著微生物进行营养竞争和拮抗竞争以实现在根际的成功定殖。在根际环境中,能够高效利用有限资源的微生物通常会占据竞争优势。比如在根际限铁的条件下,能否产生铁载体以获取铁离子对狄克氏菌[62]和青枯菌[63]等病原菌的根际入侵至关重要。细菌间的拮抗竞争作用也会通过增加病原菌的生存胁迫而降低其毒性。因此,利用病原菌的拮抗菌可达到致弱病原菌毒性的效果。刘波等[64]发现短小芽孢杆菌(Bacillus cereus)ANTI-8098A可作为青枯病的生防菌,因为ANTI-8098A处理后的青枯菌会丧失致病能力,即使回接至番茄植株上也不能致病。这可能是由于强致病力菌株对拮抗菌的拮抗物质抵御能力弱,而弱致病力的菌株对拮抗菌拮抗物质的抵抗能力强,因此,无致病力菌株在拮抗菌的胁迫下种群优势明显,从而表现出青枯菌整体致病能力的减弱。根际是微生物聚集的热区,病原菌不仅与同营养级微生物竞争,而且频繁地遭遇噬菌体的寄生和原生动物的捕食,从而降低其在根际的适应性。因此,向根际加入病原菌专性裂解性噬菌体或捕食病原菌的原生动物可降低病害的发生。Wang等[65]发现噬菌体组合处理根际病原青枯菌数量显著降低,并且噬菌体组合多样性越高,青枯菌成功突变抵御噬菌体的概率越低。而成功进化出噬菌体抗性的青枯菌,生长显著减缓,再次侵染作物根系的能力也显著降低。Xiong等[66]发现发病植株中吞噬型原生动物的相对丰度随植物生长而显著降低,而这一降低与青枯病的爆发相吻合。青枯病爆发的原因除了由于根际原生动物捕食胁迫减弱导致根际青枯菌数量增加外,也可能存在青枯菌在抵御原生动物捕食机制上投入减少、致病能力增强的情况。

|
图 3 土传病原菌在土壤中生存、定殖和侵染的过程 Fig. 3 Survival, colonization and infection processes of soil-borne pathogen in soil |
根际成功定殖后,病原细菌需要吸附在植物的根表来完成根系入侵。鞭毛不仅能增强细菌的运动能力,还能增加病原菌对寄主植物根表细胞的粘附作用[67],比如鞭毛有助于病原菌D. dadantii的粘附能力。粘附至根表后,病原细菌会释放一系列毒性因子以助其进入寄主体内[12],但也伴随着相应的生存成本的提高。根癌农杆菌的Ⅳ型分泌系统分泌的一种毒性效应蛋白可诱导植物产生有利于自身生长的物质(冠瘿碱),但是该蛋白的表达会降低自身的适应性,所以当冠瘿碱不足时,致病型根癌农杆菌的竞争能力显著低于不携带该致病基因的菌株[41]。对于病原菌的入侵,植物也会产生物质抑制病原菌生长,比如植物产生的羟基香豆素能抑制青枯菌生物膜的形成和Ⅲ型分泌系统效应蛋白的转录[68]。植物还会产生抗菌多肽和次级代谢产物,比如羟基肉桂酸(HCA)、类黄酮、类异戊二烯和植物碱。青枯菌必须分泌能够降解HCA的相关蛋白才能免受其毒害,当青枯菌的HCA降解蛋白相关基因缺陷时,其根系定殖效率大大降低[69]。此外,青枯菌还需要多药物外排泵将抗菌化合物排出体外,使其免受植物分泌的抗生物质的毒害[70]。
4 结论与展望土传病原菌侵染作物是一个时空动态过程,涉及了病原菌与土壤生物、非生物因素的互作。土壤环境本身以及病原菌与环境互作的复杂性,是制约土传病害防控效率的关键因素。掌握病原菌在土壤中的生存与致病权衡的规律和机制,对建立土传病害的精准防控策略具有重要意义。尽管本文梳理了土壤病原菌的生存与致病权衡规律、影响因素和作用机制,但相关研究证据尚亟待丰富。近期,土壤学家呼吁土壤学工作者关注土壤病原菌的研究[71],病原菌在土壤中的生存与致病权衡可作为土壤学工作者涉足土壤病原菌研究的一个重要切入点。针对土壤病原菌的生存与致病权衡,以下几方面研究值得关注。
(1)深入探究生物和非生物环境因素对病原菌存活和致病能力的影响,以及对病原菌生存-致病权衡的驱动作用;加强土壤病原菌的生态型和基因型多样性的研究及环境对病原菌多样性的影响;建立原位条件下土壤病原菌生存、根际迁移、入侵致病过程中病原菌表型-基因型的关系模型。
(2)以往的研究侧重于单一胁迫下生存-致病的权衡规律,忽略了环境本身的复杂性,导致相关理论成果不能有效指导实践。微生物与病原菌间的互作结果通常受到多种非生物因素的影响,因此在利用生物胁迫防控土传病害时需加强根际微食物网多级互作认识,需要厘清生物和非生物环境因素叠加作用对土壤病原菌生存与致病权衡的影响机制研究,构建以生存和致病权衡理论为指导的综合生态防控体系。
