土壤是陆地生物地球化学过程的产物,是人类赖以生存的重要基础。土壤微生物尤其是细菌赋予土壤生命属性,驱动着土壤中的生物地球化学循环,在改良土壤结构、提升土壤肥力、防治土壤污染、促进作物健康、应对全球气候变化及服务地球宜居性等方面发挥着重要作用[1]。土壤中的细菌主要粘附在土壤矿物和有机质表面,以微菌落或生物膜形成存在[2–4]。生物膜是指由微生物自身分泌的胞外聚合物(Extracellular Polymeric Substances,EPS)等基质包围并附着于界面上的微生物聚集体[5]。从生物进化的角度考虑,生物膜的形成是土壤细菌面临进化选择压力条件下的首选生命策略,也是细菌功能发挥的主体[3,6]。当前针对土壤中细菌群落的研究,较少考虑细菌生物膜状态。Lehmann等[7]基于279个土壤生物类群的全球Meta分析,发现细菌对土壤大团聚体和微团聚体稳定性均有重要贡献,而真菌则对土壤大团聚体有较大影响;附着型细菌较运动型细菌对土壤团聚体的影响更大,尤其是对微团聚体,这可能归因于附着型细菌产生EPS以及形成生物膜。Bystrianský等[8]利用玻璃纤维过滤器制备分离捕集阱,分离了土壤中生物膜群落和浮游细菌群落,发现两种生活模式的细菌群落之间存在显著差异,其差异主要原因可能是微环境因素的影响。Wu等[9]发现高养分输入有利于土壤生物膜形成;在形成生物膜的土壤中,微生物群落的多样性、均匀度指数及代谢活性显著提升,其中芽孢杆菌(Bacillus)和类芽孢杆菌(Paenibacillus)是土壤生物膜形成的关键菌属。因此,深入开展土壤生物膜的研究,是更好地管理生物介导的养分周转和土壤健康的关键[4,10]。
生物膜主要是由细胞和EPS组成,其中EPS约占生物膜干重的80%[11]。在土壤环境中,EPS在细胞与细胞、细胞与土壤之间起着桥接作用,与土壤矿物或有机质一起构成了生物膜中的胞外基质。细菌分泌的EPS为细胞提供了有效保护,如饥饿条件下提供营养,干燥过程中保持水分,抵抗有毒化学物质对细胞的毒害以及减缓土壤温度、pH和盐度急剧变化对细胞造成的损伤等。EPS也赋予了细菌各种生态优势,包括增强菌落粘附[12]、维持生境异质性[13]、支持互养共栖[14]、防御毒素损害[15]、改变遗传物质转移[16-17]以及提供胞外酶储存和营养捕获的作用[18]。土壤中细菌EPS的众多优势对于维护土壤健康至关重要。例如,菌落的粘附功能可提高土壤团聚体的稳定性[19];胞外酶的滞留有助于土壤的代谢稳定性[10]。然而,由于土壤环境的复杂性,土壤EPS的研究面临极大挑战,发展特异性方法提取土壤EPS是进一步理解其功能的关键。近年来,学者们克服了一些方法学上的挑战[20],评估了提取方法在不同类型土壤中的适用性[21-22],为土壤EPS的研究提供了技术上的可行性。通过量化土壤EPS组成与结构,可揭示土壤微生物对环境变化响应的微观机制,明晰微生物EPS在土壤功能方面的作用,从而有助于保障土壤健康和实现农业绿色可持续发展。鉴于此,本文重点概述细菌EPS在土壤生态功能方面的研究进展,分别从充当细胞保护层、调节土壤生物响应、缓解土壤非生物胁迫和改善土壤整体功能四个层面进行阐述(图 1),并展望未来土壤细菌EPS研究中应关注的关键科学问题,以期挖掘其在环境友好型农业发展中的潜在应用价值。
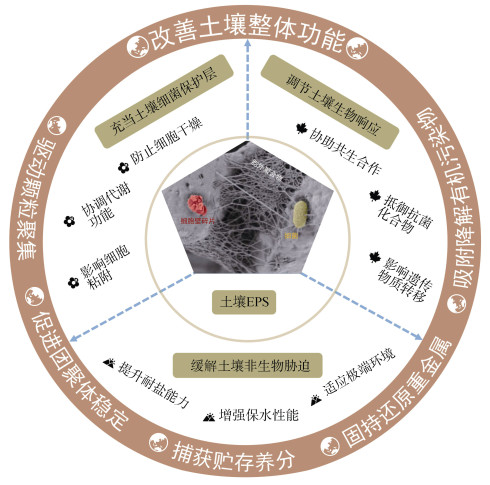
|
图 1 土壤中细菌胞外聚合物(EPS)生态功能的概念框架 Fig. 1 Conceptual framework of ecological functions of bacterial extracellular polymeric substances(EPS)in soils |
EPS是细菌在生长和代谢过程中释放的高度水合的生物大分子聚合物。EPS中的生物大分子通过分散力、静电力、氢键等相互作用,为生物膜提供机械支撑。细菌合成并分泌EPS虽然不影响细胞自身活性或代谢,但该过程对细胞而言是一个成本高昂的耗能过程,从生物学角度来看,细菌的这种行为是一种提高其在环境中存活能力的策略[23]。在土壤科学领域,“EPS”被作为胞外多糖(exopolysaccharides)的缩写[11]。然而,在生物污水处理、海洋工程、医学等科学领域,“EPS”普遍被作为胞外聚合物(extracellular polymeric substances)的缩写。为了促使不同学科之间专业术语的兼容性,笔者建议统一将EPS作为胞外聚合物的缩写,并且在定量分析时应强调概念和起源,例如使用EPS-多糖、EPS-蛋白质、EPS-氨基酸和EPS-糖醛酸等术语[20,24]。
1.2 组分EPS主要组成包括多糖、蛋白质、脂质和胞外DNA(eDNA)[23,25]。EPS-多糖是研究最多的基质组分,其既可分为中性多糖(主要是己糖)和糖醛酸(葡萄糖醛酸、半乳糖醛酸和甘露糖酸),又可分为同多糖(α-D-葡聚糖、β-D-葡聚糖和果聚糖等)和杂多糖(海藻酸盐、黄原胶、可拉酸和透明质酸等)[26]。对不同细菌菌株EPS的研究表明,EPS-多糖在组成和结构上差异较大,杂多糖的物理性质取决于单糖单元与侧链分支之间的键合,糖醛酸及其衍生物的存在决定了EPS的电荷性质[26]。EPS-蛋白质组分发挥着胞外酶和结构蛋白的功能。胞外酶既能水解可溶性/不溶性聚合物以及有机颗粒等外源底物,也可以靶向水解同源细菌或异源细菌的EPS[23]。EPS基质中的结构蛋白或非酶蛋白参与了胞外基质网络的形成,使细菌与周边环境建立起关联,例如糖蛋白凝集素有助于细菌聚集并形成絮状物[27]。eDNA是通过自溶或微生物主动分泌释放的。尽管eDNA的功能尚未完全阐明,但已有研究表明eDNA也负责生物膜的空间结构稳定,并在细菌粘附于介质表面和信号传递中起重要作用[28-29]。EPS基质还包含脂质和脂质衍生物。脂多糖参与细菌粘附过程以及发挥生物表面活性剂作用,例如促进氧化亚铁硫杆菌(Thiobacillus ferrooxidans)粘附到黄铁矿表面[23]。
1.3 影响因素细菌菌株类型、生长阶段、环境物理化学条件、底物可利用性等均会影响细菌EPS的产生以及化学组成。研究表明,EPS的生物合成依赖于细菌菌株和生长期。例如,铜绿假单胞菌(Pseudomonas aeruginosa)和表皮葡萄球菌(Staphylococcus epidermidis)只有在稳定生长阶段才开始合成EPS,而维氏固氮菌(Azotobacter vinelandii)在整个生长过程中不断产生纤维素、胶凝糖和海藻酸钠等EPS-多糖;假单胞菌(Pseudomonas)在指数期和稳定期均合成分泌EPS,但这两个生长阶段分泌的EPS化学结构不同;产碱杆菌(Alcaligenes faecalis)只有在细胞停止生长后才产生凝胶多糖[30]。土壤溶液的离子强度和离子价态会改变细菌的初始粘附行为,进而影响生物膜的形成发育过程。例如,二价阳离子(Ca2+和Mg2+)通过桥接作用使带负电荷的EPS结合在一起,从而提高了生物膜的结构稳定性[31]。大多数细菌在中性pH产生的EPS量最大[30],而在极端pH条件下,EPS的组成却较为相似,这表明在利用特定EPS组分介导抗逆性方面,细菌可能具有相似的机制[32]。细菌产生EPS的最佳生长温度主要取决于菌株本身的性质,大多数细菌在25~30 ℃产生的EPS量较高[33]。氧气张力的变化是EPS产量上调的触发机制之一,高浓度的氧气可以诱导EPS产量增加[26]。Roberson和Firestone[34]证明了在模拟土壤系统中,干燥胁迫会诱导细菌分泌更多的EPS。Kazy等[35]发现铜绿假单胞菌(Pseudomonas aeruginosa)在暴露于重金属Cu胁迫时,其EPS产量提高了4倍。EPS的产生也取决于底物的类型和浓度。不同的微生物对碳源和氮源具有不同的喜好,底物浓度决定着底物利用率和相应的EPS产生效率,并且合适的碳氮比可确保最大程度地产生EPS[36-37]。Redmile-Gordon等[20]发现易利用的甘油作为碳源,贫营养土壤中EPS-多糖产量增加。适宜碳氮比的养分供应会显著刺激土壤中细菌EPS分泌和生物膜形成[9],而过量的无机氮输入则会阻碍土壤中EPS产生[38]。
2 EPS充当土壤细菌保护层土壤细菌已进化出不同策略来应对环境胁迫,细菌分泌EPS是一种主动进化策略,是细菌响应环境压力的生物产物。EPS充当着环境压力源与细胞膜之间的物理屏障,在细菌生理特性和生态适应中起着重要作用[25,39]。
2.1 防止细胞干燥对于细菌细胞而言,维持其周围的水合环境至关重要。当面临干燥或水分受限的胁迫时,细菌会启动多种自身代谢调节开关,如上调相应功能基因表达、产生热休克蛋白、增加渗透压物质浓度和诱导细胞膜结构变化等[30]。细菌也可能会转变代谢途径和能量传输方式,促进EPS合成并分泌至外部环境[34],从而维持细菌的基本生存代谢。EPS通过类似海绵的作用保护细菌缓解干燥胁迫,从而使细菌有时间进行代谢调节。当经受干燥条件时,产生生物膜的李斯特菌(Listeria monocytogenes)较生物膜形成受限的突变株表现出更好的存活率[40]。相比于野生型的防御假单胞菌(Pseudomonas protegens),过量产生EPS的突变株使干燥条件下细菌的存活率提升了5倍[41]。此外,EPS结构在干燥过程中也会发生变化[42],EPS在快速干燥−润湿过程中诱导“水力解耦”,从而保护嵌入土壤生物膜内的细菌[43],这可能是细菌生存策略操控的水分保留方式。考虑到土壤经常遭受频繁的干湿交替,细菌细胞质的渗透调节会损害细胞功能,所以细胞的适应性反应行为除了来自细胞质渗透调节,更可能来自细菌EPS的吸湿性调节,这暗示着土壤EPS在细菌长期抵抗渗透压波动方面的作用可能更关键。
