2. 合肥工业大学土木与水利工程学院, 合肥 230009;
3. 中国科学院地理科学与资源研究所, 北京 100101
2. College of Civil Engineering, Hefei University of Technology, Hefei 230009, China;
3. Institude of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing 100101, China
解钾菌(Potassium-solubilizing bacteria,KSB)通常是指从土壤中分离出来的一类能够分解含钾矿物的细菌,它能将矿物钾转化为速效钾而供植物直接利用,对作物产量的提高和土壤肥力的改善具有重要作用[1-2]。解钾菌也被称为硅酸盐细菌或钾细菌,其分离及释钾能力的评定通常是在以钾长石[3]、白云母[4]或黑云母[5]作为唯一钾源的改良Aleksandrov培养基上进行的。由于解钾菌的植物促生、土壤培肥以及环境友好等特点,促使对其的相关研究受到国内外学者的广泛关注[6-7]。
土壤中解钾菌种类不一、功能多样。解钾菌不仅能够通过分解含钾矿物来增加土壤速效钾含量,而且还可通过活化土壤中的生物酶类(如土壤脲酶、过氧化氢酶和蔗糖酶等)并使其活性处于适宜范围,从而促进动植物残体及凋落物的分解与转化,进而提高土壤中其他营养元素的有效性[3,8]。此外,解钾菌还能够通过分泌吲哚乙酸(indole-3-acetic acid,IAA)、1-氨基环丙烷-1-羧酸(1-aminocyclopropane- 1-carboxylate,ACC)、赤霉素(gibberellin,GA)、铁载体(siderophore)和脱氨酶(deaminase)等物质来促进作物生长,同时对植株抗逆性的提高也有关键作用[9-12]。需注意的是,蒋先军等[13]的研究表明促进作物生长的解钾菌代谢产物存在适宜浓度范围。
近十年来,学者们采用室内盆栽试验和大田试验对接种解钾菌的效果进行了大量研究,发现接种确实能显著提高土壤肥力,提升作物产量和品质。Prajapati和Modi[14]的研究结果表明Enterobacter hormoechei在水培条件下能显著增加黄瓜的根长、开花结果率、果实成熟度、钾含量和叶绿素含量。Sugumaran和Janarthanam[15]通过微缩试验发现使用从印度土壤中分离得到的Bacillus mucilaginosus接种花生,可提高土壤钾含量、植株产量和种子含油量。此外,一些田间试验也证明接种解钾菌可以提高茄子作物产量[16],烟草生物量、养分含量、钾吸收量和叶片品质[17]以及水稻株高、茎粗、根长、叶面积和生物量干重[10]。此外,学者们对解钾菌作为生物钾肥的施用效果也进行了多次试验,结果表明生物钾肥能提高土壤有效氮、磷、钾含量以及作物块茎产量和品质[3,18]。因此解钾菌作为生物肥料用于农业改良确实可以有效减少化肥使用,促进生态友好型作物的生产。
近年来,大量学者聚焦于研究解钾菌与含钾矿物或其他植物促生根菌(plant growth-promoting rhizobacteria,PGPR)结合施用对作物生长的影响。许多研究表明将含钾矿物与解钾菌结合作为生物肥料施用,可能是一种替代传统化肥、降低生产成本的有效手段[4,19-20]。Sarhan和Abd El-Gayed[21]研究了将长石作为钾源并结合一些生物刺激物(解钾菌、腐殖酸和农家肥)的施用对棉花产量和收获后土壤性状的影响,结果表明解钾菌分别与长石、腐殖酸或农家肥结合施用对棉花产量的影响更显著。同时,大量试验证明解钾菌与解磷菌、丛枝菌根真菌或固氮菌等PGPR共同接种更能够促进作物生长发育,增加土壤养分供给[4,6-7,22]。
可见,前人主要围绕解钾菌宏观方面开展了大量研究,然而对影响解钾菌释钾能力的关键——解钾菌和矿物间微观互作机制——了解不多。本文主要从单个浮游解钾菌细胞入手,阐述其在含钾矿物固-液界面的聚集、吸附及生物膜形成和循环,直至最终解钾功能的发挥,并总结了影响该微观过程的关键因素,且详细介绍了与该过程相关的前沿科学技术,旨在为解钾菌微观释钾机制研究提供理论和技术参考。
1 解钾菌宏观解钾机制在过去的几十年里,研究者们从宏观角度详细阐述了解钾菌解钾机制,主要的观点有酸解[23]、酶解[24]和多糖络合溶解[23]等,该类结论均是学者们基于解钾菌能够分泌胞外聚合物(extracellular polymeric substances,EPS)而得出的。然而事实上,除细菌“EPS分泌量和组分”影响其解钾能力外(不同细菌EPS分泌种类和含量差异较大,表 1),细菌运动性也是影响解钾能力的核心因子,运动性通过调控细菌在矿物表面的黏附量和生物膜循环影响矿物释钾能力。无机酸和有机酸的产生是含钾矿物风化的主要机制[25-26]。解钾菌产生的有机酸和无机酸可以通过降低其周围环境的pH,直接促进含钾矿物的风化,导致可交换性钾的缓慢释放,提高环境速效钾含量[27-28]。另一方面,含有羟基和羧基的有机酸易与矿物中的金属离子发生反应形成金属-有机络合物,从而破坏矿物的结构,促进矿物中钾的释放[29]。Groudev[30]于1987年首次发现胞外多糖也能够提高解钾菌解钾能力。胞外多糖中含有具有络合作用的官能团,它们能与矿物中的Si4+、Al3+和K+络合从而促进含钾矿物的分解[23]。同时,Liu等[31]的研究表明,B. mucilaginosus菌株产生的多糖能够吸附有机酸并附着在矿物表面,从而导致矿物表面微区域有机酸浓度的增高。除此之外,荚膜多糖还能够通过吸附SiO2来影响固相和液相的平衡,从而加速SiO2和K+的溶解。除了有机酸和多糖外,解钾菌分泌的蛋白质(或酶)也参与含钾矿物的分解。一方面,蛋白质中含有具有络合作用的官能团(氨基、羧基等);另一方面,在矿物存在的条件下,细菌能够通过相关功能基因组的表达来实现蛋白质的调节,从而控制微生物对矿物的风化[24,32]。例如,Chen等[33]在使用双向凝胶电泳(two dimensional electrophoresis,2-DE)研究胶质芽孢杆菌分解磷矿石机理时,发现处理组(含矿物)菌体细胞的蛋白点表达量与对照组(不含矿物)相比,共有差异蛋白点496个,其中,有207个蛋白质点为新合成,214个蛋白质点表达上调。这表明细菌在风化矿石时能够激活或增加某些蛋白的表达,这些蛋白点很可能与细菌的代谢调控、增生和信号转导通路等有关。
|
|
表 1 土壤中解钾菌性能特征(胞外分泌物、革兰氏染色反应和运动性) Table 1 Characteristics of soil potassium-solubilizing bacteria(EPS secreted, gram reaction, and motility) |
解钾菌与含钾矿物的直接接触对于解钾过程至关重要。解钾菌可通过分泌多糖黏附于含钾矿物表面从而形成特殊的微环境来促进其解钾功能的发挥,同时,其生长过程中所带来的物理破坏作用也对含钾矿物的风化非常关键[32,47-48]。但直接接触并不是必需过程,孙德四等[48]研究了环状芽孢杆菌在细菌-矿物直接接触与间接接触(微孔滤膜将细菌与矿物隔离)两种模式下对铝土矿风化分解行为的影响,发现细菌及其代谢产物能够通过直接和间接作用共同促进铝土矿的分解,但直接作用的强度明显大于间接作用。此外,满李阳等[32]的研究结果也进一步证实了这一观点,并提出含钾矿物中钾的释放在接触前期主要受间接作用的影响,而在接触后期主要受直接作用的影响。
间接接触:解钾菌分泌的无机酸、有机酸、多糖以及蛋白质等物质能够通过降低环境pH、质子交换、络合或螯合等作用促进含钾矿物的分解。而从微观角度解释这些物质分泌机理的研究还处于空白阶段,目前作者设想该过程可能是由细菌的竞争(bacterial competition)或群体感应(quorum sensing)导致。在养分缺乏的环境中,细菌的竞争机制能够促使其向外分泌特殊的代谢产物、酶或胞外囊泡等,或通过占用其他菌株的EPS,来与竞争者争夺养分或抑制竞争者的生长[49-50]。例如,细菌可以通过向外分泌铁载体(特殊的代谢物)的形式占据更多的铁素资源,从而减少其竞争者的铁供应,铁载体不仅可与溶液中的铁离子形成络合物,而且还可以将一些深埋于矿物或其他螯合物中的铁离子拽出来,从而促进菌体对铁的吸收[51];细菌的运动性及其分泌的EPS(如黏附素、鼠李糖脂和脂肪酸顺-2-癸二酸等)有助于其占据生长空间,通过促使生物膜的形成来提高竞争力[50]。此外,细菌能通过向外分泌自我诱导物(autoinducers,即信号分子)来进行群体感应,当信号分子的浓度达到一定阈值时,菌体相关基因的表达就会被启动以适应或改变周围环境[52]。
