我国东北典型黑土区耕地面积约2.78亿亩(15亩合1公顷),粮食产量占全国总产量的1/4[1],素有“黑土粮仓”的美誉,是保障我国粮食安全的“稳压器”和“压舱石”。随着现代化农业的快速发展,农药已成为保障黑土粮仓粮食供给的重要生产资料。东北地区已经取代东南沿海地区成为农药施用的热点地区[2],据国家统计局年度数据[3],2019年东北四省农药使用量为19万t,占全国13.7%,且以除草剂为主,农田化学除草面积已占种植面积的90%以上。近年来,东北黑土区除草剂的高频高强度施用,导致后茬作物药害事件时有发生,成为轮作换茬、种植结构调整的关键制约因素;除草剂残留可能会造成土壤质量退化,对农田生态系统的稳定性、多样性产生影响,抑制土壤生态服务功能。此外,残留的除草剂还会通过食物链进入人体,对人体健康造成损害,严重威胁黑土地粮食安全和生态环境安全,引起了国家高度关注。《东北黑土地保护规划纲要(2017—2030年)》明确指出“推进农药减量增效,减少对黑土地的污染”。2021年中央一号文件特别强调“实施国家黑土地保护工程,推进土壤污染防治”。因此,开展黑土地农田除草剂等有机污染过程与消减关键技术研究,已成为保障黑土地农业绿色可持续发展的重要科技需求。
1 黑土地农田除草剂污染区域分布特征系统了解区域污染状况与时空分布特征是黑土地农田除草剂污染防控修复的前提。我国黑土区地处面积最大的东北平原,农业生产机械化程度高,化学除草已成为农田杂草防除的主要手段。施用的除草剂主要包括莠去津、乙草胺、烟嘧磺隆、氟磺胺草醚、精喹禾灵、灭草松、丁草胺、苄嘧磺隆等单一剂型或多种成分复配的混合剂型,但实际生产中除草剂的有效利用率仅为20%~30%,喷洒的除草剂大部分进入土壤环境中。由于黑土地农田地处我国东北高纬度地区,年平均气温较低,每年11月份至次年3月份,土壤一般处于冻结状态,该阶段微生物降解基本停滞,因此土壤中除草剂残留相比于其他地区更为严重。关于黑土地农田除草剂残留状况,在东北三省的部分区域或地区已经开展了一些调查。王万红等[4]在辽宁省北部农田采集的54个土壤表层样品中,莠去津、乙草胺及丁草胺的检出率高达100%,最大残留量分别达到21.20 μg·kg–1、203.2 μg·kg–1和30.87 μg·kg–1;黑龙江省密山地区典型农场大豆田土壤中氟磺胺草醚和灭草松的残留浓度分别为3.34~67.84 μg·kg–1和6.27~56.03 μg·kg–1 [5];蔡霖[6]调查了辽河平原、松嫩平原和三江平原部分地区110种农药残留状况,发现三大平原土壤中农药总残留浓度高达80.7~2799 μg·kg–1,在辽中南等地残留较多,总残留浓度均在1 200 μg·kg–1以上。但目前开展的调查多为零星或局部调查,仅仅关注了东北黑土地部分地区,调查的污染物也多集中于少数几种常用除草剂,而关于黑土区区域或流域尺度上农田生态系统中除草剂复合污染的时空分布特征仍不清楚,区域污染长期持续原位观测尚处空白。
我国东北黑土区存在玉米、大豆、水稻等单作与轮作多种种植体系,不同种植体系中施用除草剂的种类繁多、用量各异,用药历史存在明显差异,应用的除草剂也多从单一剂型发展到混合剂型,因此有必要详细调查各地区除草剂施用类型、施用方式、时间及用量,从源头上理清土壤中除草剂的排放清单,明确除草剂剂型及用量对土壤中除草剂污染形成的贡献,尤其要关注黑土区农田除草剂高频高剂量持续施用导致的残留问题。而且不同地区土壤类型、气候条件和环境因素也存在很大差别,这些均会影响除草剂在土壤中的迁移转化过程[7],从而导致其在土壤及作物中的残留水平产生差异。