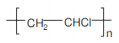2. 浙江省土壤污染生物修复重点实验室,杭州 311300;
3. 浙江农林大学集贤学院,杭州 311300;
4. 中国科学院土壤环境与污染修复重点实验室(南京土壤研究所),南京 210008
2. Key Laboratory of Soil Contamination Bioremediation of Zhejiang Province, Hangzhou 311300, China;
3. Jixian Honors College, Zhejiang Agriculture and Forestry University, Hangzhou 311300, China;
4. Key Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China
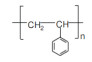
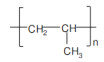
微塑料是指环境中粒径小于5 mm的塑料类污染物,包括碎片、纤维、颗粒、发泡、薄膜等类型,目前正作为一种新兴污染物受到全球关注[1]。土壤可能是环境中微塑料污染的一个重要汇集地[2-5],一些土壤中微塑料的质量丰度可高达7% [6],而农田土壤中微塑料的数量丰度也可高达42 960 ind·kg–1[7-9]。现有调查结果显示,环境中常见微塑料的聚合物类型包括聚乙烯(PE)、聚苯乙烯(PS)、聚丙烯(PP)、聚酰胺(PA)和聚氯乙烯(PVC)等[9-11]。微塑料会改变土壤理化性质,对土壤微生物、无脊椎动物或植物产生影响[12-17]。同时,微塑料由于具有复杂表面结构和较大的比表面积,对环境污染物具有“载体效应”,影响污染物在环境介质中的分配、迁移和生物有效性[18-23]。例如,风化PS微塑料在纯溶液体系中对土霉素的吸附分配系数(Kd)是未风化PS微塑料的10倍以上[24];而在模拟淡水和海水体系中,PA微塑料相比其他微塑料对四环素和磺胺嘧啶具有更强的吸附作用[23]。溶液性质对微塑料吸附抗生素具有重要影响,在天然水体条件下,PE微塑料对环丙沙星的吸附主要受pH、离子强度、可溶性有机质(DOM)等因素所控制[25-27]。
相比纯溶液体系,在土壤介质中,微塑料对抗生素等离子型污染物的吸附—解吸更为复杂。例如,土壤中含有5%的聚丙烯或聚酯微塑料情况下,微塑料可以吸附土壤中的啶虫脒等农药,并且这种吸附行为可能降低土壤自身对农药的滞留能力,导致农药移动性增加[28]。在土壤中添加微塑料进行吸附—解吸试验也发现增加了土壤中镉的解吸[29]。由于有机肥施用等因素,抗生素在土壤环境中的残留已经非常普遍[30-33],其中磺胺甲恶唑是目前畜禽养殖中使用量较大的一类抗生素,且相对稳定,在环境中具较高的检出率[34-36]。因此,微塑料进入抗生素污染土壤中是否影响土壤抗生素的吸附—解吸,以及土壤抗生素的环境风险值得进一步探究。本研究以磺胺甲恶唑为对象,通过人工制备的磺胺甲恶唑污染土壤,在土壤—溶液体系中,比较不同聚合物类型微塑料作用下,土壤磺胺甲恶唑解吸动力学特征,以及受不同溶液离子强度、水溶性有机质含量影响,土壤中磺胺甲恶唑的释放差异,为土壤—微塑料混合体系下磺胺类抗生素污染的环境风险识别提供理论依据。
1 材料与方法 1.1 供试材料供试土壤采自杭州临安城区周边的水田(30°18′ 59″ N,119°44′32″E),为红壤母质发育的水稻土,酸性较强,质地为黏壤土,是南方地区一种主要的农田土壤类型。土壤取回后风干过2 mm筛备用,土壤基本性质如表 1所示。取过2 mm筛的风干土1.0 kg,高压灭菌后添加0.5 mL ProClin 300(Sigma-Aldrich公司)广谱杀菌剂以尽可能减少老化过程中抗生素的生物降解;然后添加浓度为1 000 mg·L–1的磺胺甲恶唑母液50 mL,充分搅拌混匀后25℃避光保存5 d。老化后土壤中磺胺甲恶唑经检测含量为33.4 mg·kg–1。