直接接触:解钾菌单个细胞(single-cell)可通过主动扩散(自主运动)或被动运输(水体剪切流、重力沉降等)的方式到达矿物界面,形成生物膜。上述过程对解钾菌解钾作用的发挥和解钾效率至关重要。细菌往往生活在低雷诺系数(Reynolds numbers,约10–5)[53]的水环境(acqueous environment)中,其扩散能力可以由扩散系数(diffusion coefficient,D)来表征,能动性细菌(如Escherichia coli)在水中的扩散系数大约为4×10–10 m2·s–1,而不具有运动能力的细菌(如缺失鞭毛的E. coli)的扩散系数大约为3×10–13 m2·s–1,远小于运动细菌[54]。不具有运动能力的单个细菌在水中的运动主要依靠布朗运动,其扩散系数的值相当于与其等大小的惰性粒子的值[55],计算公式如下:
| $ D = \frac{{ < {r^2} > }}{{4t}} $ | (1) |
式中,
而同等大小的惰性粒子的布朗扩散系数可由斯托克斯-爱因斯坦方程(Stokes-Einstein equation)[56]得出:
| $ D = \frac{{KT}}{{6\pi \eta {\text{a}}}} $ | (2) |
式中,K为波尔兹曼常数(Boltzmann constant),T为绝对温度(Kelvin temperature),η为黏度(viscosity),a为粒子半径(particle radius)。目前实验已证明用以上两个公式计算不运动细菌的扩散系数,其值相差不大,约为10–13 m2·s–1数量级。
此外,Lovely和Dahlquist[57]于1975年首次给出了基于单细菌细胞运动速度计算扩散系数的方法:
| $ D = \frac{{{v^2}T}}{{3(1 - \alpha )}} $ | (3) |
式中,v为运动速率(speed),T为细胞单个运动轨迹的平均持续时间(the mean duration of a single run trajectory),α为旋转角度余弦的平均值(the mean cosine of the angle between successive trajectories),其测量需要细菌的三维运动轨迹。同时该公式的成立需要很多假设条件(如前进方向为直线、所有轨迹速度相同等),与细菌实际运动状况存在差异,还需要我们进行更多的深入研究。
上述公式是基于单个细菌细胞对细菌扩散的计算。此外,仍可利用菲克方程(Fick's equations)[56]计算基于细菌种群水平的扩散系数,
| $ {J_{\text{x}}} = - D\frac{{\partial C}}{{\partial x}} + {v_d}C $ | (4) |
式中,Jx为x轴的通量(flux at point
| $ \frac{{\partial C}}{{\partial t}} = D\frac{{{\partial ^2}C}}{{\partial {x^2}}} - {v_d}\frac{{\partial C}}{{\partial x}} $ | (5) |
式中,C为细菌种群浓度(bacterial population concentration),其值随扩散时间t变化。式(5)为菲克第二定律,适用于浓度随时间变化的非稳态扩散(none steady-state diffusion)。
同时,由式(2)的计算结果可知养分分子在水中的扩散系数大约为10–9 m2·s–1,明显大于细菌随机运动的扩散系数[58-59]。同时,在具有一定化学浓度梯度的溶液中,细菌的扩散系数会显著增加[54,60]。这说明溶液中的养分分子更容易到达固-液界面,从而诱导细菌朝固-液界面进行趋化运动并形成生物膜。这很可能是土壤中99%的细菌都吸附于生物或非生物表面(如土壤颗粒、根系等),以生物膜的形式存在并发挥作用的主要原因[61]。单个浮游解钾菌微观释钾过程可以大致认为是单个浮游细菌趋向矿物表面运动聚集、吸附及生物膜形成和循环的过程,这个过程可大致分为四个阶段(图 1e):
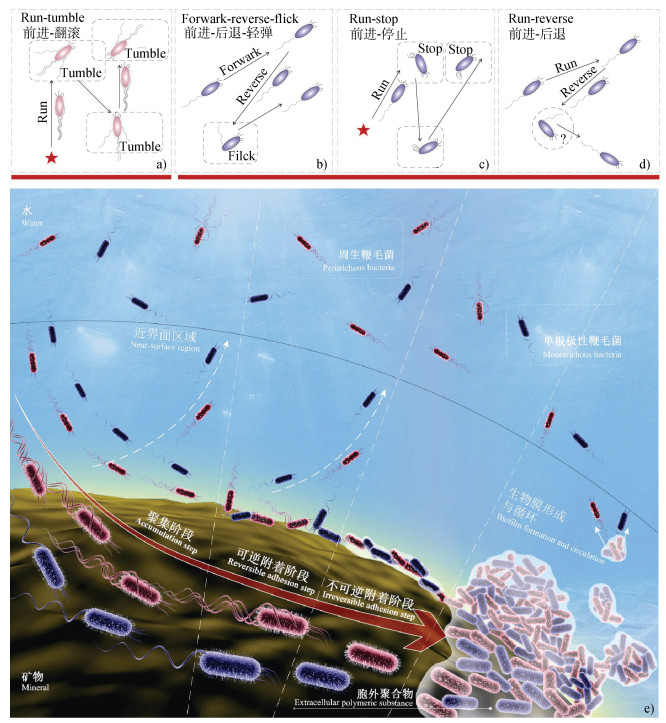
|
注:a)是以周生鞭毛菌(如E. coli)为例的自由运动,前进-翻滚(run-tumble)运动;b)、c)和d)是单根极性鞭毛菌的自由运动,分别是以Vibrio alginolyticus为例的前进-后退-轻弹(forward-reverse-flick)运动,以Rhodobacter spheroides为例的前进-停止(run-stop)运动,以P. aeruginosa(PAO1)为例的前进-后退(run-reverse)运动;e)为细菌-矿物微观作用过程。 Note: a)stands for free motion of periflagellates(such as E. coli), like run-tumble movement; and b), c)and d)for free motion of single polar flagella(V. alginolyticus, R. spheroides and P. aeruginosa), like forward-reverse-flick movement b), run-stop movement c), run-reverse movement d); and e)for bacteria-mineral microscopic interaction processes. 图 1 细菌在矿物表面生物膜形成过程 Fig. 1 Biofilm forming process on mineral surfaces |
(1)聚集阶段:大量单个浮游细菌细胞迁移至矿物-水界面并被其捕获;
(2)可逆附着阶段:细菌细胞感知固-液界面,并在“物理-化学综合作用力”下可逆附着到固-液界面上;
(3)不可逆附着阶段:细菌细胞由对固-液界面的可逆附着向不可逆附着转移;
(4)生物膜形成与循环:不可逆黏附在固-液界面上的细胞大量分裂增殖,直至形成成熟的生物膜,最后生物膜最外层的活细胞脱落并开启新循环。
2.1 聚集阶段在聚集阶段,细菌的运动性(趋化运动或随机运动)及水体的剪切流发挥着主导作用。在适宜的环境条件下,具有游动(swimming)能力的细菌能够借助自身鞭毛马达的旋转在溶液中进行运动,细菌根据自身鞭毛类型(生长位置和数量)进化了多种运动模式[62-64]。如周生鞭毛菌E. coli[65]、Salmonella typhimurium[62]和Bacillus subtilis[62]等执行的前进-翻滚(run-tumble)运动(如图 1a)。从细菌后面来看,鞭毛逆时针旋转拧成一束从而推动细胞向前运动,而细胞体顺时针旋转以保持扭矩平衡,当某些鞭毛顺时针旋转或散开时,细菌前进方向就会改变,所选择的方向取决于束相对于细胞体的变化[66-67]。而单根极性鞭毛菌由于不能通过鞭毛束的散开随机选择方向,其运动性更为复杂[68-69]。早期,学者们认为只有布朗运动可以帮助单根极性鞭毛菌完成再定位,这是因为布朗运动能够对小型单鞭毛细菌的运动产生影响,同时改变它们的前进轨迹和后退时的细胞取向[68,70],如R. spheroides[71-72]执行的前进-停止(run-stop)运动(如图 1c)以及Caulobacter crescentus[73]和P. aeruginosa(PAO1)[74]执行的前进-后退(run-reverse)运动(如图 1d)。目前,关于PAO1的运动模式还存在争议,Qian等[75]的研究表明其运动遵循run-reverse-turn模式,但Stocker[62]则认为PAO1的运动很可能按照run-reverse-flick模式。近年来,研究者们发现单根极性鞭毛菌也可以通过鞭毛离轴变形(旋转轴心偏离细胞体轴心)来改变其前进方向,如V. alginolyticus执行的前进-后退-轻弹(forward- reverse-flick)运动(如图 1b)[76]。然而,在实际情形中,细菌进行随机运动的机会并不多。一方面,环境中的水流会影响细菌的运动方向,当剪切速率不大于10 s–1时,鞭毛的推动力仍然在细菌运动中发挥主导作用,而当剪切速率大于10 s–1时,剪切流就会通过影响细菌细胞体-流体界面的作用力来主导细菌的运动方向[77-78]。另一方面,环境中常常存在化学浓度梯度,而细菌细胞膜上的跨膜蛋白能感知化学浓度变化,并将信号传递给鞭毛,促使其通过调节自身旋转方向进行具有偏向性的趋化运动[60]。矿物-水界面由于表面张力,常常吸附大量养分分子(钾素及其他养分),细菌的趋化性会驱动细菌主动向矿物-水界面靠近,以便从中获得代谢过程所需要的电子受体、钾素及其他营养物质,最终使细菌大量聚集到矿物-水的近界面区域内[79]。研究发现,相对于随机运动而言,细菌趋化运动会使细菌在沿着高养分浓度梯度上的运动时间增加[63]。相对于游动细菌而言,不具有运动能力的细菌到达矿物-水近界面区域的主要方式有布朗运动、重力沉降和剪切流[80]。除剪切流和养分浓度梯度外,环境溶液的离子强度、pH、温度及矿物表面物理化学特性(粗糙度、所带电荷性质及数量等)均会影响细菌在矿物-水界面的聚集。因此,我们推测上述物理-化学因素均是影响解钾菌在含钾矿物表面聚集的关键,且聚集过程又是影响解钾菌与含钾矿物接触并在其表面形成生物膜的关键过程。