2.2 协调代谢功能EPS基质可为胞外酶提供保护性载体,以便细菌在摄取养分前裂解高分子量的有机质[44-45]。胞外酶在EPS基质中的持留,促进了胞外消化系统的形成,从而有利于细菌捕获周围环境中的化合物,并将其作为营养物质和能源供给细胞生长代谢[10,23]。同时,EPS促使胞外酶和有机质之间紧密靠近,有助于保持较低代谢成本[38]。随着土壤水分的流动,细菌EPS可将受保护的胞外酶传递到距离较远的底物上,并在收缩时捕获胞外酶催化分解的营养物质传回到生物膜中。EPS的急剧收缩/膨胀特性也有助于维持土壤孔隙空间,促进气体扩散并保持土壤异质性。胞外酶可以通过其与胞外基质的相互作用而稳定化,Dilly和Nannipieri[46]认为胞外酶对土壤有机质的催化过程具有“酶促记忆”,并且Kemmitt等[47]曾提出“非生物调节阀控制土壤有机质矿化”假说。笔者认为土壤EPS对胞外酶的保护作用可能是解开有关土壤有机质动力学谜团的关键环节。
2.3 影响细胞粘附EPS参与细菌细胞在介质表面的粘附[24]。对于27种细菌菌株评估后发现,EPS量较少的菌株通过静电排斥作用抑制细菌粘附,而表面富含EPS的菌株则通过EPS官能团如糖醛酸基和乙酰基之间的相互作用增强细胞粘附[48]。通过对比去除表面EPS前后细菌的粘附行为,发现枯草芽孢杆菌(Bacillus subtilis)表面EPS的去除会降低细菌在黏土矿物上的粘附,而增强细菌在针铁矿上的粘附[49]。Zhao等[12]进一步发现EPS的去除会抑制猪链球菌(Streptococcus suis)在土壤颗粒上的粘附,而增强大肠杆菌(Escherichia coli)的粘附,化学键形成和静电力是控制细菌粘附到土壤胶体表面的主要机制[50-51]。细菌粘附性很大程度上取决于分子链构象、内部取代基和内部/外部相互作用。近期研究发现希瓦氏菌(Shewanella oneidensis)MR-1外膜c型细胞色素(OmcA和MtrC)在细菌-矿物界面吸附过程中具有重要贡献,OmcA蛋白和MtrC蛋白分别在细菌定殖的初期过程和后期过程中发挥主导作用[52]。因此,EPS对细胞粘附性能的贡献程度仍有待深入探究。
3 EPS调节土壤生物响应EPS在保护土壤微生物免受环境压力方面具有重要作用,除此之外,EPS也为整个生物群体提供了合适的生态位,促使各自功能发挥并产生协同效应[5]。
3.1 协助共生合作EPS在固氮根瘤菌与植物之间建立共生关系中发挥着重要作用。根瘤菌表面多糖被认为是豆科植物形成根瘤的基础。例如,为了侵入苜蓿产生结瘤并建立成功的共生关系,苜蓿中华根瘤菌(Sinorhizobium meliloti)Rm1021必须分泌琥珀酰聚糖,而不合成琥珀酰聚糖的突变株则降低了根瘤菌侵染和建立共生的能力[53]。虽然豆科根瘤菌(Rhizobium leguminosarum)的野生型菌株和葡甘露聚糖突变株均能产生结瘤,但在混合接种中野生型菌株处于优势地位,竞争性结瘤能力更强[54]。百脉根和百脉根根瘤菌(Mesorhizobium loti)R7A的EPS之间的相互作用由植物表达的受体所介导,百脉根产生的受体Epr3仅与产生特定结构EPS的细菌结合并允许侵染,EPS结构缺陷的R7A突变体则不能成功侵染百脉根[55],即植物能够特异性识别根瘤菌EPS结构。同时,EPS也充当着阻碍活性氧(ROS)扩散的物理屏障。在侵染过程中,苜蓿中华根瘤菌(Sinorhizobium meliloti)的EPS能够保护细菌抵御植物宿主产生的ROS,减缓根瘤菌氧化应激反应,从而增加侵染成功率[56]。此外,EPS过量产生的突变体可以保护EPS缺陷的突变体,共同防御ROS损伤[56]。
3.2 抵御抗菌化合物EPS的存在阻碍化合物的杀菌作用。生物膜对抗生素的耐受性涉及一系列机制,包括运输限制、营养梯度形成和表层细胞适应性应激反应等[57]。EPS具有丰富的负电荷基团,通过静电作用阻抗不同类型抗生素,从而降低抗生素的扩散速率和保护内部细胞免于接触。早期研究针对医学上重要的致病性细菌,测试了细菌EPS对抗生素的阻抗潜力。例如,由葡萄球菌(Staphylococcus sp.)产生的黏液EPS,是一种有效的全氟沙星和替考拉宁的拮抗剂[58]。EPS也能保护细菌减轻消毒剂的杀菌作用。例如,铜绿假单胞菌(Pseudomonas aeruginosa)EPS中海藻酸钠成分可提高细菌在氯水中的存活能力,而去除EPS黏液会导致细菌的耐氯性消失[59]。Wang等[60]发现暴露于磺胺甲二唑溶液中,去除EPS的生物膜中细菌的群落多样性和丰富度指数下降;EPS-蛋白质通过疏水作用结合磺胺甲二唑,从而减轻了磺胺类抗生素对生物膜中细菌的直接损害。Qiu等[61]探究了土壤细菌在抵抗磺胺嘧啶胁迫方面的机制,发现两类土壤中EPS量与施入的磺胺嘧啶浓度存在正相关关系;土壤EPS的官能团提供对磺胺嘧啶的胞外吸附位点,从而降低磺胺嘧啶的生物利用度;土壤EPS中色氨酸类物质的增加可能是微生物缓解高浓度磺胺嘧啶的一种适应机制,有效减轻土壤中磺胺嘧啶类抗生素对细菌的胁迫压力[61]。
3.3 影响遗传物质转移EPS既能促进细菌之间的水平基因转移,也能阻碍质粒进入细菌细胞。细菌主动分泌或细胞死亡裂解释放的eDNA是生物膜EPS的重要组分,可诱发生物膜内细菌发生自然转化或接合。Bae等[62]研究表明,与浮游状态细胞相比,空肠弯曲杆菌(Campylobacter jejuni)生物膜中抗生素抗性基因(ARGs)的转化频率更高。生物膜的成熟度和eDNA浓度是影响自然转化频率的主要因素[63]。然而,细胞表面EPS作为一种渗透性物理屏障,也会阻碍携带ARGs的质粒水平转移到细菌细胞。Ca2+可诱导细胞以感受态形式存在,促进水平基因转移发生,但也会通过静电作用桥接质粒和EPS,从而阻碍携带ARGs的质粒水平转移到受体细胞[16]。此外,EPS中含有一类DNA水解酶,通过降解外源质粒阻碍基因水平转移。Shou等[64]发现芳香族衍生物会协助质粒穿越EPS屏障,可能的机制是芳香族衍生物的取代基通过氢键作用结合质粒磷酸骨架上的氧位点,形成芳香烃衍生物-质粒的结合体,该结合体掩蔽了EPS水解酶作用于质粒的竞争位点,抑制EPS水解酶对外源质粒的水解,保护携带ARGs的质粒穿越EPS屏障,从而促进ARGs的迁移和扩散[64]。Qiu等[61]研究结果指出,土壤EPS可能会减缓ARGs在土壤环境中的积累,而潜在的分子机制仍有待深入探究。
4 EPS缓解土壤非生物胁迫 4.1 增强保水性能EPS的吸湿性在提高土壤保水持水方面受到广泛关注[65]。EPS-多糖和EPS-蛋白质是高度水合的分子,既能在吸湿作用下保持水分,又能通过静电力和氢键等结合机制保持土壤水分。EPS的持水量是其自身质量的15倍~20倍[66]。目前已报道的高保水性能的细菌EPS-多糖均是高分子量的化合物,如黄原胶、可拉酸和海藻酸盐等[26,67]。Rosenzweig等[68]研究了添加黄原胶的两种砂质土壤的持水量,发现加入1%的黄原胶显著增加了土壤的孔隙度和持水量。干燥可诱导接种细菌的砂粒中EPS产量增加,当直接添加1%的假单胞菌(Pseudomonas sp.)EPS后,砂粒的保水性能得到极大改善[34]。利用模拟土壤孔隙度和团聚体结构的微流控体系,学者们探究了细菌EPS对土壤孔隙中水分滞留的影响,发现EPS和土壤微孔结构对土壤保水性具有协同作用[69-70]。近期研究报道了不同碳底物及其可及性对微生物EPS的化学性质以及保水性能的影响。相比于可溶性底物N-乙酰葡糖胺,不溶性底物几丁质刺激微生物群落产生保水性能更好的EPS[65]。不同碳源底物通过影响微生物群落的结构,间接改变EPS的性质和保水性能,反之也会影响土壤微生物群落对干旱的适应性。作为最严重的非生物环境胁迫,缺水将严重影响作物的生产力,适当的管理措施间接刺激土壤微生物分泌EPS,从而提高旱地土壤的保水持水性能,是农业绿色发展的重要途径之一。
4.2 提升耐盐能力细菌通过多种机制减轻环境中的盐胁迫,如增加ACC脱氨酶活性、磷酸盐增溶作用、产生吲哚乙酸、减少盐诱导的丙二醛含量、分泌铁载体以及EPS。细菌EPS能够防止营养失衡和渗透胁迫,提升细菌对盐胁迫的耐受性,促使环境中微生物和相关植物双方受益[30]。泛生菌(Pantoea sp.)、芽孢杆菌(Bacillus sp.)、放线菌(Actinomycetes sp.)、根瘤菌(Rhizobium sp.)、节杆菌(Arthrobacter sp.)和假单胞菌(Pseudomonas sp.)均会在盐胁迫下合成分泌EPS[71-72]。细菌在盐碱条件下产生EPS的现象是一种适应性策略,可以减轻细菌细胞外膜上的胁迫应力。在高盐浓度条件下,可变盐单胞菌(Halomonas variabilis)HT1和莱比托游动球菌(Planococcus rifietoensis)RT4会刺激EPS产生和生物膜形成[73]。耐盐菌株EPS可以捕获和吸附盐离子,从而减少植物对Na+的吸收,减轻植物根系的盐分胁迫[74]。将分泌EPS的芽孢杆菌(Bacillus)接种于小麦幼苗,增加了中等盐碱土壤中小麦根系周围的土壤颗粒团聚,限制了Na+进入植物根系的被动通量,从而促进小麦的生长发育[75]。同时,添加分泌EPS的植物生长促生菌到小麦根际,小麦表现出更高的生物量产量和更好的耐盐性[74]。