|
|
表 1 供试土壤基本理化性质 Table 1 The physico-chemical properties of the soil for experiments |
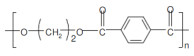
供试微塑料购买自上海冠步机电技术有限公司,包括聚乙烯(PE)、聚苯乙烯(PS)、聚丙烯(PP)、聚对苯二甲酸乙二醇酯(PET)和聚氯乙烯(PVC)5种聚合物类型,形状均为不规则颗粒。这些微塑料的粒径和表面零点电荷等基本性质如表 2所示。
|
|
表 2 供试微塑料基本理化性质 Table 2 The physico-chemical properties of the microplastics for experiments |
取老化后的模拟抗生素污染土壤3.0 g添加至50 mL聚四氟乙烯离心管,分别加入100 mg上述5种聚合物类型的微塑料,再加入0.01 mol·L–1氯化钠电解质溶液30 mL,在避光条件下,25 ℃恒温振荡培养箱以150 r·min–1振荡不同时间(0.25、0.5、1、2、4、8、12、24、36、48、72、96 h)后取上清液,用0.2 μm滤膜过滤后采用高效液相色谱—紫外光法检测上清液中磺胺甲恶唑浓度。试验设计的示意图如图 1所示。每个微塑料处理和时间处理设置3个重复,取液时每个样品取3次,每个时间梯度处理均设置不加微塑料的空白对照。
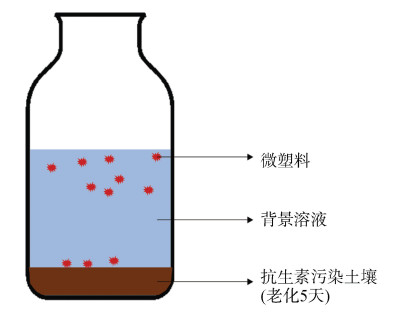
|
图 1 土壤—微塑料混合体系中抗生素解吸试验设计示意图 Fig. 1 The schematic diagram of the antibiotics desorption experiment in the soil- microplastics mixture system |
微塑料的添加量参照文献[8-9]中土壤的微塑料含量进行设置。在3.0 g模拟污染土壤与30 mL的0.01 mol·L–1的氯化钠电解质溶液中,将5种供试微塑料分别按照3种含量加入土壤—溶液混合体系中,即未添加微塑料(CK)、添加50 mg微塑料(相当于土壤中1.67%含量)、添加100 mg微塑料(相当于土壤中3.33%含量)的处理,每个处理设置3个重复。试验参照1.2的动力学解吸试验,在避光条件下,25 ℃恒温振荡培养箱以150 r·min–1振荡48 h后取上清液,用高效液相色谱—紫外光法检测上清液中磺胺甲恶唑浓度。
1.3.2 溶液离子强度试验土壤—溶液—微塑料的混合体系中,将5种微塑料的解吸试验背景溶液设置为不同浓度的氯化钠和氯化钙溶液,其中氯化钠的浓度梯度为0.01 mol·L–1、0.15 mol·L–1、0.6 mol·L–1,氯化钙的浓度为0.2 mol·L–1,与离子强度相同的0.6 mol·L–1氯化钠溶液进行对比试验。每个处理设置3个重复。其他同1.3.1微塑料添加量试验。
1.3.3 溶液中富里酸浓度试验土壤—溶液—微塑料的混合体系中,在5种微塑料的解吸试验的0.01 mol·L–1氯化钠背景溶液中添加不同浓度的富里酸,富里酸浓度梯度为:不加富里酸(CK)、10 mg·L–1、20 mg·L–1、50 mg·L–1,每个处理设置3个重复。富里酸(Sigma-Aldrich公司)直接用0.01 mol·L–1氯化钠背景溶液溶解定容。其他同1.3.1微塑料添加量试验。
1.4 数据处理将磺胺甲恶唑的动力学解吸实验数据采用准一级(式(1))、准二级(式(2))和Elovich模型(式(3))进行解吸动力学拟合,三种模型分别如下所示:
准一级动力学方程:
| $ \ln = ({Q_e} - {Q_t}) = \ln {Q_e} - {k_1}t $ | (1) |
准二级动力学方程:
| $ \frac{t}{{{Q_t}}} = \frac{1}{{{k_2}Q_e^2}} + \frac{t}{{{Q_e}}} $ | (2) |