2.2 可逆附着阶段在可逆附着阶段,细菌到达矿物-水近界面区域(距离界面20 μm以内的区域,不固定,随细菌和界面特征而变化)内,虽然自身趋化性整体效果减弱,但却被细菌与界面间的相互作用力(水动力学相互作用力和DLVO力等)捕获而难以逃离[81-82]。Molaei等[83]指出在近界面约20 μm区域内,E. coli的tumble行为降低了50%,且再定位的方向主要局限于与界面平行的方向上,这表明这时tumble并不能使细菌挣脱界面的束缚。当细菌游动时,鞭毛的运动会干扰周围液体从而形成流动,而固液界面(尤其是距离细菌几个身体长度范围内的界面)的存在又会影响这种流动,从而反过来影响细菌本身的运动,这种机制被称为水动力学相互作用,可以大大促进细菌在矿物-水界面的定殖[82]。当一个非圆形细胞从自由溶液向固液界面靠近时,由于作用于细胞体和鞭毛束下部的黏性曳力大于作用于两者上部的曳力,细菌在界面上方产生高曲率曲线运动,同时,由于其非球形而产生的曳力会使细胞保持一个相对于界面的特定倾角,因此细菌常在近界面区域以一定的角度向下倾斜着做曲线运动并逐渐向界面旋转靠近[70,84-85]。由于细菌的形状、大小、鞭毛马达的扭矩特征、鞭毛丝的变形及其与界面间的作用力在细菌的不同种类间存在差异,其旋转方向和速度也会因此存在不同[84,86]。
当细菌与界面相距大约9 a(a为细胞体半径)时,界面与细胞体及胞外运动附属物(鞭毛和Ⅳ型菌毛)间的摩擦力变得不可忽视,摩擦力会通过影响细菌的运动速度来增加细菌在界面的停留时间,从而提高细菌黏附到界面的可能性[87]。随着距离的进一步缩短,细菌鞭毛和胞外Ⅳ型菌毛(type Ⅳ pili,TFP)开始感知到界面的存在并对界面产生吸附作用,其中TFP通过自身的收缩或拉伸介导的细菌在矿物表面的运动(walking和crawling)对细菌充分利用矿物表面空间非常关键[88-89]。另一方面,与界面的接触会抑制鞭毛马达的旋转和TFP的收缩,从而促使细菌在界面的附着[90]。而当细菌与矿物-水界面的距离进一步缩短至大约50 nm时,DLVO(Derjaguin-Landau-Verwey-Overbeek)力(范德华相互吸引力和静电相互排斥力)开始发挥作用,细菌细胞被DLVO力捕获,但这个距离并不固定且取决于细胞和界面的物理化学特征[85]。当细菌与界面距离较远时,起决定作用的是范德华相互吸引力,随着距离的逐渐缩短,细菌细胞的双电层与界面的电子层(均常带有负电荷)重叠产生了静电相互排斥力[91]。当细菌细胞逐渐接近界面时会存在能量势垒(energy barrier),在能量势垒外存在一个浅层二次能量最小值(the secondary energy minimum),从界面到二次能量最小值的距离通常在几纳米或十几纳米内(不固定,取决于溶液离子强度),这个距离是细菌与界面的最佳距离[87,92]。在细菌黏附的第一步,细菌细胞通常通过自身的运动性或布朗运动到达这个位置,并可逆地附着在界面上。目前,关于DLVO力在细菌可逆附着阶段的作用仍存在争议,Vigeant等[85]认为可逆吸附的细胞距离界面的距离太远,并不能用DLVO力来解释,DLVO力只能用于描述细菌从可逆附着到不可逆附着阶段。
Li等[87]发现当细菌在界面附近运动时,布朗运动的作用会被放大,这是由于布朗运动会显著改变细菌和液-固界间的距离,进而影响两者间的水动力学作用和DLVO力等,从而促使其对界面的附着。这些物理-化学作用力很可能也是非游动细菌(non- swimming bacteria)和失去趋化感知能力的游动细菌(non-chemotactic swimming bacteria)在矿物-水界面附着的主要原因。总而言之,在细菌细胞到达矿物-水近界面区域(大约<20 μm)后,水动力学相互作用力和布朗运动首先起作用;而随着细胞与液-固界面间距离的缩小(<9 a),水动力学相互作用力、细菌与界面间的摩擦力和布朗运动起作用;当细胞进一步靠近界面(<50 nm)时,由于空间有限细菌鞭毛无法旋转,且细菌很可能感知到液-固界面并停止鞭毛旋转从而黏附到界面上,细菌与液-固体界面间的水动力学相互作用力消失,此时,DLVO力、鞭毛和菌毛对于界面的黏附作用和布朗运动发挥作用。这些力都会增加细菌细胞可逆附着到矿物表面的可能性。而在此阶段中,环境温度、溶液离子强度、pH和解钾菌胞外运动附属物的类型等均会影响解钾菌的可逆附着[77,91]。
2.3 不可逆附着阶段在不可逆附着阶段,细菌与矿物表面距离达到几十纳米,细菌由可逆附着向不可逆附着转移。此时,范德华相互吸引力和疏水相互作用(hydrophobic interactions)起主导作用[79,85]。疏水相互作用力源于水分子间氢键聚合的键能,细菌与矿物间的疏水作用为引力,有利于两者的吸附[91,93]。而在这个过程中,细菌开始向外分泌EPS,如DNA、蛋白质、脂类、脂多糖等,这些物质可以将细菌和界面连接起来,促进两者间的不可逆附着[92,94-95]。此外,由于鞭毛和TFP马达受到抑制,细菌会被促使着产生一些其他物质,如C. crescentus分泌的纳米黏合剂holdfast和大多数细菌均能够产生的胞内环二鸟苷单磷酸(cyclic diguanylate monophosphate,c-di- GMP),从而使细菌在界面的附着更牢固。除此之外,细菌鞭毛和TFP对于界面的吸附都会促进其不可逆附着[95]。环境温度和离子强度、解钾菌和含钾矿物表面的离子性质和强度及解钾菌运动附属物的类型等都会对这个与之对应的解钾过程产生影响[77,91]。
2.4 生物膜形成与循环阶段在生物膜形成与循环阶段,细菌不断生长增殖并分泌大量EPS,在矿物表面形成有组织的生物膜。而当生物膜成熟之后,最外层的活细胞脱落并开始新生物膜形成的循环过程[96]。在这个过程中,解钾菌分泌的EPS通过上述宏观解钾机制促进含钾矿物的溶解以及细菌对营养元素的吸收。因此,解钾菌分泌EPS的种类和数量对于解钾过程来说至关重要。
解钾菌的运动性在生物膜形成前期极其重要,EPS在生物膜形成后期具有不可替代的地位,而解钾菌宏观解钾机理主要是其EPS的作用,这说明解钾菌生物膜的形成过程(微观解钾机制)与解钾菌宏观解钾过程密不可分,息息相关[97]。上述四个阶段清晰地介绍了单个浮游解钾菌细胞到达含钾矿物界面并在其表面形成生物膜,以及发挥解钾机制的具体过程及其影响因素,同时还强调了解钾菌自身运动性、水动力学相互作用、DLVO理论和布朗运动在该过程中的重要性。从该视角出发能为解钾菌解钾机制的研究提供一个新方向。
3 解钾菌解钾机制研究的前沿技术和相关理论越来越多的前言技术随着科学发展应运而生,显微成像技术(数字全息显微镜、全内反射荧光显微镜、激光共聚焦显微镜、原子力显微镜和电子显微镜等)和平面光极技术等的交叉、灵活应用对解钾菌解钾机制的深入研究具有重要推动作用(图 2)。

|