4.3 适应极端环境EPS能保护细菌应对极端环境条件[76]。细菌合成分泌EPS是适应低温和高盐环境的重要策略。从北极冰川采集的样品中观察到高浓度的EPS,并且EPS具有改变冰川微观结构和脱盐的作用,提高了微生物的可居住性和生存能力[77]。当暴露于22℃和4℃的温度下,南极土壤中分离的假单胞菌(Pseudomonas sp.)30-3均具有较多的EPS分泌量,进一步利用细菌死活染色观测,发现假单胞菌30-3在4℃下形成了更密集的活细胞聚集体,从而保护了细菌免受寒冷和霜冻的损伤[78]。EPS也通过保护细菌免受高温而成为嗜热细菌的保护因子。热泉环境中分离的喜温地芽孢杆菌(Geobacillus tepidamans)V264具有较高的EPS分泌量,并且产生的EPS在高温下不易降解[79]。考虑到土壤EPS在土壤-微生物界面的位置及其特性,土壤细菌会通过分泌EPS作为响应热浪事件的反应策略之一,因此,Bérard等[43]认为土壤EPS是理想的土壤生化指标之一,可用于评估热浪事件下的土壤生物地球化学过程。
5 EPS改善土壤整体功能 5.1 驱动土壤颗粒团聚EPS在土壤颗粒团聚方面受到广泛关注,这对于土壤结构、土壤肥力和土壤健康至关重要。细菌EPS是一类具有丰富官能团的黏液,易于粘附在矿物表面(图 2),形成有机-矿物复合体[80-81]。土壤中硅酸盐黏土矿物、铁铝锰氧化物以及碳酸盐是形成有机-矿物复合体的主要矿物[82]。复合体的形成直接影响着微生物衍生有机质的归趋以及矿物的反应活性[83-84]。近年来,细菌EPS在土壤矿物表面吸附的研究取得了显著进展。枯草芽孢杆菌(Bacillus subtilis)的EPS中蛋白质和磷酸化的大分子优先吸附在针铁矿表面,且组分分布具有显著的空间异质性[83,85]。EPS-蛋白质主要通过氢键作用优先吸附在蒙脱石和高岭石表面,而EPS-核酸主要通过配位交换吸附在针铁矿表面[86–88]。有机-矿物复合体的不同形成途径(吸附与共沉淀)显著影响EPS组分在铁铝氧化物表面的选择性保留[89-90]。同时,相关研究也使用共聚焦显微镜或显微谱学可视化了EPS-矿物复合体中多糖、蛋白质和核酸的空间分布[85,88,90]。Ren等[91]研究不同pH和离子强度下EPS在土壤胶体上的吸附能力,证明了EPS-蛋白质和磷酸基团有助于EPS在土壤胶体上的吸附。

|
注:A)基于网袋法的矿物表面微生物孵育野外实验;B)实验中含有黑云母矿物的200 μm尼龙网袋;C)沉积在黑云母上的微生物EPS脱水后SEM图像。 Note: A)field experiment for microbial inoculation on mineral surfaces based on mesh bag method; B)200 μm nylon mesh bag containing biotite minerals in this experiment; C)SEM image of microbial EPS deposited on biotite after dehydration. 图 2 矿物表面微生物定殖和EPS沉积[80] Fig. 2 Microbial colonization and EPS deposition on mineral surfaces[80] |
吸附态EPS会改变矿物表面电荷,进而影响矿物颗粒的团聚行为。Chen等[92]发现在NaCl和MgCl2溶液中,模式EPS多糖成分海藻酸钠会促进赤铁矿颗粒分散,而在CaCl2溶液中海藻酸钠则有利于赤铁矿颗粒团聚,EPS与Ca2+的桥接作用有助于颗粒团聚。模式EPS成分海藻酸钠和牛血清蛋白会通过空间位阻效应降低氧化锰颗粒的团聚速率,而海藻酸钠和Ca2+结合则增加氧化锰颗粒的团聚速率[93]。EPS-多糖促进纳米颗粒团聚,而EPS-蛋白质则有助于纳米颗粒稳定[94]。EPS对矿物颗粒的团聚效果受控于体系pH、离子强度以及EPS浓度[95]。EPS既可以充当黏合剂也可以作为分散剂,主要通过静电作用和空间位阻效应所主导[96]。
5.2 促进团聚体稳定通过向土壤中添加产EPS的细菌或EPS组分,学者们将EPS与团聚体稳定性联系起来。芽孢杆菌属(Bacillus)和假单胞菌属(Pseudomonas)是众所周知的可分泌EPS和形成生物膜的土壤细菌[24]。接种恶臭假单胞菌(Pseudomonas putida)GAP-P45可提高土壤团聚体稳定性50%以上[39]。无论有无胁迫条件下,接种EPS产量高的解淀粉芽孢杆菌(Bacillus amyloliquefaciens)HYD-B17、地衣芽孢杆菌(Bacillus licheniformis)HYTAPB18和枯草芽孢杆菌(Bacillus subtilis)RMPB44均提高了土壤团聚体的稳定性[24]。HYD-B17菌株是最为有效的砂粒团聚菌株,这些砂粒团聚细菌的性能差异归因于其EPS组成成分不同,并且也取决于土壤类型[97]。早期研究测试了不同的模式EPS组分直接用作土壤改良剂的效果。相比于添加葡聚糖的土壤,添加黄原胶可以增加土壤颗粒之间的结合强度,进而稳定土壤结构以及缓解干湿交替的破坏作用,两种多糖结构的差异是其团聚效果不同的原因[98]。添加黄原胶也会显著提高砂质土壤的孔隙度和持水能力[68]。水分含量的变化造成孔隙结构的波动,使EPS与更多的矿物颗粒接触,进而增加形成稳定团聚体的可能性。Cheng等[99]从森林土壤中分离出产生EPS的绿针假单胞菌(Pseudomonas chlororaphis)A20和解蛋白芽孢杆菌(Bacillus proteolyticus)A27,测试了添加两株细菌或其EPS对土壤团聚体稳定性的影响,发现添加的两株细菌均使土壤中水稳性大团聚体含量增加一倍以上;相比添加绿针假单胞菌A20的EPS,添加解蛋白芽孢杆菌A27的EPS促进大团聚体形成的比例更高。因此,分泌EPS的土壤细菌对团聚体的形成和稳定以及土壤结构改善具有重要意义。
由于克服了一些方法学的难题,土壤EPS受到越来越多的关注,土壤EPS已经被作为土壤团聚体稳定性的指标之一[24,100]。通过向土壤中添加各种降解生物膜EPS的酶,如α-葡萄糖苷酶、β-半乳糖苷酶、DNA酶和脂肪酶,将酶预处理后的土壤进行超声处理和密度梯度分馏,测试土壤颗粒有机碳的释放动态,发现酶处理增加了土壤颗粒有机质的释放,表明土壤EPS有助于颗粒有机质附着在土壤团聚体上[100]。Redmile-Gordon等[101]发现土壤中可提取的EPS主要受当前土地利用方式的影响,并且土壤团聚体的稳定性与EPS-蛋白质含量之间存在较好的相关性。Sher等[102]通过温室培养和田间采样,发现柳枝稷草的种植可以促进土壤微生物产生EPS-多糖,随后多元线性回归分析和通径分析表明,土壤中EPS-多糖含量与团聚体稳定性之间存在正相关关系,该现象为种植柳枝稷草改善贫瘠土壤结构提供了一种潜在的解释机制。Zethof等[103]研究了半干旱草原植物对原核生物群落组成、土壤EPS含量和微团聚体稳定性的影响,发现植物根部分泌物提供了易获取的能量和养分物质,刺激了根际微生物生长以及EPS产生量,微生物群落受到植物种类和土壤母质的影响最大;土壤的团聚程度和EPS-多糖含量随着远离根际而呈现递减趋势。随后通过网络分析研究,该团队发现微生物分类群与EPS-多糖含量和/或土壤团聚之间存在正相关关系[104]。土壤EPS组分对团聚体稳定性的贡献程度可能取决于土壤质地、土地利用方式和植被类型等。此外,基于结构方程模型分析表明,土壤中多价阳离子通过增加EPS产量和改变EPS结构,进一步增强了土壤EPS对微团聚体的稳定作用[103]。
5.3 捕获贮存养分不饱和土壤环境中的微生物群落倾向于驻留在EPS包裹的生物膜中,EPS可以捕获和贮存营养物质[105-106]。降解EPS产生的小分子物质可用作养分限制条件下细胞生长的碳源和能源[26]。然而,考虑到EPS的分子结构比较复杂,EPS的完全降解必然需要多种不同的酶[23]。在碳源受限的情况下,根瘤菌(Rhizobium)NZP 2037可以利用自身分泌的EPS作为唯一的碳源[107]。当氮源可利用度较低情况下,土壤细菌会通过分泌的EPS-蛋白质从土壤有机质中进行“氮挖掘”[38]。细菌分泌EPS可作为一种碳储备的胞外策略,但很少有研究关注EPS在微生物之间营养供给或交叉喂养中的作用。稳定同位素标记技术(SIP)可用于鉴别降解EPS的微生物。Wang等[108]利用同位素标记印度贝氏固氮菌(Beijerinckia indica)的EPS,观察到EPS可被低亲缘性的细菌同化,尤其是浮霉菌门(Planctomycetes)。Costa等[109]利用Acidobacteria Granulicellas菌株WH15的EPS作为富集因子,使用SIP技术结合宏基因组技术靶向研究降解EPS的微生物群落和功能,并鉴定出产生糖苷水解酶的土壤细菌群落。WH15菌株EPS主要被浮霉菌门(Planctomycetes)、疣微菌门(Verrucomicrobia)、子囊菌门(Ascomycota)和担子菌门(Basidiomycota)细菌同化[109-110]。此外,研究人员分离出利用EPS作为唯一碳源的细菌,证明了EPS在筛选新微生物物种方面的潜在用途[108-109]。