(3)加强对病原菌适应性进化分子机制的研究。研究胁迫条件下病原菌的碱基置换、插入、缺失等响应,结合全基因组关联分析,以探究生存-致病权衡的分子机制。除此之外,应加强生态学、微生物学、进化生物学、基因组学、转录组学等多学科、多维度联合揭示病原菌的生存与致病权衡规律和作用机制,以获得对病原菌致病-生存权衡的准确认识,从而精准高效阻控土传病害。
致谢 衷心感谢南京农业大学微生态与根际健康实验室张耀予为本文图 3提供插图。
| [1] |
Mansfield J, Genin S, Magori S, et al. Top 10 plant pathogenic bacteria in molecular plant pathology[J]. Molecular Plant Pathology, 2012, 13(6): 614-629. DOI:10.1111/j.1364-3703.2012.00804.x
(  0) 0) |
| [2] |
Wallis F M, Truter S J. Histopathology of tomato plants infected with Pseudomonas solanacearum, with emphasis on ultrastructure[J]. Physiological Plant Pathology, 1978, 13(3): 307-317. DOI:10.1016/0048-4059(78)90047-4
(  0) 0) |
| [3] |
Vasse J. Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by pseudomonas solanacearum[J]. Molecular Plant-Microbe Interactions, 1995, 8(2): 241. DOI:10.1094/MPMI-8-0241
(  0) 0) |
| [4] |
van Elsas J D, Kastelein P, de Vries P M, et al. Effects of ecological factors on the survival and physiology of Ralstonia solanacearum bv. 2 in irrigation water[J]. Canadian Journal of Microbiology, 2001, 47(9): 842-854. DOI:10.1139/w01-084
(  0) 0) |
| [5] |
Vasse J, Danoun S, Trigalet A. Microscopic studies of root infection in resistant tomato cultivar Hawaii 7996[J]. Bacterial Wilt Disease and the Ralstonia Solanacearum Species Complex, 2005, 285-291.
(  0) 0) |
| [6] |
Jiang G F, Wei Z, Xu J, et al. Bacterial wilt in China: History, current status, and future perspectives[J]. Frontiers in Plant Science, 2017, 8: 1549. DOI:10.3389/fpls.2017.01549
(  0) 0) |
| [7] |
Tariq S, Saleem K. Plant defenses vs pathogen weapons: A continuation of battlefield: A mini-review[J]. International Journal of Molecular Microbiology, 2018, 1(1): 17-22.