注:EDS,能谱仪;a)是由透射电镜(TEM)拍摄的细菌形态图像[117];b)为数字全息显微镜(DHM)拍摄的细菌三维运动轨迹图像[100];c)为由全内反射荧光显微镜(TIRFM)拍摄的图像得出的细菌在近界面(100 nm内)的三维运动轨迹[87];d)为原子力显微镜(AFM)得到的近界面区域细菌受到的界面力的情况[118];e)为由平面光极技术(PO)拍摄的二维pH分布图[119];f)为激光共聚焦扫描显微镜(CLSM)拍摄的细菌在界面的分布图像[120];g)为原子力显微镜(AFM)拍摄的矿物表面生物膜图像[121];h)为使用扫描电镜(SEM)得到的矿物表型特征图像[122]。 Note: EDS, energy dispersive spectroscopy; a)Morphological image of bacteria taken by transmission electron microscope(TEM)[117]; b)Three-dimensional motion trajectory image of bacteria taken by digital holographic microscopy(DHM)[100]; c)Three-dimensional trajectories of bacteria at near interface(within 100 nm)reflected in the image obtained by total internal reflection fluorescence microscope(TIRFM)[87]; d)Image of interfacial force on bacteria in the near-interface region obtained by atomic force microscopy(AFM)[118]; e)Two-dimensional pH distribution image photographed by planar optode technology(PO)[119]; f)Image of distribution of bacteria at the interface captured by confocal laser scanning microscope(CLSM)[120]; g)Biofilm image in the mineral surface taken with atomic force microscopy(AFM)[121]; and h)Mineral phenotypic image obtained by scanning electron microscope(SEM)[122]. 图 2 解钾菌微观机制研究的相关技术图 Fig. 2 Technical drawings related to researches on microscopic mechanisms of bacteria solubilizing potassium |
光学显微镜:(1)数字全息显微镜(digital holographic microscopy,DHM)主要是以再现技术和光学显微技术满足折射或者透视物体3D轮廓,从而获取被测物体的振幅信息和相位信息,进而完成数字三维重构,其轴向分辨率为0.6 nm,横向分辨率为300 nm(取决于物镜的数值孔径),测量范围可达4 mm(1.25倍)[89-99]。该显微镜目前已应用于无荧光条件下生物样本和活体细胞的三维运动轨迹观测、三维形貌测量和变形测量[100-101]。在解钾菌解钾机制的研究中,DHM可以用于观测解钾菌由浮游状态运动至含钾矿物界面附近并在其界面形成微菌落的3D运动轨迹,同时也能测定该过程中含钾矿物表面形态的变化。(2)全内反射荧光显微镜(total internal reflection fluorescence microscope,TIRFM)是利用全内反射产生的隐失波照明样品,使照明厚度限定在几百纳米厚(200 nm,甚至是100 nm)的光学薄层内的显微术。该显微镜可以用于活细胞单分子成像、生物大分子在界面吸附行为的研究和酶反应的观测等[102-103]。TIRFM能够用于测定解钾菌在含钾矿物界面附近几百个纳米范围内的运动特征,且能够观察到运动特征随着与界面距离的改变而产生的变化情况[87]。(3)激光共聚焦扫描显微镜(confocal laser scanning microscope,CLSM)是一种集显微技术、高速激光扫描和图像处理于一体的新型光学显微术,放大倍数可达32 000倍,分辨率可以达到普通显微镜的1.4倍[104]。该显微镜可用于测定矿物三维结构,表征细菌在界面形成的生物膜,获得高分辨率的细菌彩色荧光图像[105-106]。结合细胞染色或荧光标记,CLSM可以用于观测解钾菌的实时运动变化和在含钾矿物表面形成的生物膜形貌变化以及矿物表面粗糙度的实时变化。(4)原子力显微镜(atomic force microscopy,AFM)是用一端固定而另一端装有纳米级针尖的弹性微悬臂来检测样品表面结构三维形貌或原位定量测量界面和细菌细胞之间界面力的显微术[107-108]。该显微镜可以用于测量含钾矿物表面粗糙度以及解钾菌与含钾矿物间的DLVO力等,对于理解生物膜的形成以及解钾菌对于特定矿物表面的亲和力至关重要[109]。
电子显微镜是使用波长极短的电子束做光源,电磁场做透镜,分辨率可达0.1 nm的电子光学仪器[110]。电子显微镜又分为透射电子显微镜(transmission electron microscope,TEM)和扫描电子显微镜(scanning electron microscope,SEM),前者是用电磁透镜对透射电子聚焦成像,而后者是利用末级透镜上面的扫描线圈(其作用是使电子束偏转,在样品表面做有规则扫描)来调节成像。由于胞外运动附属物一般极细,鞭毛直径约几十纳米[111-112],菌毛直径几纳米到几十纳米[113-114],只能通过电子显微镜才能观察到。同时,电子显微镜也可以用于矿物表面粗糙度和细菌在矿物界面生物膜形成情况的测定。
平面光极(planar optode,PO)是一种基于荧光分析原理,利用光学数字成像实时测定待测分析物(pH、O2、CO2、NH4+、温度等)二维浓度的技术。该仪器采用简单低成本的光电子器件,具有响应速度快、精度高、抗干扰能力强、稳定性好等优点[115-116]。利用平面光极技术可以观测解钾菌-含钾矿物反应界面pH分布及动态变化,从而获取有机酸等物质的分泌时间点。
4 展望结合光学显微术、电子显微术和荧光定量技术等各个前沿技术手段,可以跨尺度、多维度研究“功能性菌株对养分循环的影响机制”。例如:数字全息显微镜实时记录细菌在界面三维运动行为变化;原子力显微镜微尺度量化细菌和矿物界面间空间受力;激光扫描共聚焦显微镜定量生物膜三维形貌及其随时间变化情况;PO实时微尺度表征环境物化指标变化情况。科学工作者们可结合自身目标,将上述技术巧妙结合。本文主要从土壤钾素出发综述了“解钾菌及其释钾微观机制的研究进展”,为同行们提出了新的研究思路和视角。事实上,对于常见功能性菌株而言(如:解钾菌、解磷菌、固氮菌等系列根际促生菌),其影响养分循环即存在共性又有细微差别,科学工作者们可结合本文提出的解钾菌微观机制适当的应用到其他课题中去,共同、逐步推进“功能性菌株对养分循环的影响机制”研究。
基于目前关于解钾菌的知识,我们提出以下几点未来的研究方向:
1)本文在探究解钾菌微观解钾机制过程中所应用的细菌与固-液界面微观相互作用知识同样适用于其他土壤植物促生根菌(可能会有细微差异),研究者们可结合本文思路进一步探究根际促生菌作用机理;
2)目前本文仅将细菌与固-液界面的相关研究以理论形式应用于解释解钾菌微观解钾机理之中,有关其具体的实际应用还需要进一步探索;
3)在研究解钾菌微观释钾过程中,探究水动力学相互作用、DLVO理论、布朗运动以及细菌运动性在解钾的各个阶段的作用力大小,进一步预测解钾菌解钾能力强弱。
致谢 感谢朱堃老师对文章写作的建议,感谢博士生韩天富对文献框架和撰写思路方面的指导,感谢硕士生冉洪芋、肖鹏和雍路路对语言表述方面提供的帮助。
| [1] |
Lian B, Smith D L, Fu P Q. Application and mechanism of silicate bacteria in agriculture and industry (In Chinese)[J]. Guizhou Science, 2000, 18(Z1): 43-53. [连宾, Smith D L, 傅平秋. 硅酸盐细菌在工农业生产中的应用及其作用机理[J]. 贵州科学, 2000, 18(Z1): 43-53.]
(  0) 0) |
| [2] |
Sharma A, Shankhdhar D, Shankhdhar S C. Potassium- solubilizing microorganisms: Mechanism and their role in potassium solubilization and uptake[M]//Meena V S, Maurya B R, Verma J P, et al. Potassium solubilizing microorganisms for sustainable agriculture. India: Springer, 2016: 203-219.