5.4 固持还原重金属细菌分泌的EPS可以增强对重金属的吸附固持。大量研究已探讨EPS对金属离子的生物吸附潜力[111-112],以期为重金属污染土壤的微生物修复提供理论支撑。EPS基质的生物吸附能力归因于丰富的官能团,例如羧基、磷酸基、巯基、酚基和羟基等,能与阳离子之间产生较强的静电引力。生物吸附涉及EPS官能团与金属之间的多种机制,包括物理吸附、离子交换、络合和沉淀作用[26,113–114]。EPS的结合位点数量和络合能力与蛋白质、多糖和脂质含量相关[115]。研究指出EPS与重金属之间的结合能力和键合强度很高,并且吸附遵循Langmuir或Freundlich方程[26,116]。同时,分泌的EPS会吸附到土壤矿物表面,影响矿物固定重金属的能力。Mikutta等[117]研究了Pb2+、Cu2+和Zn2+在枯草芽孢杆菌(Bacillus subtilis)EPS-膨润土/水铁矿复合物上的吸附动力学;膨润土选择性吸附EPS的低分子量组分和含N组分,矿物结合态EPS增加了膨润土对重金属的吸附程度和速率;水铁矿选择性地保留了EPS的高分子量组分和富P组分,并且对水铁矿吸附重金属具有负效应[117]。EPS-蛋白质增强了针铁矿对Hg(Ⅱ)的吸附能力[118]。EPS对氧化铝吸附Zn的影响具有pH依赖性,并且EPS的羧基和磷酰基在此过程中发挥着重要作用[119]。Nkoh等[120]发现添加细菌EPS增加了土壤胶体表面的负电荷,导致对Cu2+和Cd2+的吸附量增加,静电力、疏水力、范德华力以及络合作用均有助于可变电荷土壤对重金属的吸附。因此,土壤EPS的生物吸附作用显著影响着重金属的环境行为。
细菌EPS的渗透性屏障作用可以保护细胞免受高金属浓度的毒性。通过对比Cu2+胁迫下荧光假单胞菌(Pseudomonas fluorescens)生物膜和浮游细胞的代谢响应,发现浮游细胞启动氧化应激反应,而金属胁迫诱导了生物膜中EPS相关代谢途径表达。代谢过程的差异证明了EPS的存在增加了细胞对重金属的耐受性[121]。近年来,研究发现EPS也是电子传递介质和电子供体[122]。EPS可以通过半缩醛基团将金属离子(Ag+和Au3+)还原为元素纳米颗粒,从而降低了金属离子的生物有效性[123-124]。然而,Zhang等[125]发现EPS会显著干扰硫化汞的沉淀,并导致可用于微生物甲基化的黒辰砂形成,从而增加了神经毒素甲基汞的环境风险。最新研究中将土壤EPS纳入土壤生化指标之一,Redmile-Gordon和Chen[126]发现酸性土壤中细菌通过分泌EPS-多糖和可溶性糖醛酸,作为一种应对Zn2+胁迫的耐受机制。多元统计分析评估了场地污染土壤中细菌群落的多金属抗性和排毒途径,发现土壤细菌可通过分泌EPS-多糖和EPS-蛋白质作为应对Cr胁迫的响应策略[127]。
5.5 吸附降解有机污染物细菌EPS的分泌能够有效缓解有机污染环境的胁迫压力。EPS含有亲水基团和疏水基团,有利于带正/负电荷的有机污染物吸附。有机多溴二苯醚污染物在土壤中的命运、迁移和生物利用度主要取决于土壤有机质的吸附作用[128]。土壤EPS中的蛋白质组分会增加土壤对多溴二苯醚的螯合,而多糖组分的影响则具有浓度依赖性[129]。EPS作为一种生物表面活性剂,能够降低表面张力和界面张力,从而提高有机污染物的分散性、乳化性和生物利用度。相比于Tween 80等常见表面活性剂,根瘤菌EPS表现出更好的乳化活性[130]。土壤固氮菌产生的EPS可增加难溶有机污染物的分散性,增强疏水性污染物与细胞之间的亲和力,促使污染物更容易被降解[131]。微生物降解多环芳烃被认为是行之有效的生物修复技术,其中EPS增加了难溶性多环芳烃的生物利用度,加速了多环芳烃的生物降解[132]。EPS和多环芳烃之间的相互作用被认为是自发放热过程,两者的结合主要由疏水相互作用决定[26]。Han等[133]在模式土壤组分蒙脱石和腐殖酸载体上构建生物膜群落,探究土壤生物膜超微结构对苯并(a)芘吸附和生物降解的影响。研究结果表明,无机和有机载体均有利于生物膜的形成,并且有机载体上的生物膜具有更致密的EPS基质,加速了苯并(a)芘的生物降解[133]。许多烃类降解细菌均具有产生乳化活性EPS的能力,从而为生物修复有机污染环境提供了潜在的应用价值[134]。
6 结论与展望土壤细菌已经发展出一系列适应生存环境的策略。细菌EPS的分泌是一种提供湿润环境、捕获营养物质、促进化学反应以及保护细胞应对非生物胁迫、抗生素消杀和掠食者侵袭的重要策略。细菌EPS是高度多样化的混合物,其功能取决于组成和结构。EPS可以促进土壤团聚、改善土壤质量和提升土壤肥力。土壤和植物根际中EPS还可以改善植物和微生物对养分和水分的利用率,从而有益于土壤-微生物-植物的整体功能。土壤细菌具有巨大的环境功能潜力,通过促进土壤生物膜的形成和改进土壤EPS的组成,助力可持续和环境友好型农业发展。
EPS是一类复杂的生物聚合物,对其组成结构、环境功能和遗传调控的理解虽然较为广泛,但还远不完整。进一步研究需要阐明EPS生物合成和调控所涉及的基因和相关机制。同时,新型EPS的发现和表征可能会带来潜在的应用价值,尤其是在污染环境修复、土壤地力恢复和土壤肥力提升等方面。现代仪器分析技术与表征方法(如核磁共振、原子力红外光谱、激光共聚焦显微拉曼光谱、环境扫描电子显微镜、激光扫描共聚焦显微镜、稳定同位素探针、纳米二次离子质谱技术、傅里叶变换离子回旋共振质谱和同步辐射技术等)以及与微生物组学技术相结合,将更全面揭示EPS的组成结构和遗传调控,并加速发掘EPS在土壤生态系统中的功能潜力。通过调控环境因素,EPS的产生可能被触发、停止或逆转,实现土壤EPS对养分存储和释放的调控,进而调节土壤的物理结构和养分状况,为保障粮食安全和保护环境健康提供新策略与新途径。
土壤EPS的研究正处于方兴未艾的阶段,以下关键科学问题有待进一步明晰,以增进对土壤EPS的理解:
1)EPS成分的理化性质:量化土壤EPS及其成分EPS-多糖、EPS-蛋白质和EPS-氨基酸等,可以阐明微生物群落的生物膜表型对环境条件的适应性。土壤中总糖、总肽及总氨基酸等的测定分析提供了丰富的土壤生化物质信息,但由于无法准确区分这些土壤生化物质的来源,从而难以解答许多土壤科学的关键问题[135-136]。对土壤生化物质来源的模糊划分和忽视行为阻碍了当前土壤学科的发展,亟需进一步区分生化物质的来源。因此,土壤EPS的特异性提取和精准分析将为理解相关问题提供有效途径[38,137],从而深刻揭示土壤EPS与土壤功能之间的关联性。
2)EPS-蛋白质与球囊霉素相关土壤蛋白(Glomalin-related soil proteins,GRSP):球囊霉素是丛枝菌根真菌的假设基因产物。GRSP的提取方法值得重新审视,即对土壤进行柠檬酸盐缓冲溶液结合高压灭菌步骤的提取方法。该方法的提取物被证明包含更多来源于细菌而非真菌的蛋白质[138],并且未发现与GRSP之间存在必然的关联性[139]。同时,该方法裂解了微生物细胞,并共同提取了微生物胞内成分和土壤有机质。学者们进一步推测GRSP与土壤团聚体稳定性的良好关系可能归因于土壤EPS-蛋白质的有效共提取[20]。Redmile-Gordon等[101]发现EPS-蛋白质对团聚体稳定性的潜在贡献大于土壤有机质或EPS-多糖。因此,阐明EPS-蛋白质与GRSP对土壤团聚体稳定性的相对贡献是必要的,有助于引导土壤科学工作者聚焦于更为关键的研究对象。
3)EPS作为敏感的土壤健康指标:基于避免微生物胞内污染和降低腐殖质共提取的判断标准,进一步优化阳离子交换树脂(Cation exchange resin,CER)法提取土壤EPS[20–22]。对土壤EPS的认知,有助于解析微生物细胞与土壤矿物/土壤有机质之间的生物物理化学界面过程,揭开被隐藏的微生物界面响应机制,推进土壤EPS发展为一类敏感的生物学指标。建议将土壤EPS的量化分析包含在土壤理化分析过程中,将其发展为土壤健康指标之一,并评估土壤EPS与其他土壤健康指标之间的相对重要性和相互关联性。
4)EPS推动新型生物肥料研发:过度使用化学肥料和农药的环境危害,迫使世界各地的研究人员寻求替代方法来提高作物的生产力。生物有机肥料的出现为减少化肥和农药使用提供了有效途径,有机肥与微生物接种剂的结合可以提供养分并改善土壤结构[30]。微生物接种剂已经研究了几十年,但仍然需要改善微生物的生长条件,实现接种剂的高生物量和高定殖存活率。考虑到EPS对土壤细菌和土壤结构的增益效应,可以利用EPS对微生物菌株进行包封,进而制备新型生物有机肥。EPS既能有效地增加生物菌剂在土壤中的定殖与存活,又能改善土壤结构[140]。同时,将农业废弃物料作为大规模生产EPS的底物,既可增加经济收益,也能解决废弃物料堆积或焚烧带来的环境问题。
| [1] |
Chu H Y, Ma Y Y, Yang T, et al. The strategies for development of the subdiscipline of Soil Biology for the 14th Five-Year Plan (In Chinese)[J]. Acta Pedologica Sinica, 2020, 57(5): 1105-1116. [褚海燕, 马玉颖, 杨腾, 等. "十四五"土壤生物学分支学科发展战略[J]. 土壤学报, 2020, 57(5): 1105-1116.]