Elovich模型:
| $ {Q_t} = a + {\text{b}}\ln (t) $ | (3) |
式中,Qe和Qt分别表示平衡时和t取样时刻上清液中的磺胺甲恶唑浓度(mg·L–1),t为解吸反应的时间(h);式(1)和式(2)中k1和k2分别表示准一级动力学常数和准二级动力学常数,单位分别为h–1和mg·L–1·h–1;式(3)中a和b为模型拟合参数。
采用Origin 2017进行数据制图,SPSS 22软件进行统计分析;不同处理之间的差异显著性分析采用单因素方差分析中的Turkey统计检验法,以0.05水平统计差异的显著性。
2 结果与讨论 2.1 不同类型微塑料作用下土壤磺胺甲恶唑的解吸动力学图 2为不同类型微塑料作用下土壤磺胺甲恶唑的解吸动力学曲线,表 3是采用准一级、准二级和Elovich三种模型拟合后的解吸动力学特征参数。从解吸动力学曲线看,土壤中磺胺甲恶唑的解吸均在48 h内达到解吸平衡。但与不添加微塑料的对照(CK)相比,添加PE和PS微塑料的土壤磺胺甲恶唑经历了三个解吸阶段,即:10 h以内快速解吸、10~48 h缓慢解吸、48 h以后逐渐达到平衡;而添加其他3种微塑料的土壤磺胺甲恶唑在10 h内快速解吸阶段后至48 h左右达到解吸平衡之间变幅较小,与CK基本上无差异。从模型拟合的R2可以看出,准二级动力学对土壤磺胺甲恶唑的解吸拟合最佳,其次为Elovich模型,准一级动力学模型拟合相对较差(表 3)。通过准二级动力学解吸常数(k2)反映出添加PE和PS微塑料处理的磺胺甲恶唑准二级动力学解吸常数(k2)分别为1.54 mg·L–1·h–1和1.531 mg·L–1·h–1,显著(P < 0.05)低于对照和其他添加微塑料处理,表明添加PE和PS微塑料使土壤中磺胺甲恶唑的解吸变慢。这可能是由于被PE和PS微塑料吸附的磺胺甲恶唑又重新缓慢释放到溶液,从而减缓了整个解吸速率,而其他三种微塑料由于对磺胺甲恶唑吸附较弱或过强导致未出现吸附后磺胺甲恶唑再释放的过程[37-38]。
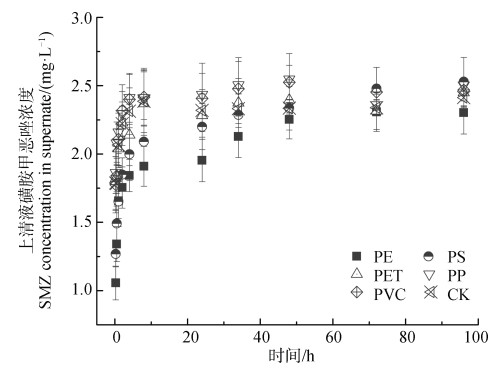
|
注:PE,聚乙烯Polyethylene;PS,聚苯乙烯Polystyrene;PP,聚丙烯Polypropylene;PVC,聚氯乙烯Polyvinyl chloride;PET,聚对苯二甲酸乙二醇酯Polyethylene terephthalate. 图 2 微塑料存在下土壤中磺胺甲恶唑的解吸动力学 Fig. 2 Desorption kinetic curves of sulfamethoxazole(SMZ)from the soil in the presence of microplastic |
|
|
表 3 微塑料存在下土壤中磺胺甲恶唑解吸的动力学拟合参数 Table 3 Fitting parameters using different models to the desorption kinetic curves of sulfamethoxazole from the soil in the presence of microplastic |
表 4比较了5种微塑料的不同添加量对土壤中磺胺甲恶唑平衡解吸量的影响。除添加PS微塑料外,其他4种微塑料添加后溶液中的磺胺甲恶唑平衡解吸浓度都要显著(P < 0.05)低于对照处理,并且添加PE、PP和PVC微塑料时,随添加量增加,平衡解吸浓度均有减小趋势;但总体上50 mg和100 mg微塑料添加量之间对平衡解吸浓度没有产生显著性差异。比较这5种微塑料在同一个添加量下对溶液中磺胺甲恶唑平衡解吸浓度的影响可见,添加PVC微塑料中的磺胺甲恶唑平衡解吸浓度最低,其次为添加PE微塑料处理;并且这两种微塑料处理后磺胺甲恶唑平衡解吸浓度均呈现随添加量增加而降低的趋势。
|
|