(  0) 0) |
| [3] |
Zhang C S, Kong F Y. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants[J]. Applied Soil Ecology, 2014, 82: 18-25. DOI:10.1016/j.apsoil.2014.05.002
(  0) 0) |
| [4] |
Basak B B, Biswas D R. Co-inoculation of potassium solubilizing and nitrogen fixing bacteria on solubilization of waste mica and their effect on growth promotion and nutrient acquisition by a forage crop[J]. Biology and Fertility of Soils, 2010, 46: 641-648. DOI:10.1007/s00374-010-0456-x
(  0) 0) |
| [5] |
Sarikhani M R, Oustan S, Ebrahimi M, et al. Isolation and identification of potassium-releasing bacteria in soil and assessment of their ability to release potassium for plants[J]. European Journal of Soil Science, 2018, 69: 1078-1086. DOI:10.1111/ejss.12708
(  0) 0) |
| [6] |
Liu X Q, Du X R, Tan Y J, et al. The effects of different ratios between phosphate-solubilizing bacteria and potassium-solubilizing bacteria fertilizers on the flue-cured tobacco growth and the enzyme activities in the rhizospheric soil (In Chinese)[J]. Journal of Yunnan Agricultural University: Natural Science, 2019, 34(5): 845-851. [刘晓倩, 杜杏蓉, 谭玉娇, 等. 增施不同配比解磷菌、解钾菌生物菌肥对烤烟生长发育和根际土壤酶活性的影响[J]. 云南农业大学学报: 自然科学, 2019, 34(5): 845-851.]
(  0) 0) |
| [7] |
Shen H, He X H, Liu Y Q, et al. A complex inoculant of N2-fixing, P-and K-solubilizing bacteria from a purple soil improves the growth of kiwifruit(Actinidia chinensis)plantlets[J]. Frontiers in Microbiology, 2016, 7: 841.
(  0) 0) |
| [8] |
党雯. 解钾菌的筛选及其对矿区复垦土壤肥力的影响[D]. 山西: 山西大学生物工程学院, 2015. Dang W. The screening of potassim bacteria and its effects to soil fertility of mine area reclaimation[D]. Shanxi: Bioengineering College of Shanxi University, 2015. (  0) 0) |
| [9] |
Chandrakala C, Voleti S R, Bandeppa S, et al. Silicate solubilization and plant growth promoting potential of Rhizobium sp. isolated from rice rhizosphere[J]. Silicon, 2019, 11: 2895-2906. DOI:10.1007/s12633-019-0079-2
(  0) 0) |
| [10] |
Bakhshandeh E, Pirdashti H, Lendeh K S. Phosphate and potassium-solubilizing bacteria effect on the growth of rice[J]. Ecological Engineering, 2017, 103: 164-169. DOI:10.1016/j.ecoleng.2017.03.008
(  0) 0) |
| [11] |
Anjanadevi I P, John N S, John K S, et al. Rock inhabiting potassium solubilizing bacteria from Kerala, India: Characterization and possibility in chemical K fertilizer substitution[J]. Journal of Basic Microbiology, 2016, 56(1): 67-77. DOI:10.1002/jobm.201500139
(  0) 0) |
| [12] |
Feng K, Cai Z, Ding T, et al. Effects of potassium-solubulizing and photosynthetic bacteria on tolerance to salt stress in maize[J]. Journal of Applied Microbiology, 2019, 126: 1530-1540. DOI:10.1111/jam.14220
(  0) 0) |
| [13] |
Jiang X J, Huang Z X, Xie D T, et al. Promoting effects of the metabolites of silicate for plant growth (In Chinese)[J]. Journal of Southwest Agricultural University, 2000, 22(2): 116-119. DOI:10.3969/j.issn.1673-9868.2000.02.006 [蒋先军, 黄昭贤, 谢德体, 等. 硅酸盐细菌代谢产物对植物生长的促进作用[J]. 西南农业大学学报, 2000, 22(2): 116-119.]
(  0) 0) |
| [14] |
Prajapati K, Modi H A. Growth promoting effect of potassium solubilizing Enterobacter hormaechei(KSB-8)on cucumber(Cucumis sativus)under hydroponic conditions[J]. International Journal of Advanced Research in Biological Sciences, 2016, 3(5): 168-173.
(  0) 0) |
| [15] |
Sugumaran P, Janarthanam B. Solubilization of potassium containing minerals by bacteria and their effect on plant growth[J]. World Journal of Agricultural Sciences, 2007, 3(3): 350-355.
(  0) 0) |
| [16] |
Ramarethinam S, Chandra K. Studies on the effect of potash solubilizing/mobilizing bacteria Frateuria aurantia(symbion-K-liquid formulation)on brinjal growth and yield[J]. Pestology, 2006, 30(11): 35-39.
(  0) 0) |
| [17] |
Subhashini D V. Growth promotion and increased potassium uptake of tobacco by potassium-mobilizing bacterium Frateuria aurantia grown at different potassium levels in vertisols[J]. Communications in Soil Science and Plant Analysis, 2015, 46(2): 210-220. DOI:10.1080/00103624.2014.967860
(  0) 0) |
| [18] |
Ahmed A A, El-Baky M M H A, El-Baky F S A, et al. Comparative studies of application both mineral and bio-potassium fertilizers on the growth, yield and quality of potato plant[J]. Research Journal of Agriculture and Biological Sciences, 2009, 5(6): 1061-1069.
(  0) 0) |
| [19] |
Sheng X F. Growth promotion and increased potassium uptake of cotton and rape by a potassium releasing strain of Bacillus edaphicus[J]. Soil Biology and Biochemistry, 2005, 37(10): 1918-1922. DOI:10.1016/j.soilbio.2005.02.026
(  0) 0) |
| [20] |
Badr M A, Shafei A M, Sharaf E S H. The dissolution of K and P-bearing minerals by silicate dissolving bacteria and their effect on sorghum growth[J]. Research Journal of Agriculture and Biological Sciences, 2006, 2(1): 5-11.
(  0) 0) |
| [21] |
Sarhan M G R, Abd El-Gayed S S. The possibility of using feldspar as alernative potassium for cotton fertilization combined with silicate dissolving bacteria, humic acids and farmyard manure and its effect on soil properties[J]. Journal of Soil Sciences and Agricutural Engineering, Mansoura University, 2017, 8(12): 761-767.
(  0) 0) |
| [22] |
彩花. 菌根真菌与解磷钾细菌的相互作用及共接种对樟子松幼苗生长的影响[D]. 内蒙古: 内蒙古农业大学生命科学学院, 2013. Cai H. Interaction between Mycorrhizal Fungi and phosphorus/potassium dissolved bacteria and effects of coinoculation on the seedlings growth of Pinus sylvestnis var. mongolica Litv. [D]. Mongolia: College of Life Sciences Inner Mongolia Agricultural University, 2013. (  0) 0) |
| [23] |
Sheng X F, Huang W Y. Mechanism of potassium release from feldspar affected by the strain NBT of silicate bacterium (In Chinese)[J]. Acta Pedologica Sinica, 2002, 39(6): 863-871. DOI:10.3321/j.issn:0564-3929.2002.06.012 [盛下放, 黄为一. 硅酸盐细菌NBT菌株解钾机理初探[J]. 土壤学报, 2002, 39(6): 863-871.]
(  0) 0) |
| [24] |
Hu J, Yu J P, Lian B. Capability and mechanism of potassium releasing from potassium-bearing minerals by Aspergillis niger (In Chinese)[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2011, 30(3): 277-285. DOI:10.3969/j.issn.1007-2802.2011.03.006 [胡婕, 郁建平, 连宾. 黑曲霉对含钾矿物的解钾作用与机理分析[J]. 矿物岩石地球化学通报, 2011, 30(3): 277-285.]
(  0) 0) |
| [25] |
Hiebert F K, Bennett P C. Microbial control of silicate weathering in organic-rich ground water[J]. Science, 1992, 258: 278-281. DOI:10.1126/science.258.5080.278
(  0) 0) |
| [26] |
Štyriaková I, Štyriak I, Galko I, et al. The release of iron-bearing minerals and dissolution of feldspars by heterotrophic bacteria of Bacillus species[J]. Ceramics Silikaty, 2003, 47(47): 20-26.
(  0) 0) |
| [27] |
Sheng X F, He L Y. Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat[J]. Canadian Journal of Microbiology, 2006, 52(1): 66-72. DOI:10.1139/w05-117
(  0) 0) |
| [28] |
Basak B B, Sarkar B, Biswas D R, et al. Bio-intervention of naturally occurring silicate minerals for alternative source of potassium: Challenges and opportunities[J]. Advances in Agronomy, 2016, 141: 115-145.
(  0) 0) |
| [29] |
Wang J, Li X K, Lu J W, et al. Effects of different low-molecular weight organic acids and cations on release of potassium from potassium-bearing minerals (In Chinese)[J]. Journal of Huazhong Agricultural University, 2013, 32(3): 67-71. DOI:10.3969/j.issn.1000-2421.2013.03.013 [王瑾, 李小坤, 鲁剑巍, 等. 小分子有机酸和阳离子对含钾矿物钾素释放的影响[J]. 华中农业大学学报, 2013, 32(3): 67-71.]