(  0) 0) |
| [2] |
Song C Q, Wu J S, Lu Y H, et al. Advances of Soil Microbiology in the last decade in China (In Chinese)[J]. Advance in Earth Science, 2013, 28(10): 1087-1105. DOI:10.11867/j.issn.1001-8166.2013.10.1087 [宋长青, 吴金水, 陆雅海, 等. 中国土壤微生物学研究十年回顾[J]. 地球科学进展, 2013, 28(10): 1087-1105.]
(  0) 0) |
| [3] |
Burmølle M, Kjøller A, Sørensen S J. Biofilms in soil//Glinski J, Horabik J, Lipiec J. Encyclopedia of Agrophysics[M]. Dordrecht: Springer Netherlands, 2011: 70-75.
(  0) 0) |
| [4] |
Cai P, Sun X, Wu Y, et al. Soil biofilms: Microbial interactions, challenges, and advanced techniques for ex-situ characterization[J]. Soil Ecology Letters, 2019, 1(3): 85-93.
(  0) 0) |
| [5] |
Flemming H-C, Wingender J, Szewzyk U, et al. Biofilms: An emergent form of bacterial life[J]. Nature Reviews Microbiology, 2016, 14(9): 563-575. DOI:10.1038/nrmicro.2016.94
(  0) 0) |
| [6] |
Flemming H-C, Wuertz S. Bacteria and archaea on Earth and their abundance in biofilms[J]. Nature Reviews Microbiology, 2019, 17(4): 247-260. DOI:10.1038/s41579-019-0158-9
(  0) 0) |
| [7] |
Lehmann A, Zheng W, Rillig M C. Soil biota contributions to soil aggregation[J]. Nature Ecology & Evolution, 2017, 1(12): 1828-1835.
(  0) 0) |
| [8] |
Bystrianský L, Hujslová M, Hršelová H, et al. Observations on two microbial life strategies in soil: Planktonic and biofilm-forming microorganisms are separable[J]. Soil Biology and Biochemistry, 2019, 136: 107535. DOI:10.1016/j.soilbio.2019.107535
(  0) 0) |
| [9] |
Wu Y, Cai P, Jing X, et al. Soil biofilm formation enhances microbial community diversity and metabolic activity[J]. Environment International, 2019, 132: 105116. DOI:10.1016/j.envint.2019.105116
(  0) 0) |
| [10] |
Burns R G, DeForest J L, Marxsen J, et al. Soil enzymes in a changing environment: Current knowledge and future directions[J]. Soil Biology and Biochemistry, 2013, 58: 216-234. DOI:10.1016/j.soilbio.2012.11.009
(  0) 0) |
| [11] |
Chenu C. Clay- or sand-polysaccharide associations as models for the interface between micro-organisms and soil: Water related properties and microstructure//Brussaard L, Kooistra M J. Soil structure/Soil biota interrelationships[M]. Amsterdam: Elsevier, 1993: 143-156.
(  0) 0) |
| [12] |
Zhao W, Walker S L, Huang Q, et al. Contrasting effects of extracellular polymeric substances on the surface characteristics of bacterial pathogens and cell attachment to soil particles[J]. Chemical Geology, 2015, 410: 79-88. DOI:10.1016/j.chemgeo.2015.06.013
(  0) 0) |
| [13] |
Davis C A, Pyrak-Nolte L J, Atekwana E A, et al. Microbial-induced heterogeneity in the acoustic properties of porous media[J]. Geophysical Research Letters, 2009, 36: L21405. DOI:10.1029/2009GL039569
(  0) 0) |
| [14] |
Ren D, Madsen J S, Sørensen S J, et al. High prevalence of biofilm synergy among bacterial soil isolates in cocultures indicates bacterial interspecific cooperation[J]. The ISME Journal, 2015, 9(1): 81-89. DOI:10.1038/ismej.2014.96
(  0) 0) |
| [15] |
Aquino S F, Stuckey D C. Soluble microbial products formation in anaerobic chemostats in the presence of toxic compounds[J]. Water Research, 2004, 38(2): 255-266. DOI:10.1016/j.watres.2003.09.031
(  0) 0) |
| [16] |
Hu X, Kang F, Yang B, et al. Extracellular polymeric substances acting as a permeable barrier hinder the lateral transfer of antibiotic resistance genes[J]. Frontiers in Microbiology, 2019, 10: 736. DOI:10.3389/fmicb.2019.00736
(  0) 0) |
| [17] |
Wu S, Wu Y, Cao B, et al. An invisible workforce in soil: The neglected role of soil biofilms in conjugative transfer of antibiotic resistance genes[J]. Critical Reviews in Environmental Science and Technology, 2021. DOI:10.1080/10643389.2021.1892015
(  0) 0) |
| [18] |
Flemming H-C. The perfect slime[J]. Colloids and Surfaces B: Biointerfaces, 2011, 86(2): 251-259. DOI:10.1016/j.colsurfb.2011.04.025
(  0) 0) |
| [19] |
Tang J, Mo Y, Zhang J, et al. Influence of biological aggregating agents associated with microbial population on soil aggregate stability[J]. Applied Soil Ecology, 2011, 47(3): 153-159. DOI:10.1016/j.apsoil.2011.01.001
(  0) 0) |
| [20] |
Redmile-Gordon M A, Brookes P C, Evershed R P, et al. Measuring the soil-microbial interface: Extraction of extracellular polymeric substances(EPS)from soil biofilms[J]. Soil Biology and Biochemistry, 2014, 72: 163-171. DOI:10.1016/j.soilbio.2014.01.025
(  0) 0) |
| [21] |
Wang S, Redmile-Gordon M, Mortimer M, et al. Extraction of extracellular polymeric substances(EPS)from red soils(Ultisols)[J]. Soil Biology and Biochemistry, 2019, 135: 283-285. DOI:10.1016/j.soilbio.2019.05.014
(  0) 0) |
| [22] |
Bérard A, Clavel T, Le Bourvellec C, et al. Exopolysaccharides in the rhizosphere: A comparative study of extraction methods. Application to their quantification in Mediterranean soils[J]. Soil Biology and Biochemistry, 2020, 149: 107961. DOI:10.1016/j.soilbio.2020.107961
(  0) 0) |
| [23] |
Flemming H-C, Wingender J. The biofilm matrix[J]. Nature Reviews Microbiology, 2010, 8(9): 623-633. DOI:10.1038/nrmicro2415
(  0) 0) |
| [24] |
Costa O Y A, Raaijmakers J M, Kuramae E E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation[J]. Frontiers in Microbiology, 2018, 9: 1636. DOI:10.3389/fmicb.2018.01636
(  0) 0) |
| [25] |
Wingender J, Neu T R, Flemming H C. What are bacterial extracellular polymeric substances?//Wingender J, Neu T R, Flemming H C. Microbial extracellular polymeric substances: Characterization, structure and function[M]. Berlin, Heidelberg: Springer, 1999: 1-19.
(  0) 0) |
| [26] |
More T T, Yadav J S S, Yan S, et al. Extracellular polymeric substances of bacteria and their potential environmental applications[J]. Journal of Environmental Management, 2014, 144: 1-25.