(  0) 0) |
| [8] |
von Bodman S B, Bauer W D, Coplin D L. Quorum sensing in plant-pathogenic bacteria[J]. Annual Review of Phytopathology, 2003, 41(1): 455-482. DOI:10.1146/annurev.phyto.41.052002.095652
(  0) 0) |
| [9] |
Lenoir T. The eternal laws of form: Morphotypes and the conditions of existence in Goethe's biological thought[J]. Journal of Social and Biological Systems, 1984, 7(4): 317-324. DOI:10.1016/0140-1750(84)90005-8
(  0) 0) |
| [10] |
Stearns S C. Trade-offs in life-history evolution[J]. Functional Ecology, 1989, 3(3): 259. DOI:10.2307/2389364
(  0) 0) |
| [11] |
Ferenci T. Trade-off mechanisms shaping the diversity of bacteria[J]. Trends in Microbiology, 2016, 24(3): 209-223. DOI:10.1016/j.tim.2015.11.009
(  0) 0) |
| [12] |
Leonard S, Hommais F, Nasser W, et al. Plant-phytopathogen interactions: Bacterial responses to environmental and plant stimuli[J]. Environmental Microbiology, 2017, 19(5): 1689-1716. DOI:10.1111/1462-2920.13611
(  0) 0) |
| [13] |
Heim S, del Mar Lleo M, Bonato B, et al. The viable but nonculturable state and starvation are different stress responses of Enterococcus faecalis, as determined by proteome analysis[J]. Journal of Bacteriology, 2002, 184(23): 6739-6745. DOI:10.1128/JB.184.23.6739-6745.2002
(  0) 0) |
| [14] |
Kong H G, Bae J Y, Lee H J, et al. Induction of the viable but nonculturable state of Ralstonia solanacearum by low temperature in the soil microcosm and its resuscitation by catalase[J]. PLoS One, 2014, 9(10): e109792. DOI:10.1371/journal.pone.0109792
(  0) 0) |
| [15] |
Alexander E, Pham D, Steck T R. The viable-but-nonculturable condition is induced by copper in Agrobacterium tumefaciens and Rhizobium leguminosarum[J]. Applied and Environmental Microbiology, 1999, 65(8): 3754-3756. DOI:10.1128/AEM.65.8.3754-3756.1999
(  0) 0) |
| [16] |
Ghezzi J I, Steck T R. Induction of the viable but non-culturable condition in Xanthomonas campestris pv. campestris in liquid microcosms and sterile soil[J]. FEMS Microbiology Ecology, 1999, 30(3): 203-208. DOI:10.1111/j.1574-6941.1999.tb00648.x
(  0) 0) |
| [17] |
del Campo R, Russi P, Mara P, et al. Xanthomonas axonopodis pv. citriâ fenters the VBNC state after copper treatment and retains its virulence[J]. FEMS Microbiology Letters, 2009, 298(2): 143-148. DOI:10.1111/j.1574-6968.2009.01709.x
(  0) 0) |
| [18] |
Ordax M, Biosca E G, Wimalajeewa S C, et al. Survival of Erwinia amylovorain mature apple fruit calyces through the viable but nonculturable(VBNC)state[J]. Journal of Applied Microbiology, 2009, 107(1): 106-116. DOI:10.1111/j.1365-2672.2009.04187.x
(  0) 0) |
| [19] |
Pinto D, Santos M A, Chambel L. Thirty years of viable but nonculturable state research: Unsolved molecular mechanisms[J]. Critical Reviews in Microbiology, 2015, 41(1): 61-76. DOI:10.3109/1040841X.2013.794127
(  0) 0) |
| [20] |
Zhao F, Bi X F, Hao Y L, et al. Induction of viable but nonculturable Escherichia coli O157:H7 by high pressure CO2 and its characteristics[J]. PLoS One, 2013, 8(4): e62388. DOI:10.1371/journal.pone.0062388
(  0) 0) |
| [21] |
Grey B E, Steck T R. The viable but nonculturable state of Ralstonia solanacearum may be involved in long-term survival and plant infection[J]. Applied and Environmental Microbiology, 2001, 67(9): 3866-3872. DOI:10.1128/AEM.67.9.3866-3872.2001
(  0) 0) |
| [22] |
Kussell E. Phenotypic diversity, population growth, and information in fluctuating environments[J]. Science, 2005, 309(5743): 2075-2078. DOI:10.1126/science.1114383
(  0) 0) |
| [23] |
Jain N, Fries B C. Phenotypic switching of Cryptococcus neoformans and Cryptococcus gattii[J]. Mycopathologia, 2008, 166(4): 181-188. DOI:10.1007/s11046-008-9137-9
(  0) 0) |
| [24] |
Chin C Y, Tipton K A, Farokhyfar M, et al. A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii[J]. Nature Microbiology, 2018, 3(5): 563-569. DOI:10.1038/s41564-018-0151-5
(  0) 0) |
| [25] |
Kelman A. The relationship of pathogenicity in Pseudononas solanacearum to colony appearance on tetrazolium medium[J]. Phytopathology, 1954, 44: 693-695.
(  0) 0) |
| [26] |
Buddenhagen I, Kelman A. Biological and physiological aspects of bacterial wilt caused by Pseudomonas solanacearum[J]. Annual Review of Phytopathology, 1964, 2(1): 203-230. DOI:10.1146/annurev.py.02.090164.001223
(  0) 0) |
| [27] |
Kelman A, Hruschka J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum[J]. Journal of General Microbiology, 1973, 76(1): 177-188. DOI:10.1099/00221287-76-1-177
(  0) 0) |
| [28] |
Shekhawat G S. Factors affecting survival in soil and virulence of Pseudomonas solanacearum[J]. Journal of Plant Diseases and Protection, 1991, 98.