表 4 微塑料存在下土壤磺胺甲恶唑的平衡解吸量比较 Table 4 Comparison of the equilibrium desorption concentration of sulfamethoxazole in the presence of microplastic |
本研究中,添加微塑料后溶液中磺胺甲恶唑的平衡解吸浓度总体上呈现降低趋势,这与微塑料对磺胺甲恶唑的吸附有关。不同吸附能力的微塑料导致了溶液中平衡解吸浓度的差异。磺胺甲恶唑的解离常数pka1和pka2分别为1.4和5.8,供试土壤pH为5.02,由此可知在该pH条件下磺胺甲恶唑主要以不带电荷的双性离子存在,静电作用对微塑料吸附磺胺甲恶唑不起主导作用。有研究认为亲水性化合物在PE、PS、PVC等微塑料表面的吸附受到微塑料的极性特征影响[39]。磺胺甲恶唑的logKow值小于2,是低疏水性化合物[40],因此,PVC微塑料的强极性特征成为影响磺胺甲恶唑吸附的关键因素。除此以外,微塑料表面的吸附域也起到重要的作用,橡胶状结构域相对于玻璃态结构域对有机污染物的吸附性更强[41-42]。在供试的5种微塑料类型中,PE在常温下具有最为丰富的橡胶体吸附域,也被认为对有机污染物具有较强的吸附能力[37]。因此,本研究中发现的PE微塑料添加后磺胺甲恶唑平衡解吸浓度下降与PE微塑料的橡胶体吸附域的作用机制有关。
2.3 离子类型及其强度对土壤磺胺甲恶唑解吸的影响图 3和图 4分别为不同浓度氯化钠背景溶液以及相同离子强度的氯化钠与氯化钙背景溶液中磺胺甲恶唑平衡解吸浓度的比较。不同类型微塑料添加下,氯化钠和氯化钙背景溶液对土壤磺胺甲恶唑解吸的影响趋势基本一致。随着氯化钠浓度增加,溶液中磺胺甲恶唑的平衡解吸浓度显著(P < 0.05)降低(图 3),而相同离子强度的氯化钙与氯化钠背景溶液相比,氯化钙背景溶液中的磺胺甲恶唑的平衡解吸浓度要显著(P < 0.05)高于氯化钠背景溶液(图 4)。
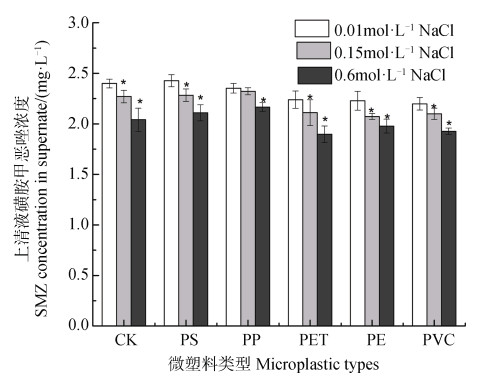
|
注:*号表示与常规氯化钠背景浓度(0.01 mol·L–1)溶液相比具有显著性(P < 0.05)差异。 Note: * over the columns indicates the significant(P < 0.05)difference between the treatment and the normal background(0.01 mol·L–1)NaCl solution. 图 3 不同浓度氯化钠溶液条件下土壤中磺胺甲恶唑的解吸情况 Fig. 3 Desorption concentration of sulfamethoxazole(SMZ)from the soil in the presence of microplastics under different concentration of NaCl solution |
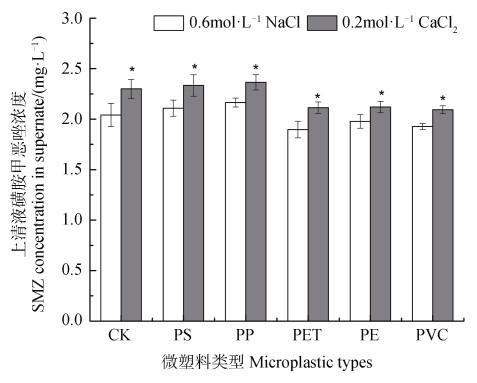
|
注:*号表示与常规氯化钠溶液相比具有显著性(P < 0.05)差异。 Note: * over the columns indicates the significant(P < 0.05)difference between the CaCl2 and NaCl solution. 图 4 相同离子强度的氯化钠与氯化钙溶液条件下土壤中磺胺甲恶唑的解吸情况 Fig. 4 Effects of CaCl2 and NaCl solution with same ionic strength on the sulfamethoxazole desorption in the presence of microplastics |