(  0) 0) |
| [27] |
Park C, Novak J T. Characterization of lectins and bacterial adhesins in activated sludge flocs[J]. Water Environment Research, 2009, 81(8): 755-764. DOI:10.2175/106143008X370421
(  0) 0) |
| [28] |
Okshevsky M, Meyer R L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms[J]. Critical Reviews in Microbiology, 2015, 41(3): 341-352. DOI:10.3109/1040841X.2013.841639
(  0) 0) |
| [29] |
Peng N, Cai P, Mortimer M, et al. The exopolysaccharide-eDNA interaction modulates 3D architecture of Bacillus subtilis biofilm[J]. BMC Microbiology, 2020, 20(1): 115. DOI:10.1186/s12866-020-01789-5
(  0) 0) |
| [30] |
Saha I, Datta S, Biswas D. Exploring the role of bacterial extracellular polymeric substances for sustainable development in agriculture[J]. Current Microbiology, 2020, 77(11): 3224-3239. DOI:10.1007/s00284-020-02169-y
(  0) 0) |
| [31] |
Bystrianský L, Hujslová M, Gryndler M. Study of the effects of mineral salts on the biofilm formation on polypropylene fibers using three quantification methods[J]. Folia Microbiologica, 2021, 66(1): 133-143. DOI:10.1007/s12223-020-00833-1
(  0) 0) |
| [32] |
Blanco Y, Rivas L A, González-Toril E, et al. Environmental parameters, and not phylogeny, determine the composition of extracellular polymeric substances in microbial mats from extreme environments[J]. Science of the Total Environment, 2019, 650: 384-393. DOI:10.1016/j.scitotenv.2018.08.440
(  0) 0) |
| [33] |
Nichols C M, Bowman J P, Guezennec J. Effects of incubation temperature on growth and production of exopolysaccharides by an Antarctic sea ice bacterium grown in batch culture[J]. Applied and Environmental Microbiology, 2005, 71(7): 3519-3523. DOI:10.1128/AEM.71.7.3519-3523.2005
(  0) 0) |
| [34] |
Roberson E B, Firestone M K. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp.[J]. Applied and Environmental Microbiology, 1992, 58(4): 1284-1291. DOI:10.1128/aem.58.4.1284-1291.1992
(  0) 0) |
| [35] |
Kazy S K, Sar P, Singh S P, et al. Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain: Synthesis, chemical nature and copper binding[J]. World Journal of Microbiology and Biotechnology, 2002, 18(6): 583-588. DOI:10.1023/A:1016354713289
(  0) 0) |
| [36] |
Laspidou C S, Rittmann B E. A unified theory for extracellular polymeric substances, soluble microbial products, and active and inert biomass[J]. Water Research, 2002, 36(11): 2711-2720. DOI:10.1016/S0043-1354(01)00413-4
(  0) 0) |
| [37] |
Sengupta D, Datta S, Biswas D. Towards a better production of bacterial exopolysaccharides by controlling genetic as well as physico-chemical parameters[J]. Applied Microbiology and Biotechnology, 2018, 102(4): 1587-1598. DOI:10.1007/s00253-018-8745-7
(  0) 0) |
| [38] |
Redmile-Gordon M A, Evershed R P, Hirsch P R, et al. Soil organic matter and the extracellular microbial matrix show contrasting responses to C and N availability[J]. Soil Biology and Biochemistry, 2015, 88: 257-267. DOI:10.1016/j.soilbio.2015.05.025
(  0) 0) |
| [39] |
Sandhya V, Ali Sk Z. The production of exopolysaccharide by Pseudomonas putida GAP-P45 under various abiotic stress conditions and its role in soil aggregation[J]. Microbiology, 2015, 84(4): 512-519. DOI:10.1134/S0026261715040153
(  0) 0) |
| [40] |
Truelstrup Hansen L, Vogel B F. Desiccation of adhering and biofilm Listeria monocytogenes on stainless steel: Survival and transfer to salmon products[J]. International Journal of Food Microbiology, 2011, 146(1): 88-93. DOI:10.1016/j.ijfoodmicro.2011.01.032
(  0) 0) |
| [41] |
Krause L, Biesgen D, Treder A, et al. Initial microaggregate formation: Association of microorganisms to montmorillonite-goethite aggregates under wetting and drying cycles[J]. Geoderma, 2019, 351: 250-260. DOI:10.1016/j.geoderma.2019.05.001
(  0) 0) |
| [42] |
Roberson E B, Chenu C, Firestone M K. Microstructural changes in bacterial exopolysaccharides during desiccation[J]. Soil Biology and Biochemistry, 1993, 25(9): 1299-1301. DOI:10.1016/0038-0717(93)90230-9
(  0) 0) |
| [43] |
Bérard A, Sassi M B, Kaisermann A, et al. Soil microbial community responses to heat wave components: Drought and high temperature[J]. Climate Research, 2015, 66(3): 243-264. DOI:10.3354/cr01343
(  0) 0) |
| [44] |
Flemming H-C, Neu T R, Wozniak D J. The EPS matrix: The "House of Biofilm Cells"[J]. Journal of Bacteriology, 2007, 189(22): 7945-7947. DOI:10.1128/JB.00858-07
(  0) 0) |
| [45] |
Geisseler D, Horwath W R, Joergensen R G, et al. Pathways of nitrogen utilization by soil microorganisms-A review[J]. Soil Biology and Biochemistry, 2010, 42(12): 2058-2067. DOI:10.1016/j.soilbio.2010.08.021
(  0) 0) |
| [46] |
Dilly O, Nannipieri P. Response of ATP content, respiration rate and enzyme activities in an arable and a forest soil to nutrient additions[J]. Biology and Fertility of Soils, 2001, 34(1): 64-72. DOI:10.1007/s003740100375
(  0) 0) |
| [47] |
Kemmitt S J, Lanyon C V, Waite I S, et al. Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass-A new perspective[J]. Soil Biology and Biochemistry, 2008, 40(1): 61-73. DOI:10.1016/j.soilbio.2007.06.021
(  0) 0) |
| [48] |
Tsuneda S, Aikawa H, Hayashi H, et al. Extracellular polymeric substances responsible for bacterial adhesion onto solid surface[J]. FEMS Microbiology Letters, 2003, 223(2): 287-292. DOI:10.1016/S0378-1097(03)00399-9
(  0) 0) |
| [49] |
Hong Z, Chen W, Rong X, et al. The effect of extracellular polymeric substances on the adhesion of bacteria to clay minerals and goethite[J]. Chemical Geology, 2013, 360: 118-125.
(  0) 0) |
| [50] |
Ren L, Hong Z, Liu Z, et al. ATR-FTIR investigation of mechanisms of Bacillus subtilis adhesion onto variable- and constant-charge soil colloids[J]. Colloids and Surfaces B: Biointerfaces, 2018, 162: 288-295. DOI:10.1016/j.colsurfb.2017.11.067
(  0) 0) |
| [51] |
Nkoh N J, Liu Z-D, Yan J, et al. The role of extracellular polymeric substances in bacterial adhesion onto variable charge soils[J]. Archives of Agronomy and Soil Science, 2020, 66(13): 1780-1793. DOI:10.1080/03650340.2019.1696016
(  0) 0) |
| [52] |
Jing X, Wu Y, Shi L, et al. Outer membrane c-type cytochromes OmcA and MtrC play distinct roles in enhancing the attachment of Shewanella oneidensis MR-1 cells to goethite[J]. Applied and Environmental Microbiology, 2020, 86(23): e01941-20.
(  0) 0) |
| [53] |
Cheng H-P, Walker G C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of Alfalfa by Rhizobium meliloti[J]. Journal of Bacteriology, 1998, 180(19): 5183-5191. DOI:10.1128/JB.180.19.5183-5191.1998
(  0) 0) |
| [54] |
Williams A, Wilkinson A, Krehenbrink M, et al. Glucomannan-mediated attachment of Rhizobium leguminosarum to Pea root hairs is required for competitive nodule infection[J]. Journal of Bacteriology, 2008, 190(13): 4706-4715. DOI:10.1128/JB.01694-07
(  0) 0) |
| [55] |
Kawaharada Y, Kelly S, Nielsen M W, et al. Receptor-mediated exopolysaccharide perception controls bacterial infection[J]. Nature, 2015, 523(7560): 308-312. DOI:10.1038/nature14611
(  0) 0) |
| [56] |
Lehman A P, Long S R. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage[J]. Journal of Bacteriology, 2013, 195(23): 5362-5369. DOI:10.1128/JB.00681-13
(  0) 0) |
| [57] |
Stewart P S. Mechanisms of antibiotic resistance in bacterial biofilms[J]. International Journal of Medical Microbiology, 2002, 292(2): 107-113. DOI:10.1078/1438-4221-00196
(  0) 0) |
| [58] |
Souli M, Giamarellou H. Effects of slime produced by clinical isolates of coagulase-negative staphylococci on activities of various antimicrobial agents[J]. Antimicrobial Agents and Chemotherapy, 1998, 42(4): 939-941. DOI:10.1128/AAC.42.4.939
(  0) 0) |
| [59] |
Grobe S, Wingender J, Flemming H-C. Capability of mucoid Pseudomonas aeruginosa to survive in chlorinated water[J]. International Journal of Hygiene and Environmental Health, 2001, 204(2): 139-142.
(  0) 0) |
| [60] |
Wang L, Li Y, Wang L, et al. Extracellular polymeric substances affect the responses of multi-species biofilms in the presence of sulfamethizole[J]. Environmental Pollution, 2018, 235: 283-292. DOI:10.1016/j.envpol.2017.12.060
(  0) 0) |
| [61] |
Qiu L, Wu J, Du W, et al. Response of soil bacterial communities to sulfadiazine present in manure: Protection and adaptation mechanisms of extracellular polymeric substances[J]. Journal of Hazardous Materials, 2021, 408: 124887. DOI:10.1016/j.jhazmat.2020.124887
(  0) 0) |
| [62] |
Bae J, Oh E, Jeon B. Enhanced transmission of antibiotic resistance in Campylobacter jejuni biofilms by natural transformation[J]. Antimicrobial Agents and Chemotherapy, 2014, 58(12): 7573-7575. DOI:10.1128/AAC.04066-14
(  0) 0) |
| [63] |
Hendrickx L, Hausner M, Wuertz S. Natural genetic transformation in monoculture Acinetobacter sp. strain BD413 biofilms[J]. Applied and Environmental Microbiology, 2003, 69(3): 1721-1727. DOI:10.1128/AEM.69.3.1721-1727.2003
(  0) 0) |
| [64] |
Shou W, Kang F, Huang S, et al. Substituted aromatic-facilitated dissemination of mobile antibiotic resistance genes via an antihydrolysis mechanism across an extracellular polymeric substance permeable barrier[J]. Environmental Science & Technology, 2019, 53(2): 604-613.
(  0) 0) |
| [65] |
Bhattacharjee A, Thompson A M, Schwarz K C, et al. Soil microbial EPS resiliency is influenced by carbon source accessibility[J]. Soil Biology and Biochemistry, 2020, 151: 108037. DOI:10.1016/j.soilbio.2020.108037
(  0) 0) |
| [66] |
Or D, Phutane S, Dechesne A. Extracellular polymeric substances affecting pore-scale hydrologic conditions for bacterial activity in unsaturated soils[J]. Vadose Zone Journal, 2007, 6(2): 298-305. DOI:10.2136/vzj2006.0080
(  0) 0) |
| [67] |
Flemming H-C. EPS-Then and now[J]. Microorganisms, 2016, 4(4): 41. DOI:10.3390/microorganisms4040041
(  0) 0) |
| [68] |
Rosenzweig R, Shavit U, Furman A. Water retention curves of biofilm-affected soils using xanthan as an analogue[J]. Soil Science Society of America Journal, 2012, 76(1): 61-69. DOI:10.2136/sssaj2011.0155
(  0) 0) |
| [69] |
Deng J, Orner E P, Chau J F, et al. Synergistic effects of soil microstructure and bacterial EPS on drying rate in emulated soil micromodels[J]. Soil Biology and Biochemistry, 2015, 83: 116-124. DOI:10.1016/j.soilbio.2014.12.006
(  0) 0) |
| [70] |
Guo Y-S, Furrer J M, Kadilak A L, et al. Bacterial extracellular polymeric substances amplify water content variability at the pore scale[J]. Frontiers in Environmental Science, 2018, 6: 93. DOI:10.3389/fenvs.2018.00093
(  0) 0) |
| [71] |
Zhang S, Fan C, Wang Y, et al. Salt-tolerant and plant-growth-promoting bacteria isolated from high-yield paddy soil[J]. Canadian Journal of Microbiology, 2018, 64(12): 968-978. DOI:10.1139/cjm-2017-0571
(  0) 0) |
| [72] |
Vaishnav A, Shukla A K, Sharma A, et al. Endophytic bacteria in plant salt stress tolerance: Current and future prospects[J]. Journal of Plant Growth Regulation, 2019, 38(2): 650-668. DOI:10.1007/s00344-018-9880-1
(  0) 0) |
| [73] |
Qurashi A W, Sabri A N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress[J]. Brazilian Journal of Microbiology, 2012, 43(3): 1183-1191. DOI:10.1590/S1517-83822012000300046
(  0) 0) |
| [74] |
Upadhyay S K, Singh J S, Singh D P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition[J]. Pedosphere, 2011, 21(2): 214-222. DOI:10.1016/S1002-0160(11)60120-3
(  0) 0) |
| [75] |
Ashraf M, Hasnain S, Berge O, et al. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress[J]. Biology and Fertility of Soils, 2004, 40(3): 157-162.