(  0) 0) |
| [29] |
[Álvarez B, Biosca E G, López M M. On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen[J]. Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, 2010, 267-279.]
(  0) 0) |
| [30] |
Zhu Y J, Xiao R F, Liu B. Growth and pathogenicity characteristics of Ralstonia solanacearum strain RS1100 in long-term stationary phase culture[J]. Journal of Plant Diseases and Protection, 2010, 117(4): 156-161. DOI:10.1007/BF03356353
(  0) 0) |
| [31] |
Brumbley S M, Denny T P. Cloning of wild-type Pseudomonas solanacearum phcA, a gene that when mutated alters expression of multiple traits that contribute to virulence[J]. Journal of Bacteriology, 1990, 172(10): 5677-5685. DOI:10.1128/jb.172.10.5677-5685.1990
(  0) 0) |
| [32] |
Denny T P. Characterization of Pseudomonas solanacearum Tn5 Mutants deficient in extracellular polysaccharide[J]. Molecular Plant-Microbe Interactions, 1988, 1(5): 215. DOI:10.1094/MPMI-1-215
(  0) 0) |
| [33] |
Peyraud R, Cottret L, Marmiesse L, et al. A resource allocation trade-off between virulence and proliferation drives metabolic versatility in the plant pathogen Ralstonia solanacearum[J]. PLoS Pathogens, 2016, 12(10): e1005939. DOI:10.1371/journal.ppat.1005939
(  0) 0) |
| [34] |
Poussier S, Thoquet P, Trigalet-Demery D, et al. Host plant-dependent phenotypic reversion of Ralstonia solanacearum from non-pathogenic to pathogenic forms via alterations in the phcA gene[J]. Molecular Microbiology, 2003, 49(4): 991-1003. DOI:10.1046/j.1365-2958.2003.03605.x
(  0) 0) |
| [35] |
Battesti A, Majdalani N, Gottesman S. The RpoS-mediated general stress response in Escherichia coli[J]. Annual Review of Microbiology, 2011, 65(1): 189-213. DOI:10.1146/annurev-micro-090110-102946
(  0) 0) |
| [36] |
Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the σS(RpoS)subunit of RNA polymerase[J]. Microbiology and Molecular Biology Reviews, 2002, 66(3): 373-395. DOI:10.1128/MMBR.66.3.373-395.2002
(  0) 0) |
| [37] |
Pu Y Y, Zhao Z L, Li Y X, et al. Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells[J]. Molecular Cell, 2016, 62(2): 284-294. DOI:10.1016/j.molcel.2016.03.035
(  0) 0) |
| [38] |
Pasquaroli S, Zandri G, Vignaroli C, et al. Antibiotic pressure can induce the viable but non-culturable state in Staphylococcus aureus growing in biofilms[J]. Journal of Antimicrobial Chemotherapy, 2013, 68(8): 1812-1817. DOI:10.1093/jac/dkt086
(  0) 0) |
| [39] |
Cappelier J M, Besnard V, Roche S, et al. Avirulence of viable but non-culturable Listeria monocytogenes cells demonstrated by in vitro and in vivo models[J]. Veterinary Research, 2005, 36(4): 589-599. DOI:10.1051/vetres:2005018
(  0) 0) |
| [40] |
Cappelier J M, Besnard V, Roche S M, et al. Avirulent viable but non culturable cells of Listeria monocytogenes need the presence of an embryo to be recovered in egg yolk and regain virulence after recovery[J]. Veterinary Research, 2007, 38(4): 573-583. DOI:10.1051/vetres:2007017
(  0) 0) |
| [41] |
Platt T G, Fuqua C, Bever J D. Resource and competitive dynamics shape the benefits of public goods cooperation in a plant pathogen[J]. Evolution, 2012, 66(6): 1953-1965. DOI:10.1111/j.1558-5646.2011.01571.x
(  0) 0) |
| [42] |
Gray J V, Petsko G A, Johnston G C, et al. "Sleeping beauty": Quiescence in Saccharomyces cerevisiae[J]. Microbiology and Molecular Biology Reviews, 2004, 68(2): 187-206. DOI:10.1128/MMBR.68.2.187-206.2004
(  0) 0) |
| [43] |