盐度或离子强度对磺胺甲恶唑解吸的影响与吸附作用机制有关,当土壤或微塑料对磺胺甲恶唑的吸附以静电作用主导时,其吸附能力会随着盐度或离子强度的增加而降低[37];但也有研究表明,PE微塑料与磺胺甲恶唑的吸附由于受到范德华力主导,其在0.05%到3.5%的盐度范围内均未受到影响[43]。甚至Guo等[37]发现在低盐度范围内,PS微塑料对磺胺甲恶唑的吸附还略有增加。也有研究发现,随着离子强度的增加,降低抗生素的亲水性,使其更容易分配在微塑料等固相介质中,即所谓的“盐析效应”[44]。因此,如前所述,本研究的土壤pH条件下,非静电作用主导磺胺甲恶唑的吸附,氯化钠浓度的增加可能通过盐析效应降低其从土壤中的解吸。与钠离子相比,钙离子在本试验中增加了溶液中磺胺甲恶唑的平衡解吸浓度,这同钙离子与磺胺甲恶唑在土壤或微塑料表面的竞争吸附行为有关[45]。
2.4 富里酸对土壤磺胺甲恶唑解吸的影响图 5为微塑料存在时,溶液中添加不同浓度的富里酸对磺胺甲恶唑解吸的影响。在未添加微塑料的对照处理(CK)中,溶液中磺胺甲恶唑的平衡解吸浓度随着富里酸浓度的增加而降低。但添加微塑料处理中,磺胺甲恶唑的平衡解吸浓度随着富里酸浓度的增加未发生明显变化,特别是添加PS、PP和PET处理。
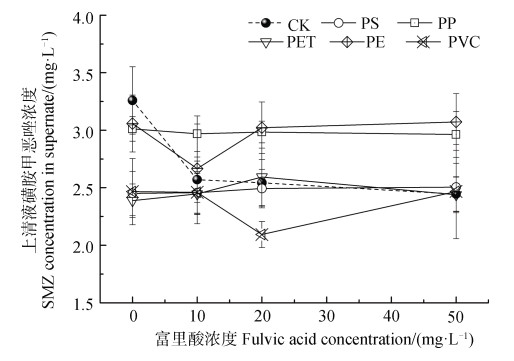
|
图 5 不同富里酸浓度条件下土壤中磺胺甲恶唑的解吸 Fig. 5 Change of sulfamethoxazole(SMZ)desorption from the soil with the increase of fulvic acid concentration in the presence of microplastics |
在未添加塑料的情况下,加入的富里酸会首先吸附在土壤上,并可能会进一步通过疏水作用和π-π键与磺胺甲恶唑作用,从而减少土壤中磺胺甲恶唑的解吸[46]。Lian等[47]研究胡敏酸对生物炭吸附磺胺甲恶唑的影响时发现,当胡敏酸浓度增加到30 mg·L–1以上时,磺胺甲恶唑在生物炭上的吸附随着胡敏酸浓度的增加而增加。所以,当体系中加入微塑料后,富里酸可能首先被疏水性较强的微塑料表面所吸附,从而使土壤中的磺胺甲恶唑受富里酸的影响较小[48]。
3 结论在土壤—溶液中添加聚乙烯和聚苯乙烯微塑料可使土壤中磺胺甲恶唑的解吸速率变慢,解吸过程与未添加微塑料相比,在10~48 h之间存在明显的缓慢解吸阶段。氯化钠浓度增加降低了供试土壤中磺胺甲恶唑的解吸,而相同离子强度的钙离子却增加了磺胺甲恶唑的解吸;但是,体系中加入微塑料未改变离子类型及其强度对土壤磺胺甲恶唑解吸的影响。添加微塑料总体上可减小富里酸对土壤磺胺甲恶唑解吸的影响,使磺胺甲恶唑解吸量不会随着富里酸浓度的增加而降低。总体而言,在土壤—微塑料混合体系下,微塑料可改变土壤抗生素的解吸,尤其是在富含水溶性有机质土壤中,微塑料的存在能增加抗生素解吸。因此,需要进一步关注微塑料对土壤抗生素迁移性和生物有效性的影响。
| [1] |
Thompson R C, Olsen Y, Mitchell R P, et al. Lost at sea: Where is all the plastic?[J]. Science, 2004, 304(5672): 838. DOI:10.1126/science.1094559
(  0) 0) |
| [2] |
Horton A A, Walton A, Spurgeon D J, et al. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities[J]. Science of the Total Environment, 2017, 586: 127-141. DOI:10.1016/j.scitotenv.2017.01.190
(  0) 0) |
| [3] |
Hurley R R, Nizzetto L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks[J]. Current Opinion in Environmental Science & Health, 2018, 1: 6-11.