(  0) 0) |
| [76] |
Flemming H-C. Biofilms and environmental protection[J]. Water Science and Technology, 1993, 27(7): 1-10.
(  0) 0) |
| [77] |
Krembs C, Eicken H, Deming J W. Exopolymer alteration of physical properties of sea ice and implications for ice habitability and biogeochemistry in a warmer Arctic[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(9): 3653-3658. DOI:10.1073/pnas.1100701108
(  0) 0) |
| [78] |
Panicker G, Aislabie J, Bej A K. Analysis of aggregative behavior of Pseudomonas sp. 30-3 isolated from Antarctic soil[J]. Soil Biology and Biochemistry, 2006, 38(10): 3152-3157. DOI:10.1016/j.soilbio.2006.02.006
(  0) 0) |
| [79] |
Kambourova M, Mandeva R, Dimova D, et al. Production and characterization of a microbial glucan, synthesized by Geobacillus tepidamans V264 isolated from Bulgarian hot spring[J]. Carbohydrate Polymers, 2009, 77(2): 338-343. DOI:10.1016/j.carbpol.2009.01.004
(  0) 0) |
| [80] |
Dohnalkova A C, Chu R K, Tfaily M, et al. Investigation into the stabilization of soil organic matter by microbes[J]. Microscopy and Microanalysis, 2015, 21(S3): 863-864. DOI:10.1017/S1431927615005115
(  0) 0) |
| [81] |
Kleber M, Eusterhues K, Keiluweit M, et al. Mineral-organic associations: Formation, properties, and relevance in soil environments[J]. Advances in Agronomy, 2015, 130: 1-140.
(  0) 0) |
| [82] |
Totsche K U, Amelung W, Gerzabek M H, et al. Microaggregates in soils[J]. Journal of Plant Nutrition and Soil Science, 2018, 181(1): 104-136. DOI:10.1002/jpln.201600451
(  0) 0) |
| [83] |
Omoike A, Chorover J. Adsorption to goethite of extracellular polymeric substances from Bacillus subtilis[J]. Geochimica et Cosmochimica Acta, 2006, 70(4): 827-838. DOI:10.1016/j.gca.2005.10.012
(  0) 0) |
| [84] |
Kikuchi S, Kashiwabara T, Shibuya T, et al. Molecular-scale insights into differences in the adsorption of cesium and selenium on biogenic and abiogenic ferrihydrite[J]. Geochimica et Cosmochimica Acta, 2019, 251: 1-14. DOI:10.1016/j.gca.2019.02.001
(  0) 0) |
| [85] |
Liu X, Eusterhues K, Thieme J, et al. STXM and NanoSIMS investigations on EPS fractions before and after adsorption to goethite[J]. Environmental Science & Technology, 2013, 47(7): 3158-3166.
(  0) 0) |
| [86] |
Cao Y, Wei X, Cai P, et al. Preferential adsorption of extracellular polymeric substances from bacteria on clay minerals and iron oxide[J]. Colloids and Surfaces B: Biointerfaces, 2011, 83(1): 122-127. DOI:10.1016/j.colsurfb.2010.11.018
(  0) 0) |
| [87] |
Fang L, Cao Y, Huang Q, et al. Reactions between bacterial exopolymers and goethite: A combined macroscopic and spectroscopic investigation[J]. Water Research, 2012, 46(17): 5613-5620. DOI:10.1016/j.watres.2012.07.046
(  0) 0) |
| [88] |
Lin D, Ma W, Jin Z, et al. Interactions of EPS with soil minerals: A combination study by ITC and CLSM[J]. Colloids and Surfaces B: Biointerfaces, 2016, 138: 10-16. DOI:10.1016/j.colsurfb.2015.11.026
(  0) 0) |
| [89] |
Mikutta R, Zang U, Chorover J, et al. Stabilization of extracellular polymeric substances(Bacillus subtilis)by adsorption to and coprecipitation with Al forms[J]. Geochimica et Cosmochimica Acta, 2011, 75(11): 3135-3154. DOI:10.1016/j.gca.2011.03.006
(  0) 0) |
| [90] |
Zhang M, Peacock C L, Cai P, et al. Selective retention of extracellular polymeric substances induced by adsorption to and coprecipitation with ferrihydrite[J]. Geochimica et Cosmochimica Acta, 2021, 299: 15-34. DOI:10.1016/j.gca.2021.02.015
(  0) 0) |
| [91] |
Ren L, Hong Z, Qian W, et al. Adsorption mechanism of extracellular polymeric substances from two bacteria on Ultisol and Alfisol[J]. Environmental Pollution, 2018, 237: 39-49. DOI:10.1016/j.envpol.2018.01.075
(  0) 0) |
| [92] |
Chen K L, Mylon S E, Elimelech M. Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes[J]. Environmental Science & Technology, 2006, 40(5): 1516-1523.
(  0) 0) |
| [93] |
Huangfu X, Jiang J, Ma J, et al. Aggregation kinetics of manganese dioxide colloids in aqueous solution: Influence of humic substances and biomacromolecules[J]. Environmental Science & Technology, 2013, 47(18): 10285-10292.
(  0) 0) |
| [94] |
Lin D, Drew Story S, Walker S L, et al. Influence of extracellular polymeric substances on the aggregation kinetics of TiO2 nanoparticles[J]. Water Research, 2016, 104: 381-388. DOI:10.1016/j.watres.2016.08.044
(  0) 0) |
| [95] |
Lin D, Cai P, Peacock C L, et al. Towards a better understanding of the aggregation mechanisms of iron(hydr)oxide nanoparticles interacting with extracellular polymeric substances: Role of pH and electrolyte solution[J]. Science of the Total Environment, 2018, 645: 372-379. DOI:10.1016/j.scitotenv.2018.07.136
(  0) 0) |
| [96] |
Guhra T, Ritschel T, Totsche K U. Formation of mineral-mineral and organo-mineral composite building units from microaggregate-forming materials including microbially produced extracellular polymeric substances[J]. European Journal of Soil Science, 2019, 70(3): 604-615. DOI:10.1111/ejss.12774
(  0) 0) |
| [97] |
Wu N, Pan H-X, Qiu D, et al. Feasibility of EPS-producing bacterial inoculation to speed up the sand aggregation in the Gurbantunggut Desert, Northwestern China[J]. Journal of Basic Microbiology, 2014, 54(12): 1378-1386. DOI:10.1002/jobm.201400355
(  0) 0) |
| [98] |
Czarnes S, Hallett P D, Bengough A G, et al. Root- and microbial-derived mucilages affect soil structure and water transport[J]. European Journal of Soil Science, 2000, 51(3): 435-443. DOI:10.1046/j.1365-2389.2000.00327.x
(  0) 0) |
| [99] |
Cheng C, Shang-Guan W, He L, et al. Effect of exopolysaccharide-producing bacteria on water-stable macro-aggregate formation in soil[J]. Geomicrobiology Journal, 2020, 37(8): 738-745. DOI:10.1080/01490451.2020.1764677
(  0) 0) |
| [100] |
Büks F, Kaupenjohann M. Enzymatic biofilm digestion in soil aggregates facilitates the release of particulate organic matter by sonication[J]. Soil, 2016, 2(4): 499-509. DOI:10.5194/soil-2-499-2016
(  0) 0) |
| [101] |
Redmile-Gordon M, Gregory A S, White R P, et al. Soil organic carbon, extracellular polymeric substances(EPS), and soil structural stability as affected by previous and current land-use[J]. Geoderma, 2020, 363: 114143. DOI:10.1016/j.geoderma.2019.114143
(  0) 0) |
| [102] |
Sher Y, Baker N R, Herman D, et al. Microbial extracellular polysaccharide production and aggregate stability controlled by switchgrass(Panicum virgatum)root biomass and soil water potential[J]. Soil Biology and Biochemistry, 2020, 143: 107742. DOI:10.1016/j.soilbio.2020.107742
(  0) 0) |
| [103] |
Zethof J H T, Bettermann A, Vogel C, et al. Prokaryotic community composition and extracellular polymeric substances affect soil microaggregation in carbonate containing semiarid grasslands[J]. Frontiers in Environmental Science, 2020, 8: 51. DOI:10.3389/fenvs.2020.00051
(  0) 0) |
| [104] |
Bettermann A, Zethof J H T, Babin D, et al. Importance of microbial communities at the root-soil interface for extracellular polymeric substances and soil aggregation in semiarid grasslands[J]. Soil Biology and Biochemistry, 2021, 108301.
(  0) 0) |
| [105] |
Or D, Smets B F, Wraith J M, et al. Physical constraints affecting bacterial habitats and activity in unsaturated porous media-A review[J]. Advances in Water Resources, 2007, 30(6): 1505-1527.
(  0) 0) |
| [106] |
Miltner A, Bombach P, Schmidt-Brücken B, et al. SOM genesis: Microbial biomass as a significant source[J]. Biogeochemistry, 2012, 111(1): 41-55.