Ouafa Z A, Reverchon S, Lautier T, et al. The nucleoid-associated proteins H-NS and FIS modulate the DNA supercoiling response of the pel genes, the major virulence factors in the plant pathogen bacterium Dickeya dadantii[J]. Nucleic Acids Research, 2012, 40(10): 4306-4319. DOI:10.1093/nar/gks014
(  0) 0) |
| [44] |
Zghidi-Abouzid O, Hérault E, Rimsky S, et al. Regulation of pel genes, major virulence factors in the plant pathogen bacterium Dickeya dadantii, is mediated by cooperative binding of the nucleoid-associated protein H-NS[J]. Research in Microbiology, 2016, 167(4): 247-253. DOI:10.1016/j.resmic.2016.02.001
(  0) 0) |
| [45] |
Rakhuba D V, Kolomiets E I, Dey E S, et al. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell[J]. Polish Journal of Microbiology, 2010, 59(3): 145-155. DOI:10.33073/pjm-2010-023
(  0) 0) |
| [46] |
Young G M, Schmiel D H, Miller V L. A new pathway for the secretion of virulence factors by bacteria: The flagellar export apparatus functions as a protein-secretion system[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(11): 6456-6461. DOI:10.1073/pnas.96.11.6456
(  0) 0) |
| [47] |
Tans-Kersten J, Brown D, Allen C. Swimming motility, a virulence trait of Ralstonia solanacearum, is regulated by FlhDC and the plant host environment[J]. Molecular Plant-Microbe Interactions, 2004, 17(6): 686-695. DOI:10.1094/MPMI.2004.17.6.686
(  0) 0) |
| [48] |
Francis G, Brennan L, Stretton S, et al. Genetic mapping of starch-and lambda-receptor sites in maltoporin: Identification of substitutions causing direct and indirect effects on binding sites by cysteine mutagenesis[J]. Molecular Microbiology, 1991, 5(9): 2293-2301. DOI:10.1111/j.1365-2958.1991.tb02160.x
(  0) 0) |
| [49] |
Meyer J R, Gudelj I, Beardmore R. Biophysical mechanisms that maintain biodiversity through trade-offs[J]. Nature Communications, 2015, 6: 6278. DOI:10.1038/ncomms7278
(  0) 0) |
| [50] |
Carroll A C, Wong A. Plasmid persistence: Costs, benefits, and the plasmid paradox[J]. Canadian Journal of Microbiology, 2018, 64(5): 293-304. DOI:10.1139/cjm-2017-0609
(  0) 0) |
| [51] |
Vogwill T, MacLean R C. The genetic basis of the fitness costs of antimicrobial resistance: A meta-analysis approach[J]. Evolutionary Applications, 2015, 8(3): 284-295. DOI:10.1111/eva.12202
(  0) 0) |
| [52] |
Phan K, Ferenci T. A design-constraint trade-off underpins the diversity in ecologically important traits in species Escherichia coli[J]. The ISME Journal, 2013, 7(10): 2034-2043. DOI:10.1038/ismej.2013.82
(  0) 0) |
| [53] |
Raza W, Wei Z, Ling N, et al. Effect of organic fertilizers prepared from organic waste materials on the production of antibacterial volatile organic compounds by two biocontrol Bacillus amyloliquefaciens strains[J]. Journal of Biotechnology, 2016, 227: 43-53. DOI:10.1016/j.jbiotec.2016.04.014
(  0) 0) |
| [54] |
Friman V P, Lindstedt C, Hiltunen T, et al. Predation on multiple trophic levels shapes the evolution of pathogen virulence[J]. PLoS One, 2009, 4(8): e6761. DOI:10.1371/journal.pone.0006761
(  0) 0) |
| [55] |
Hibbing M E, Fuqua C, Parsek M R, et al. Bacterial competition: Surviving and thriving in the microbial jungle[J]. Nature Reviews Microbiology, 2010, 8(1): 15-25. DOI:10.1038/nrmicro2259
(  0) 0) |
| [56] |
Schell M A. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network[J]. Annual Review of Phytopathology, 2000, 38(1): 263-292. DOI:10.1146/annurev.phyto.38.1.263
(  0) 0) |