(  0) 0) |
| [4] |
de Souza Machado A A, Kloas W, Zarfl C, et al. Microplastics as an emerging threat to terrestrial ecosystems[J]. Global Change Biology, 2018, 24(4): 1405-1416. DOI:10.1111/gcb.14020
(  0) 0) |
| [5] |
Nizzetto L, Futter M, Langaas S. Are agricultural soils dumps for microplastics of urban origin?[J]. Environmental Science & Technology, 2016, 50(20): 10777-10779.
(  0) 0) |
| [6] |
Fuller S, Gautam A. A procedure for measuring microplastics using pressurized fluid extraction[J]. Environmental Science & Technology, 2016, 50(11): 5774-5780.
(  0) 0) |
| [7] |
Liu M T, Lu S B, Song Y, et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China[J]. Environmental Pollution, 2018, 242: 855-862. DOI:10.1016/j.envpol.2018.07.051
(  0) 0) |
| [8] |
Zhang G S, Liu Y F. The distribution of microplastics in soil aggregate fractions in southwestern China[J]. Science of the Total Environment, 2018, 642: 12-20. DOI:10.1016/j.scitotenv.2018.06.004
(  0) 0) |
| [9] |
Zhou B Y, Wang J Q, Zhang H B, et al. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, East China: Multiple sources other than plastic mulching film[J]. Journal of Hazardous Materials, 2020, 388: 121814. DOI:10.1016/j.jhazmat.2019.121814
(  0) 0) |
| [10] |
Fok L, Cheung P K, Tang G D, et al. Size distribution of stranded small plastic debris on the Coast of Guangdong, South China[J]. Environmental Pollution, 2017, 220: 407-412. DOI:10.1016/j.envpol.2016.09.079
(  0) 0) |
| [11] |
Hidalgo-Ruz V, Gutow L, Thompson R C, et al. Microplastics in the marine environment: A review of the methods used for identification and quantification[J]. Environmental Science & Technology, 2012, 46(6): 3060-3075.
(  0) 0) |
| [12] |
Liu H F, Yang X M, Liu G B, et al. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil[J]. Chemosphere, 2017, 185: 907-917. DOI:10.1016/j.chemosphere.2017.07.064
(  0) 0) |
| [13] |
de Souza Machado A A, Lau C W, Kloas W, et al. Microplastics can change soil properties and affect plant performance[J]. Environmental Science & Technology, 2019, 53(10): 6044-6052.
(  0) 0) |
| [14] |
Rodriguez-Seijo A, Lourenço J, Rocha-Santos T A P, et al. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché[J]. Environmental Pollution, 2017, 220: 495-503. DOI:10.1016/j.envpol.2016.09.092
(  0) 0) |
| [15] |
Wan Y, Wu C X, Xue Q, et al. Effects of plastic contamination on water evaporation and desiccation cracking in soil[J]. Science of the Total Environment, 2019, 654: 576-582. DOI:10.1016/j.scitotenv.2018.11.123
(  0) 0) |
| [16] |
Wang J, Huang M K, Wang Q, et al. LDPE microplastics significantly alter the temporal turnover of soil microbial communities[J]. Science of the Total Environment, 2020, 726: 138682. DOI:10.1016/j.scitotenv.2020.138682
(  0) 0) |
| [17] |
Feng X Y, Sun Y H, Zhang S W, et al. Ecological effects of microplastics on soil-plant systems (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(2): 299-313. [冯雪莹, 孙玉焕, 张书武, 等. 微塑料对土壤-植物系统的生态效应[J]. 土壤学报, 2021, 58(2): 299-313.]
(  0) 0) |
| [18] |
Guo X Y, Wang X L, Zhou X Z, et al. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition[J]. Environmental Science & Technology, 2012, 46(13): 7252-7259.