(  0) 0) |
| [107] |
Patel J J, Gerson T. Formation and utilisation of carbon reserves by Rhizobium[J]. Archives of Microbiology, 1974, 101(1): 211-220. DOI:10.1007/BF00455939
(  0) 0) |
| [108] |
Wang X, Sharp C E, Jones G M, et al. Stable-isotope probing identifies uncultured Planctomycetes as primary degraders of a complex heteropolysaccharide in soil[J]. Applied and Environmental Microbiology, 2015, 81(14): 4607-4615. DOI:10.1128/AEM.00055-15
(  0) 0) |
| [109] |
Costa O Y A, Pijl A, Kuramae E E. Dynamics of active potential bacterial and fungal interactions in the assimilation of acidobacterial EPS in soil[J]. Soil Biology and Biochemistry, 2020, 148: 107916. DOI:10.1016/j.soilbio.2020.107916
(  0) 0) |
| [110] |
Costa O Y A, de Hollander M, Pijl A, et al. Cultivation-independent and cultivation-dependent metagenomes reveal genetic and enzymatic potential of microbial community involved in the degradation of a complex microbial polymer[J]. Microbiome, 2020, 8(1): 76. DOI:10.1186/s40168-020-00836-7
(  0) 0) |
| [111] |
Joshi P M, Juwarkar A A. In vivo studies to elucidate the role of extracellular polymeric substances from Azotobacter in immobilization of heavy metals[J]. Environmental Science & Technology, 2009, 43(15): 5884-5889.
(  0) 0) |
| [112] |
Nkoh J N, Yan J, Hong Z, et al. An electrokinetic perspective into the mechanism of divalent and trivalent cation sorption by extracellular polymeric substances of Pseudomonas fluorescens[J]. Colloids and Surfaces B: Biointerfaces, 2019, 183: 110450. DOI:10.1016/j.colsurfb.2019.110450
(  0) 0) |
| [113] |
Ha J, Gelabert A, Spormann A M, et al. Role of extracellular polymeric substances in metal ion complexation on Shewanella oneidensis: Batch uptake, thermodynamic modeling, ATR-FTIR, and EXAFS study[J]. Geochimica et Cosmochimica Acta, 2010, 74(1): 1-15. DOI:10.1016/j.gca.2009.06.031
(  0) 0) |
| [114] |
Fang L, Yang S, Huang Q, et al. Biosorption mechanisms of Cu(Ⅱ)by extracellular polymeric substances from Bacillus subtilis[J]. Chemical Geology, 2014, 386: 143-151. DOI:10.1016/j.chemgeo.2014.08.017
(  0) 0) |
| [115] |
Wei L, Li Y, Noguera D R, et al. Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances(EPS)in different sludges: Effect of EPS fractional polarity on binding mechanism[J]. Journal of Hazardous Materials, 2017, 321: 473-483. DOI:10.1016/j.jhazmat.2016.05.016
(  0) 0) |
| [116] |
Shou W, Kang F, Lu J. Nature and value of freely dissolved EPS ecosystem services: Insight into molecular coupling mechanisms for regulating metal toxicity[J]. Environmental Science & Technology, 2018, 52(2): 457-466.
(  0) 0) |
| [117] |
Mikutta R, Baumgärtner A, Schippers A, et al. Extracellular polymeric substances from Bacillus subtilis associated with minerals modify the extent and rate of heavy metal sorption[J]. Environmental Science & Technology, 2012, 46(7): 3866-3873.
(  0) 0) |
| [118] |
Song W, Pan X, Mu S, et al. Biosorption of Hg(Ⅱ)onto goethite with extracellular polymeric substances[J]. Bioresource Technology, 2014, 160: 119-122. DOI:10.1016/j.biortech.2013.12.052
(  0) 0) |
| [119] |
Li C-C, Wang Y-J, Du H, et al. Influence of bacterial extracellular polymeric substances on the sorption of Zn on γ-alumina: A combination of FTIR and EXAFS studies[J]. Environmental Pollution, 2017, 220: 997-1004. DOI:10.1016/j.envpol.2016.11.048
(  0) 0) |
| [120] |
Nkoh J N, Xu R K, Yan J, et al. Mechanism of Cu(Ⅱ)and Cd(Ⅱ)immobilization by extracellular polymeric substances(Escherichia coli)on variable charge soils[J]. Environmental Pollution, 2019, 247: 136-145. DOI:10.1016/j.envpol.2019.01.038
(  0) 0) |
| [121] |
Booth S C, Workentine M L, Wen J, et al. Differences in metabolism between the biofilm and planktonic response to metal stress[J]. Journal of Proteome Research, 2011, 10(7): 3190-3199. DOI:10.1021/pr2002353
(  0) 0) |
| [122] |
Xiao Y, Zhang E, Zhang J, et al. Extracellular polymeric substances are transient media for microbial extracellular electron transfer[J]. Science Advances, 2017, 3(7): e1700623. DOI:10.1126/sciadv.1700623
(  0) 0) |
| [123] |
Kang F, Alvarez P J, Zhu D. Microbial extracellular polymeric substances reduce Ag+ to silver nanoparticles and antagonize bactericidal activity[J]. Environmental Science & Technology, 2014, 48(1): 316-322.
(  0) 0) |
| [124] |
Kang F, Qu X, Alvarez P J J, et al. Extracellular saccharide-mediated reduction of Au3+ to gold nanoparticles: New insights for heavy metals biomineralization on microbial surfaces[J]. Environmental Science & Technology, 2017, 51(5): 2776-2785.
(  0) 0) |
| [125] |
Zhang Z, Si R, Lv J, et al. Effects of extracellular polymeric substances on the formation and methylation of mercury sulfide nanoparticles[J]. Environmental Science & Technology, 2020, 54(13): 8061-8071.
(  0) 0) |
| [126] |
Redmile-Gordon M, Chen L. Zinc toxicity stimulates microbial production of extracellular polymers in a copiotrophic acid soil[J]. International Biodeterioration & Biodegradation, 2017, 119: 413-418.
(  0) 0) |
| [127] |
Zhang J, Shi Q, Fan S, et al. Distinction between Cr and other heavy-metal-resistant bacteria involved in C/N cycling in contaminated soils of copper producing sites[J]. Journal of Hazardous Materials, 2021, 402: 123454. DOI:10.1016/j.jhazmat.2020.123454
(  0) 0) |
| [128] |
Liu W, Cheng F, Li W, et al. Desorption behaviors of BDE-28 and BDE-47 from natural soils with different organic carbon contents[J]. Environmental Pollution, 2012, 163: 235-242. DOI:10.1016/j.envpol.2011.12.043
(  0) 0) |
| [129] |
Liu G, Bian Y, Jia M, et al. Effect of extracellular polymeric substance components on the sorption behavior of 2, 2', 4, 4'-tetrabromodiphenyl ether to soils: Kinetics and isotherms[J]. Science of the Total Environment, 2017, 609: 144-152. DOI:10.1016/j.scitotenv.2017.07.089
(  0) 0) |
| [130] |
Bhattacharyya R, Das S, Bhattacharya R, et al. Rhizobial exopolysaccharides: A novel biopolymer for legume-rhizobia symbiosis and environmental monitoring//Zaidi A, Khan M S, Musarrat J. Microbes for legume improvement[M]. Cham: Springer International Publishing, 2017: 119-133.
(  0) 0) |
| [131] |
Gauri S S, Mandal S M, Pati B R. Impact of Azotobacter exopolysaccharides on sustainable agriculture[J]. Applied Microbiology and Biotechnology, 2012, 95(2): 331-338. DOI:10.1007/s00253-012-4159-0
(  0) 0) |
| [132] |
Zhang Y, Wang F, Yang X, et al. Extracellular polymeric substances enhanced mass transfer of polycyclic aromatic hydrocarbons in the two-liquid-phase system for biodegradation[J]. Applied Microbiology and Biotechnology, 2011, 90(3): 1063-1071. DOI:10.1007/s00253-011-3134-5
(  0) 0) |
| [133] |
Han C, Zhang Y, Redmile-Gordon M, et al. Organic and inorganic model soil fractions instigate the formation of distinct microbial biofilms for enhanced biodegradation of benzo[J]. Journal of Hazardous Materials, 2021, 404: 124071. DOI:10.1016/j.jhazmat.2020.124071
(  0) 0) |
| [134] |
Kielak A M, Castellane T C L, Campanharo J C, et al. Characterization of novel Acidobacteria exopolysaccharides with potential industrial and ecological applications[J]. Scientific Reports, 2017, 7: 41193. DOI:10.1038/srep41193
(  0) 0) |
| [135] |
Gunina A, Kuzyakov Y. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate[J]. Soil Biology and Biochemistry, 2015, 90: 87-100. DOI:10.1016/j.soilbio.2015.07.021
(  0) 0) |
| [136] |
Marchus K A, Blankinship J C, Schimel J P. Environmental controls on extracellular polysaccharide accumulation in a California grassland soil[J]. Soil Biology and Biochemistry, 2018, 125: 86-92. DOI:10.1016/j.soilbio.2018.07.009
(  0) 0) |
| [137] |
Redmile-Gordon M A, Evershed R P, Kuhl A, et al. Engineering soil organic matter quality: Biodiesel Co-Product(BCP)stimulates exudation of nitrogenous microbial biopolymers[J]. Geoderma, 2015, 259: 205-212.
(  0) 0) |
| [138] |
Gillespie A W, Farrell R E, Walley F L, et al. Glomalin-related soil protein contains non-mycorrhizal-related heat-stable proteins, lipids and humic materials[J]. Soil Biology and Biochemistry, 2011, 43(4): 766-777. DOI:10.1016/j.soilbio.2010.12.010
(  0) 0) |
| [139] |
Holátko J, Brtnický M, Kučerík J, et al. Glomalin-Truths, myths, and the future of this elusive soil glycoprotein[J]. Soil Biology and Biochemistry, 2021, 153: 108116. DOI:10.1016/j.soilbio.2020.108116
(  0) 0) |
| [140] |
Bharadwaj A. Role of microbial extracellular polymeric substances in soil fertility//Vaishnav A, Choudhary D K. Microbial polymers: Applications and ecological perspectives[M]. Singapore: Springer, 2021: 341-354.
(  0) 0) |
 2022, Vol. 59
2022, Vol. 59