(  0) 0) |
| [30] |
Groudev S N. Use of heterotrophic microorganisms in mineral biotechnology[J]. Acta Biotechnologica, 1987, 7(4): 299-306. DOI:10.1002/abio.370070404
(  0) 0) |
| [31] |
Liu W X, Xu X S, Wu X H, et al. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture[J]. Environmental Geochemistry and Health, 2006, 28(1-2): 133-140. DOI:10.1007/s10653-005-9022-0
(  0) 0) |
| [32] |
Man L Y, Cao X Y, Sun D S. Effect of potassium-solubilizing bacteria-mineral contact mode on decomposition behavior of potassium-rich shale (In Chinese)[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(4): 1099-1109. [满李阳, 曹晓燕, 孙德四. 钾细菌-矿物接触模式对富钾页岩分解行为的影响[J]. 中国有色金属学报, 2014, 24(4): 1099-1109.]
(  0) 0) |
| [33] |
Chen S, Lian B, Liu C Q. Effect of Bacillus mucilaginosus on weathering of phosphorite and a preliminary analysis of bacterial proteins[J]. Chinese Journal of Geochemistry, 2008, 27(2): 209-216. DOI:10.1007/s11631-008-0209-9
(  0) 0) |
| [34] |
Liao Y X, Fu X C, Cai R L, et al. The phenotype characteristics of a silicate bacterium (In Chinese)[J]. Jiangxi Science, 2000, 18(3): 149-153. DOI:10.3969/j.issn.1001-3679.2000.03.007 [廖延雄, 傅筱冲, 蔡汝林, 等. 一株硅酸盐细菌的表型特征[J]. 江西科学, 2000, 18(3): 149-153.]
(  0) 0) |
| [35] |
Sheng X F, Zhao F, He L Y, et al. Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar[J]. Canadian Journal of Microbiology, 2008, 54(12): 1064-1068. DOI:10.1139/W08-089
(  0) 0) |
| [36] |
Rüger H J. Differentiation of Bacillus globisporus, Bacillus marinus comb. nov. Bacillus aminovorans, and Bacillus insolitus[J]. International Journal of Systematic Bacteriology, 1983, 33(2): 157-161. DOI:10.1099/00207713-33-2-157
(  0) 0) |
| [37] |
Zahra M K, Monib M, Abdel-Al S I, et al. Significance of soil inoculation with silicate bacteria[J]. Zentralblatt für Mikrobiologie, 1983, 139(5): 349-357.
(  0) 0) |
| [38] |
Mehta P, Walia A, Kulshrestha S, et al. Efficiency of plant growth-promoting P-solubilizing Bacillus circulans CB7 for enhancement of tomato growth under net house conditions[J]. Journal of Basic Microbiology, 2015, 55(1): 33-44. DOI:10.1002/jobm.201300562
(  0) 0) |
| [39] |
Logan N A, Old D C, Dick H M. Isolation of Bacillus circulans from a wound infection[J]. Journal of Clinical Pathology, 1985, 38(7): 838-839. DOI:10.1136/jcp.38.7.838
(  0) 0) |
| [40] |
Hu X F, Li S X, Wu J G, et al. Transfer of Bacillus mucilaginosus and Bacillus edaphicus to the genus Paenibacillus as Paenibacillus mucilaginosus comb. nov. and Paenibacillus edaphicus comb. nov.[J]. International Journal of Systematic and Evolutionary Microbiology, 2010, 60(1): 8-14. DOI:10.1099/ijs.0.008532-0
(  0) 0) |
| [41] |
Meena V S, Maurya B R, Verma J P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils?[J]. Microbiological Research, 2014, 169(5-6): 337-347. DOI:10.1016/j.micres.2013.09.003
(  0) 0) |
| [42] |
Stover C K, Pham X Q, Erwin A L, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen[J]. Nature, 2000, 406: 959-964. DOI:10.1038/35023079
(  0) 0) |
| [43] |
Vater S M, Weiße S, Maleschlijski S, et al. Swimming behavior of Pseudomonas aeruginosa studied by holographic 3D tracking[J]. Public Library of Science One, 2014, 9(1): e87765.
(  0) 0) |
| [44] |
Prajapati K B, Modi H A. Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil[J]. CIB Tech Journal of Microbiology, 2012, 1(2-3): 8-14.
(  0) 0) |
| [45] |
Matias P C, Mattiello E M, Santos W O, et al. Solubilization of a K-silicate rock by Acidithiobacillus thiooxidans[J]. Minerals Engineering, 2019, 132: 69-75. DOI:10.1016/j.mineng.2018.11.050
(  0) 0) |
| [46] |
Valdés J, Pedroso I, Quatrini R, et al. Comparative genome analysis of Acidithiobacillus ferrooxidans, A. thiooxidans and A. caldus: Insights into their metabolism and ecophysiology[J]. Hydrometallurgy, 2008, 94: 180-184. DOI:10.1016/j.hydromet.2008.05.039
(  0) 0) |
| [47] |
Zhou Y F, Wang R C, Lu X C, et al. Influence of microbe-mineral contact model on mineral dissolution: A primary study on microperthite dissolution by Paenibacillus polymyxa (In Chinese)[J]. Geological Journal of China Universities, 2007, 13(4): 657-661. DOI:10.3969/j.issn.1006-7493.2007.04.007 [周跃飞, 王汝成, 陆现彩, 等. 微生物-矿物接触模式影响矿物溶解机制的实验研究-以多粘芽孢杆菌参与下的微纹长石溶解为例[J]. 高校地质学报, 2007, 13(4): 657-661.]
(  0) 0) |
| [48] |
Sun D S, Wang H J, Zhang Q. Effects of Bacillus circulans on decomposition behavior of bauxite (In Chinese)[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(4): 1119-1129. [孙德四, 王化军, 张强. 环状芽孢杆菌对铝土矿浸出分解行为的影响[J]. 中国有色金属学报, 2013, 23(4): 1119-1129.]
(  0) 0) |
| [49] |
Stubbendieck R M, Straight P D. Multifaceted interfaces of bacterial competition[J]. Journal of Bacteriology, 2016, 198(16): 2145-2155. DOI:10.1128/JB.00275-16
(  0) 0) |
| [50] |
Hibbing M E, Fuqua C, Parsek M R, et al. Bacterial competition: Surviving and thriving in the microbial jungle[J]. Nature Reviews Microbiology, 2010, 8(1): 15-25. DOI:10.1038/nrmicro2259
(  0) 0) |
| [51] |
Xie X J. Siderophores-the powerful weapon of bacterial nutrition competition (In Chinese)[J]. Microbiology, 2007, 34(5): 1026-1026. [谢小军. 铁载体-细菌营养竞争的有力武器[J]. 微生物学通报, 2007, 34(5): 1026-1026.]
(  0) 0) |
| [52] |
Stephens K, Bentley W E. Synthetic biology for manipulating quorum sensing in microbial consortia[J]. Trends in Microbiology, 2020, 28(8): 633-643. DOI:10.1016/j.tim.2020.03.009
(  0) 0) |
| [53] |
Purcell E M. Life at low Reynolds number[J]. American Journal of Physics, 1977, 45(1): 3-11. DOI:10.1119/1.10903
(  0) 0) |
| [54] |
Berg H C, Turner L. Chemotaxis of bacteria in glass capillary arrays. Escherichia coli, motility, microchannel plate, and light scattering[J]. Biophysical journal, 1990, 58(4): 919-930. DOI:10.1016/S0006-3495(90)82436-X
(  0) 0) |
| [55] |
Saxton M J, Jacobson K. Single-particle tracking: Applications to membrane dynamics[J]. Annual Review of Biophysics and Biomolecular Structure, 1997, 26: 373-399. DOI:10.1146/annurev.biophys.26.1.373
(  0) 0) |
| [56] |
Berg H C. Random walks in biology . Princeton: Princeton University Press, 1993.
(  0) 0) |
| [57] |
Lovely P S, Dahlquist F W. Statistical measures of bacterial motility and chemotaxis[J]. Journal of Theoretical Biology, 1975, 50(2): 477-496. DOI:10.1016/0022-5193(75)90094-6
(  0) 0) |
| [58] |
Xu M G, Zhang Y P. The influencing factors of soil nutrient diffusion and their correlation (In Chinese)[J]. Progress in Soil Science, 1995(3): 13-20. [徐明岗, 张一平. 土壤养分扩散的影响因素及其相互关系[J]. 土壤学进展, 1995(3): 13-20.]
(  0) 0) |
| [59] |
Berg H C. E. coil in motion . New York: Springer, 2004.
(  0) 0) |
| [60] |
Zhu X Y, Shen C Y, Chen G W, et al. Advancement in research on bacterial chemotaxis in soil (In Chinese)[J]. Acta Pedologica Sinica, 2019, 56(2): 259-275. [朱晓艳, 沈重阳, 陈国炜, 等. 土壤细菌趋化性研究进展[J]. 土壤学报, 2019, 56(2): 259-275.]