(  0) 0) |
| [19] |
Hüffer T, Hofmann T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution[J]. Environmental Pollution, 2016, 214: 194-201. DOI:10.1016/j.envpol.2016.04.018
(  0) 0) |
| [20] |
Turner A, Holmes L A. Adsorption of trace metals by microplastic pellets in fresh water[J]. Environmental Chemistry, 2015, 12(5): 600-610. DOI:10.1071/EN14143
(  0) 0) |
| [21] |
Ziccardi L M, Edgington A, Hentz K, et al. Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: A state-of-the-science review[J]. Environmental Toxicology and Chemistry, 2016, 35(7): 1667-1676. DOI:10.1002/etc.3461
(  0) 0) |
| [22] |
Yang J, Li L Z, Zhou Q, et al. Microplastics contamination of soil environment: Sources, processes and risks (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(2): 281-298. [杨杰, 李连祯, 周倩, 等. 土壤环境中微塑料污染: 来源、过程及风险[J]. 土壤学报, 2021, 58(2): 281-298.]
(  0) 0) |
| [23] |
Zhou Q, Zhang H B, Zhou Y, et al. Surface weathering and changes in components of microplastics from estuarine beaches (In Chinese)[J]. Chinese Science Bulletin, 2018, 63(2): 214-224. [周倩, 章海波, 周阳, 等. 滨海河口潮滩中微塑料的表面风化和成分变化[J]. 科学通报, 2018, 63(2): 214-224.]
(  0) 0) |
| [24] |
Zhang H B, Wang J Q, Zhou B Y, et al. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors[J]. Environmental Pollution, 2018, 243: 1550-1557. DOI:10.1016/j.envpol.2018.09.122
(  0) 0) |
| [25] |
Li J, Zhang K N, Zhang H. Adsorption of antibiotics on microplastics[J]. Environmental Pollution, 2018, 237: 460-467. DOI:10.1016/j.envpol.2018.02.050
(  0) 0) |
| [26] |
Atugoda T, Wijesekara H, Werellagama D R I B, et al. Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: Implications for vector transport in water[J]. Environmental Technology & Innovation, 2020, 19: 100971.
(  0) 0) |
| [27] |
Shen X C, Li D C, Sima X F, et al. The effects of environmental conditions on the enrichment of antibiotics on microplastics in simulated natural water column[J]. Environmental Research, 2018, 166: 377-383. DOI:10.1016/j.envres.2018.06.034
(  0) 0) |
| [28] |
Šunta U, Prosenc F, Trebše P, et al. Adsorption of acetamiprid, chlorantraniliprole and flubendiamide on different type of microplastics present in alluvial soil[J]. Chemosphere, 2020, 261: 127762. DOI:10.1016/j.chemosphere.2020.127762
(  0) 0) |
| [29] |
Zhang S W, Han B, Sun Y H, et al. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil[J]. Journal of Hazardous Materials, 2020, 388: 121775. DOI:10.1016/j.jhazmat.2019.121775
(  0) 0) |
| [30] |
Han Q F, Zhao S, Zhang X R, et al. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China[J]. Environment International, 2020, 138: 105551. DOI:10.1016/j.envint.2020.105551
(  0) 0) |
| [31] |
Tasho R P, Cho J Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review[J]. Science of the Total Environment, 2016, 563/564: 366-376. DOI:10.1016/j.scitotenv.2016.04.140
(  0) 0) |
| [32] |
Zeng Q Y, Ding D, Tan X. Pollution status and sources of tetracycline antibiotics in agricultural soil in China: A review (In Chinese)[J]. Ecology and Environmental Sciences, 2018, 27(9): 1774-1782. [曾巧云, 丁丹, 檀笑. 中国农业土壤中四环素类抗生素污染现状及来源研究进展[J]. 生态环境学报, 2018, 27(9): 1774-1782.]
(  0) 0) |
| [33] |
Yuan X X, Liang J Y, Fan L X, et al. Effects of manure application on source and transport of antibiotic resistant genes in soil and their affecting factors (In Chinese)[J]. Acta Pedologica Sinica, 2020, 57(1): 36-47. [苑学霞, 梁京芸, 范丽霞, 等. 粪肥施用土壤抗生素抗性基因来源、转移及影响因素[J]. 土壤学报, 2020, 57(1): 36-47.]