| [57] |
van Elsas J D, Kastelein P, van Bekkum P, et al. Survival of Ralstonia solanacearum biovar 2, the causative agent of potato brown rot, in field and microcosm soils in temperate climates[J]. Phytopathology, 2000, 90(12): 1358-1366. DOI:10.1094/PHYTO.2000.90.12.1358
(  0) 0) |
| [58] |
Yao J, Allen C. Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum[J]. Journal of Bacteriology, 2006, 188(10): 3697-3708. DOI:10.1128/JB.188.10.3697-3708.2006
(  0) 0) |
| [59] |
Hoffman L R, D'Argenio D A, MacCoss M J, et al. Aminoglycoside antibiotics induce bacterial biofilm formation[J]. Nature, 2005, 436(7054): 1171-1175. DOI:10.1038/nature03912
(  0) 0) |
| [60] |
Harrison J J, Ceri H, Turner R J. Multimetal resistance and tolerance in microbial biofilms[J]. Nature Reviews Microbiology, 2007, 5(12): 928-938. DOI:10.1038/nrmicro1774
(  0) 0) |
| [61] |
Yao J, Allen C. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host[J]. Journal of Bacteriology, 2007, 189(17): 6415-6424. DOI:10.1128/JB.00398-07
(  0) 0) |
| [62] |
Franza T, Expert D. Role of iron homeostasis in the virulence of phytopathogenic bacteria: An 'à la carte' menu[J]. Molecular Plant Pathology, 2013, 14(4): 429-438. DOI:10.1111/mpp.12007
(  0) 0) |
| [63] |
Gu S H, Wei Z, Shao Z Y, et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes[J]. Nature Microbiology, 2020, 5(8): 1002-1010. DOI:10.1038/s41564-020-0719-8
(  0) 0) |
| [64] |
刘波, 林营志, 朱育菁, 等. [J]. 农业生物技术学报, 2004, 12(3): 322-329. Liu B, Lin Y Z, Zhu Y J, et al. Attenuation characteristics of bacterial-wilt-disease biocontrol strain Anti-8098A(Bacillus cereus)to Ralstonia solanacearum[J]. Journal of Agricultural Biotechnology, 2004, 12(3): 322-329. (  0) 0) |
| [65] |
Wang X F, Wei Z, Yang K M, et al. Phage combination therapies for bacterial wilt disease in tomato[J]. Nature Biotechnology, 2019, 37(12): 1513-1520. DOI:10.1038/s41587-019-0328-3
(  0) 0) |
| [66] |
Xiong W, Song Y, Yang K M, et al. Rhizosphere protists are key determinants of plant health[J]. Microbiome, 2020, 8(1): 27. DOI:10.1186/s40168-020-00799-9
(  0) 0) |
| [67] |
Rossez Y, Wolfson E B, Holmes A, et al. Bacterial flagella: Twist and stick, or dodge across the kingdoms[J]. PLoS Pathogens, 2015, 11(1): e1004483. DOI:10.1371/journal.ppat.1004483
(  0) 0) |
| [68] |
Yang L, Li S L, Qin X Y, et al. Exposure to umbelliferone reduces Ralstonia solanacearum biofilm formation, transcription of type Ⅲ secretion system regulators and effectors and virulence on tobacco[J]. Frontiers in Microbiology, 2017, 8: 1234. DOI:10.3389/fmicb.2017.01234
(  0) 0) |
| [69] |
Lowe T M, Ailloud F, Allen C. Hydroxycinnamic acid degradation, a broadly conserved trait, protects Ralstonia solanacearum from chemical plant defenses and contributes to root colonization and virulence[J]. Molecular Plant-Microbe Interactions, 2015, 28(3): 286-297. DOI:10.1094/MPMI-09-14-0292-FI
(  0) 0) |
| [70] |
Brown D G, Swanson J K, Allen C. Two host-induced Ralstonia solanacearum genes, acrA and dinF, encode multidrug efflux pumps and contribute to bacterial wilt virulence[J]. Applied and Environmental Microbiology, 2007, 73(9): 2777-2786. DOI:10.1128/AEM.00984-06
(  0) 0) |
| [71] |
Huang X Q, Cai Z C. Soil microbes and control of soil-borne diseases (In Chinese)[J]. Bulletin of the Chinese Academy of Sciences, 2017, 32(6): 593-600. [黄新琦, 蔡祖聪. 土壤微生物与作物土传病害控制[J]. 中国科学院院刊, 2017, 32(6): 593-600.]
(  0) 0) |
 2022, Vol. 59
2022, Vol. 59