(  0) 0) |
| [61] |
Wu Y C, Cai P, Jing X X, et al. Soil biofilm formation enhances microbial community diversity and metabolic activity[J]. Environment International, 2019, 132: 105116. DOI:10.1016/j.envint.2019.105116
(  0) 0) |
| [62] |
Stocker R. Reverse and flick: Hybrid locomotion in bacteria[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(7): 2635-2636. DOI:10.1073/pnas.1019199108
(  0) 0) |
| [63] |
Berg H C, Brown D A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking[J]. Nature, 1972, 239(5374): 500-504. DOI:10.1038/239500a0
(  0) 0) |
| [64] |
Taylor B L, Koshland D E J. Reversal of flagellar rotation in monotrichous and peritrichous bacteria: Generation of changes in direction[J]. Journal of Bacteriology, 1974, 119(2): 640-642. DOI:10.1128/jb.119.2.640-642.1974
(  0) 0) |
| [65] |
Darnton N C, Turner L, Rojevsky S, et al. On torque and tumbling in swimming Escherichia coli[J]. Journal of Bacteriology, 2007, 189(5): 1756-1764. DOI:10.1128/JB.01501-06
(  0) 0) |
| [66] |
Lowe G, Meister M, Berg H C. Rapid rotation of flagellar bundles in swimming bacteria[J]. Nature, 1987, 325(6105): 637-640. DOI:10.1038/325637a0
(  0) 0) |
| [67] |
Macnab R M. Bacterial flagella rotating in bundles: A study in helical geometry[J]. Proceedings of the National Academy of Sciences of the United States of America, 1977, 74(1): 221-225. DOI:10.1073/pnas.74.1.221
(  0) 0) |
| [68] |
McCarter L L. Polar flagellar motility of the Vibrionaceae[J]. Microbiology and Molecular Biology Reviews, 2001, 65(3): 445-462. DOI:10.1128/MMBR.65.3.445-462.2001
(  0) 0) |
| [69] |
Hintsche M, Waljor V, Großmann R, et al. A polar bundle of flagella can drive bacterial swimming by pushing, pulling, or coiling around the cell body[J]. Scientific Reports, 2017, 7: 16771. DOI:10.1038/s41598-017-16428-9
(  0) 0) |
| [70] |
Ping L Y. Cell orientation of swimming bacteria: From theoretical simulation to experimental evaluation[J]. Science China: Life Sciences, 2012, 55(3): 202-209. DOI:10.1007/s11427-012-4298-7
(  0) 0) |
| [71] |
Packer H L, Lawther H, Armitage J P. The Rhodobacter sphaeroide flagellar motor is a variable-speed rotor[J]. Federation of European Biochemical Societies Letters, 1997, 409(1): 37-40. DOI:10.1016/S0014-5793(97)00473-0
(  0) 0) |
| [72] |
Armitage J P, Macnab R M. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides[J]. Journal of Bacteriology, 1987, 169(2): 514-518. DOI:10.1128/jb.169.2.514-518.1987
(  0) 0) |
| [73] |
Li G L, Tang J X. Low flagellar motor torque and high swimming efficiency of Caulobacter crescentus swarmer cells[J]. Biophysical Journal, 2006, 91(7): 2726-2734. DOI:10.1529/biophysj.106.080697
(  0) 0) |
| [74] |
Sampedro I, Parales R E, Krell T, et al. Pseudomonas chemotaxis[J]. Federation of European Microbiological Societies Microbiology Reviews, 2015, 39(1): 17-46.
(  0) 0) |
| [75] |
Qian C, Wong C C, Swarup S, et al. Bacterial tethering analysis reveals a "run-reverse-turn" mechanism for Pseudomonas species motility[J]. Applied and Environmental Microbiology, 2013, 79(15): 4734-4743. DOI:10.1128/AEM.01027-13
(  0) 0) |
| [76] |
Xie L, Altindal T, Chattopadhyay S, et al. Bacterial flagellum as a propeller and as a rudder for efficient chemotaxis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(6): 2246-2251. DOI:10.1073/pnas.1011953108
(  0) 0) |
| [77] |
Conrad J C. Physics of bacterial near-surface motility using flagella and type Ⅳ pili: Implications for biofilm formation[J]. Research in Microbiology, 2012, 163: 619-629. DOI:10.1016/j.resmic.2012.10.016
(  0) 0) |
| [78] |
Hill J, Kalkanci O, Mcmurry J L, et al. Hydrodynamic surface interactions enable Escherichia coli to seek efficient routes to swim upstream[J]. Physical Review Letters, 2007, 98(6): 068101. DOI:10.1103/PhysRevLett.98.068101
(  0) 0) |
| [79] |
Tuson H H, Weibel D B. Bacteria-surface interactions[J]. Soft Matter, 2013, 9(18): 4368-4380.
(  0) 0) |
| [80] |
Berg H C, Dyson F. Random walks in biology[J]. Physics Today, 1987, 40(3): 73-74. DOI:10.1063/1.2819954
(  0) 0) |
| [81] |
Berke A P, Turner L, Berg H C, et al. Hydrodynamic attraction of swimming microorganisms by surfaces[J]. Physical review letters, 2008, 101(3): 038102. DOI:10.1103/PhysRevLett.101.038102
(  0) 0) |
| [82] |
Desai N, Shaik V A, Ardekani A M. Hydrodynamic interaction enhances colonization of sinking nutrient sources by motile microorganisms[J]. Frontiers in Microbiology, 2019, 10: 289. DOI:10.3389/fmicb.2019.00289
(  0) 0) |
| [83] |
Molaei M, Barry M, Stocker R, et al. Failed escape: Solid surfaces prevent tumbling of Escherichia coli[J]. Physical Review Letters, 2014, 113(6): 068103. DOI:10.1103/PhysRevLett.113.068103
(  0) 0) |
| [84] |
Lauga E, DiLuzio W R, Whitesides G M, et al. Swimming in circles: Motion of bacteria near solid boundaries[J]. Biophysical Journal, 2006, 90(2): 400-412. DOI:10.1529/biophysj.105.069401
(  0) 0) |
| [85] |
Vigeant M A S, Ford R M, Wagner M, et al. Reversible and irreversible adhesion of motile Escherichia coli cells analyzed by total internal reflection aqueous fluorescence microscopy[J]. Applied and Environmental Microbiology, 2002, 68(6): 2794-2801. DOI:10.1128/AEM.68.6.2794-2801.2002
(  0) 0) |
| [86] |
Magariyama Y, Ichiba M, Nakata K, et al. Difference in bacterial motion between forward and backward swimming caused by the wall effect[J]. Biophysical Journal, 2005, 88(5): 3648-3658. DOI:10.1529/biophysj.104.054049
(  0) 0) |
| [87] |
Li G L, Tam L K, Tang J X. Amplified effect of Brownian motion in bacterial near-surface swimming[J]. Proceedings of the National Academy of Sciences, 2008, 105(47): 18355-18359. DOI:10.1073/pnas.0807305105
(  0) 0) |
| [88] |
O'Toole G A, Wong G C. Sensational biofilms: Surface sensing in bacteria[J]. Current Opinion in Microbiology, 2016, 30: 139-146. DOI:10.1016/j.mib.2016.02.004
(  0) 0) |
| [89] |
Utada A S, Bennett R R, Fong J C N, et al. Vibrio cholerae use pili and flagella synergistically to effect motility switching and conditional surface attachment[J]. Nature Communications, 2014, 5: 4913. DOI:10.1038/ncomms5913
(  0) 0) |
| [90] |
Gordon V D, Wang L Y. Bacterial mechanosensing: The force will be with you, always[J]. Journal of Cell Science, 2019, 132(7): jcs227694. DOI:10.1242/jcs.227694
(  0) 0) |
| [91] |
Rong X M, Huang Q Y, Chen W L, et al. Interaction mechanisins of soil minerals with microorganisms and their environmental significance (In Chinese)[J]. Acta Ecologica Sinica, 2008, 28(1): 376-387. DOI:10.3321/j.issn:1000-0933.2008.01.044 [荣兴民, 黄巧云, 陈雯莉, 等. 土壤矿物与微生物相互作用的机理及其环境效应[J]. 生态学报, 2008, 28(1): 376-387.]
(  0) 0) |
| [92] |
Hori K, Matsumoto S. Bacterial adhesion: From mechanism to control[J]. Biochemical Engineering Journal, 2010, 48(3): 424-434. DOI:10.1016/j.bej.2009.11.014
(  0) 0) |
| [93] |
Rong X M, Huang Q Y, Chen W L, et al. Surface thermodynamical analysis of adsorption of bacteria on soil clay minerals (In Chinese)[J]. Acta Pedologica Sinica, 2011, 48(2): 331-337. [荣兴民, 黄巧云, 陈雯莉, 等. 细菌在两种土壤矿物表面吸附的热力学分析[J]. 土壤学报, 2011, 48(2): 331-337.]