(  0) 0) |
| [34] |
Qi H, Ma S, Zhang Y H, et al. A review on behaviour and ecological toxicity of antibiotics residue in soil environments (In Chinese)[J]. Journal of Anhui Agricultural Sciences, 2011, 39(18): 10906-10908. DOI:10.3969/j.issn.0517-6611.2011.18.069 [漆辉, 马莎, 张乙涵, 等. 抗生素残留在土壤环境中的行为及其生态毒性研究进展[J]. 安徽农业科学, 2011, 39(18): 10906-10908.]
(  0) 0) |
| [35] |
Kümmerer K. Antibiotics in the aquatic environment-A review-Part I[J]. Chemosphere, 2009, 75(4): 417-434. DOI:10.1016/j.chemosphere.2008.11.086
(  0) 0) |
| [36] |
Chen Y, Xu Y N, Pang Y. Advances in research on the occurrence, source and risk assessment of antibiotics (In Chinese)[J]. Journal of Environmental Engineering Technology, 2020. DOI:10.12153/j.issn.1674-991X.20200180 [陈宇, 许亚南, 庞燕. 抗生素赋存、来源及风险评估研究进展[J]. 环境工程技术学报, 2020.]
(  0) 0) |
| [37] |
Guo X, Chen C, Wang J L. Sorption of sulfamethoxazole onto six types of microplastics[J]. Chemosphere, 2019, 228: 300-308. DOI:10.1016/j.chemosphere.2019.04.155
(  0) 0) |
| [38] |
Guo X, Liu Y, Wang J L. Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation study[J]. Marine Pollution Bulletin, 2019, 145: 547-554. DOI:10.1016/j.marpolbul.2019.06.063
(  0) 0) |
| [39] |
Wang F, Shih K M, Li X Y. The partition behavior of perfluorooctanesulfonate(PFOS)and perfluorooctanesulfonamide(FOSA)on microplastics[J]. Chemosphere, 2015, 119: 841-847. DOI:10.1016/j.chemosphere.2014.08.047
(  0) 0) |
| [40] |
Razanajatovo R M, Ding J N, Zhang S S, et al. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics[J]. Marine Pollution Bulletin, 2018, 136: 516-523. DOI:10.1016/j.marpolbul.2018.09.048
(  0) 0) |
| [41] |
Salloum M J, Chefetz B, Hatcher P G. Phenanthrene sorption by aliphatic-rich natural organic matter[J]. Environmental Science & Technology, 2002, 36(9): 1953-1958.
(  0) 0) |
| [42] |
Wang K J, Xing B S. Chemical extractions affect the structure and phenanthrene sorption of soil humin[J]. Environmental Science & Technology, 2005, 39(21): 8333-8340.
(  0) 0) |
| [43] |
Xu B L, Liu F, Brookes P C, et al. The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics[J]. Marine Pollution Bulletin, 2018, 131: 191-196. DOI:10.1016/j.marpolbul.2018.04.027
(  0) 0) |
| [44] |
Wu X W, Liu P, Huang H, et al. Adsorption of triclosan onto different aged polypropylene microplastics: Critical effect of cations[J]. Science of the Total Environment, 2020, 717: 137033. DOI:10.1016/j.scitotenv.2020.137033
(  0) 0) |
| [45] |
Huang X F, Xiong Y J, Peng K M, et al. The progress of antibiotics removal performance under the complexion effect of metal ions (In Chinese)[J]. Environmental Chemistry, 2016, 35(1): 133-140. [黄翔峰, 熊永娇, 彭开铭, 等. 金属离子络合对抗生素去除特性的影响研究进展[J]. 环境化学, 2016, 35(1): 133-140.]
(  0) 0) |
| [46] |
Xu J, Hu Y Y, Li X Y, et al. Rapidly probing the interaction between sulfamethazine antibiotics and fulvic acids[J]. Environmental Pollution, 2018, 243: 752-757. DOI:10.1016/j.envpol.2018.09.002
(  0) 0) |
| [47] |
Lian F, Sun B B, Chen X, et al. Effect of humic acid(HA)on sulfonamide sorption by biochars[J]. Environmental Pollution, 2015, 204: 306-312. DOI:10.1016/j.envpol.2015.05.030
(  0) 0) |
| [48] |
Xu B L, Liu F, Brookes P C, et al. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter[J]. Environmental Pollution, 2018, 240: 87-94. DOI:10.1016/j.envpol.2018.04.113
(  0) 0) |
 2022, Vol. 59
2022, Vol. 59