(  0) 0) |
| [94] |
Johanson J J, Feriancikova L, Xu S P. Influence of enterococcal surface protein(esp)on the transport of Enterococcus faecium within saturated quartz sands[J]. Environmental Science and Technology, 2012, 46(3): 1511-1518. DOI:10.1021/es203265m
(  0) 0) |
| [95] |
Flemming H C, Wingender J. The biofilm matrix[J]. Nature Reviews Microbiology, 2010, 8(9): 623-633. DOI:10.1038/nrmicro2415
(  0) 0) |
| [96] |
Rasamiravaka T, Labtani Q, Duez P, et al. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms[J]. BioMed Research International, 2015, 759348: 1-17.
(  0) 0) |
| [97] |
Du B, Gu Y, Chen G W, et al. Flagellar motility mediates early-stage biofilm formation in oligotrophic aquatic environment[J]. Ecotoxicology and Environmental Safety, 2020, 194: 110340. DOI:10.1016/j.ecoenv.2020.110340
(  0) 0) |
| [98] |
Lee S H, Grier D G. Holographic microscopy of holographically trapped three-dimensional structures[J]. Optics Express, 2007, 15(4): 1505-1512. DOI:10.1364/OE.15.001505
(  0) 0) |
| [99] |
Zhou W J, Yu Y J, Chen M Y. Recent developments and applications of digital micro-holography (In Chinese)[J]. Optical Technique, 2007, 33(6): 870-874. DOI:10.3321/j.issn:1002-1582.2007.06.012 [周文静, 于瀛洁, 陈明仪. 数字全息显微测量技术的发展与最新应用[J]. 光学技术, 2007, 33(6): 870-874.]
(  0) 0) |
| [100] |
Peng Q M, Zhou X, Wang Z, et al. Three-dimensional bacterial motions near a surface investigated by digital holographic microscopy: Effect of surface stiffness[J]. Langmuir, 2019, 35(37): 12257-12263. DOI:10.1021/acs.langmuir.9b02103
(  0) 0) |
| [101] |
Marquet P, Rappaz B, Magistretti P J. Digital holographic microscopy: A noninvasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy[J]. Optics Letters, 2005, 30(5): 468-470. DOI:10.1364/OL.30.000468
(  0) 0) |
| [102] |
He H, Ren J C. Progress in total internal reflection fluorescence microscopy and its applications in single-molecule detection (In Chinese)[J]. Journal of Instrumental Analysis, 2007, 26(3): 445-449. DOI:10.3969/j.issn.1004-4957.2007.03.037 [何化, 任吉存. 全内反射荧光成像技术及其在单分子检测中的研究进展[J]. 分析测试学报, 2007, 26(3): 445-449.]
(  0) 0) |
| [103] |
Chen Y Z. Visualizing the single molecule in live cells (In Chinese)[J]. Chinese Bulletin of Life Sciences, 2003, 15(2): 79-83. DOI:10.3969/j.issn.1004-0374.2003.02.004 [陈宜张. 活细胞单分子实时视见研究[J]. 生命科学, 2003, 15(2): 79-83.]
(  0) 0) |
| [104] |
Sun X D, Suo L M, Zhang M Z, et al. New progress of reservoir research by the technology of laser confocal scanning microscope analysis in the Daqing exploration area (In Chinese)[J]. Acta Petrologica Sinica, 2005, 21(5): 1479-1488. [孙先达, 索丽敏, 张民志, 等. 激光共聚焦扫描显微检测技术在大庆探区储层分析研究中的新进展[J]. 岩石学报, 2005, 21(5): 1479-1488.]
(  0) 0) |
| [105] |
Rautureau M, Cooke R U, Boyde A. The application of confocal microscopy to the study of stone weathering[J]. Earth Surface Processes and Landforms, 2006, 18(9): 769-775.
(  0) 0) |
| [106] |
Song M, Peng Y X, Israel P, et al. Bioweathering and K release of K-bearing minerals by Penicillium oxalicum (In Chinese)[J]. Microbiology China, 2015, 42(7): 1410-1417. [宋淼, 彭云湘, Israel P, 等. 草酸青霉对含钾矿物风化及钾溶出的影响[J]. 微生物学通报, 2015, 42(7): 1410-1417.]
(  0) 0) |
| [107] |
Xing Y W, Xu M D, Gui X H, et al. The application of atomic force microscopy in mineral flotation[J]. Advances in Colloid and Interface Science, 2018, 256: 373-392. DOI:10.1016/j.cis.2018.01.004
(  0) 0) |
| [108] |
Zhu J, Sun R G. Introduction to atomic force microscope and its manipulation (In Chinese)[J]. Life Science Instruments, 2005, 3(1): 22-26. DOI:10.3969/j.issn.1671-7929.2005.01.006 [朱杰, 孙润广. 原子力显微镜的基本原理及其方法学研究[J]. 生命科学仪器, 2005, 3(1): 22-26.]
(  0) 0) |
| [109] |
Rodrigues S, Paillard C, Pennec G L, et al. Vibrio tapetis, the causative agent of brown ring disease, forms biofilms with spherical components[J]. Frontiers in Microbiology, 2015, 6: 1384.
(  0) 0) |
| [110] |
Yao J E. State and prospects of electron microscopes (In Chinese)[J]. Journal of Chinese Electron Microscopy Society, 1998, 17(6): 767-773. DOI:10.3969/j.issn.1000-6281.1998.06.016 [姚骏恩. 电子显微镜的现状与展望[J]. 电子显微学报, 1998, 17(6): 767-773.]
(  0) 0) |
| [111] |
Li D, Zhu Y, Yang T, et al. Bacterial flagella as an osteogenic differentiation nano-promoter[J]. Nanoscale Horizons, 2019, 4(6): 1286-1292. DOI:10.1039/C9NH00124G
(  0) 0) |
| [112] |
Schmitt R, Raska I, Mayer F. Plain and complex flagella of Pseudomonas rhodos: Analysis of fine structure and composition[J]. Journal of Bacteriology, 1974, 117(2): 844-857. DOI:10.1128/jb.117.2.844-857.1974
(  0) 0) |
| [113] |
Beaussart A, Baker A E, Kuchma S L, et al. Nanoscale adhesion forces of Pseudomonas aeruginosa type Ⅳ Pili[J]. American Chemical Society Nanotechnology, 2014, 8(10): 10723-10733.
(  0) 0) |
| [114] |
Anda J D, Lee E Y, Lee C K, et al. High-speed "4D" computational microscopy of bacterial surface motility[J]. American Chemical Society Nanotechnology, 2017, 11(9): 9340-9351.
(  0) 0) |
| [115] |
Sánchez-Barragán I, Costa-Fernández J M, Sanz-Medel A, et al. A ratiometric approach for pH optosensing with a single fluorophore indicator[J]. Analytica Chimica Acta, 2006, 562(2): 197-203. DOI:10.1016/j.aca.2006.01.033
(  0) 0) |
| [116] |
Shen W B, Zhou N N, Li Y N, et al. Application of pH planar optode in water/sediment system with the presence of bioturbation (In Chinese)[J]. Journal of Jilin University(Earth Science Edition), 2013, 43(3): 931-938. [沈万斌, 周楠楠, 李一楠, 等. pH平面光极在生物扰动存在下水/沉积物体系中的应用[J]. 吉林大学学报(地球科学版), 2013, 43(3): 931-938.]
(  0) 0) |
| [117] |
Klee S R, Özel M, Appel B, et al. Characterization of Bacillus anthracis-like bacteria isolated from wild great apes from Côte d'Ivoire and Cameroon[J]. Journal of Bacteriology, 2006, 188(15): 5333-5344. DOI:10.1128/JB.00303-06
(  0) 0) |
| [118] |
Lower S K, Tadanier C J, Hochella M F J. Measuring interfacial and adhesion forces between bacteria and mineral surfaces with biological force microscopy[J]. Geochimica Et Cosmochimica Acta, 2000, 64(18): 3133-3139. DOI:10.1016/S0016-7037(00)00430-0
(  0) 0) |
| [119] |
Han C, Yao L, Xu D, et al. High-resolution imaging of pH in alkaline sediments and water based on a new rapid response fluorescent planar optode[J]. Scientific Reports, 2016, 6: 26417. DOI:10.1038/srep26417
(  0) 0) |
| [120] |
Lüdecke C, Jandt K D, Siegismund D, et al. Reproducible biofilm cultivation of chemostat-grown Escherichia coli and investigation of bacterial adhesion on biomaterials using a non-constant-depth film fermenter[J]. The Public Library of Science One, 2014, 9(1): e84837.
(  0) 0) |
| [121] |
Ma W T, Peng D H, Walker S L, et al. Bacillus subtilis biofilm development in the presence of soil clay minerals and iron oxides[J]. Nature Partner Journals Biofilms and Microbiomes, 2017, 3: 4.
(  0) 0) |
| [122] |
Wu J F, Wang L B, Meng L J. Analysis of mineral composition and microstructure of gravel aggregate based on XRD and SEM[J]. Road Materials and Pavement Design, 2017, 18(3): 139-148.
(  0) 0) |
 2022, Vol. 59
2022, Vol. 59