此外,残留于土壤中水溶性较强的除草剂(如莠去津、氟磺胺草醚等),易随降雨淋溶或地表径流等途径进入地下水或地表水[8-9],从而对水生生态系统和人类饮用水源构成威胁。近年来在欧美等多个国家长期使用莠去津的地区,地下水、河流、湖泊和港湾中均检测到不同含量的莠去津及其代谢物残留[10-12]。我国东辽河流域地旱田和非旱田分布区内地表水中也能检测到莠去津的大量残留,平均含量分别为9.71 μg·L–1和8.85 μg·L–1,雨季流域地表水中莠去津含量最高,可达18.93 μg·L–1 [13]。目前关于东北黑土区除草剂在地表水和地下水中时空分布特征的报道较为有限,尤其是区域尺度上土壤-水体系统中除草剂污染的演变规律有待进一步深入研究,可为黑土区除草剂污染分区分类分级管控提供基础数据和理论支持。
2 黑土地农田除草剂环境行为及其驱动机制阐明污染过程与驱动机制是黑土地农田除草剂污染防控修复的理论基础。土壤中各种矿物、有机质(尤其是土壤胶体)与土壤溶液交界处形成的界面是土壤颗粒与污染物相互作用最密切的部位,对土壤中除草剂的迁移、转化/降解及其生态环境效应起决定性作用。目前关于土壤颗粒界面除草剂的吸附解吸、降解转化等过程已经开展了大量研究[14-15],系统探讨了东北黑土区广泛应用的长残效除草剂(如莠去津、氯嘧磺隆等磺酰脲类除草剂)在不同类型土壤及其有机无机组分(可溶性有机质、腐殖酸、胡敏酸等有机质;蒙脱石、高岭石、铁铝氧化物等黏土矿物)上的吸附解吸行为[16-20],采用红外光谱、X射线衍射光谱、同步扫描荧光光谱、高分辨率核磁共振和电子自旋共振等技术探明了典型除草剂与土壤有机、无机组分间主要存在范德华力、氢键、疏水分配、配位交换、电荷-偶极和偶极-偶极等吸附作用力[21-22]。除草剂在土壤中的吸附过程通常是多种机制共同作用的结果,但目前关于各种机制对吸附的相对贡献尚未有明确结论。温度、土壤pH、离子强度等环境因子以及施用生物质炭等农艺管理措施对典型除草剂在土壤及其组分中吸附解吸及降解转化均存在不同程度的影响[23-26]。目前关于土壤颗粒界面除草剂污染研究多聚焦于吸附、降解等单一过程,而多过程耦合作用及其驱动机制研究仍较为有限。相比于其他土壤类型,黑土含有较高含量的有机质,有机质对土壤中除草剂的氧化还原转化及微生物降解过程可能具有更强的介导作用,黑土区特定气候条件(如冻融循环交替等)对土壤中有机质介导除草剂迁移转化过程的驱动作用有待进一步深入研究。
解析农田生态系统中除草剂的迁移转化规律是开展其生态环境和健康风险评估的基础。除草剂在土壤-作物系统中的迁移归趋,直接关系着农产品质量安全,对农药的合理利用也具有重要的现实意义。目前关注较多的是黑土区广泛应用的除草剂(如莠去津、乙草胺等)在不同土壤-作物系统(玉米、大豆、小麦、水稻等)中的消减动态和残留规律[27-29],探索了植物体内莠去津等典型除草剂的吸收代谢途径[30-31],发现细胞色素P450、谷胱甘肽转移酶等参与了作物体内典型除草剂的代谢转化[32],揭示了主要作物对典型除草剂的部分解毒代谢机制[33-34]。作物通过根部吸收土壤中残留的除草剂,并在作物体内或可食农产品部位进行累积,影响作物根部吸收除草剂的主要因素包括土壤理化性质、除草剂自身性质和作物种类等[35]。我国于2021年发布了《食品安全国家标准食品中农药最大残留限量》(GB2763-2021),全面覆盖了我国已批准使用的农药品种和主要植物源性农产品,但目前关于黑土区粮食作物中除草剂的的残留状况研究仍相当有限。土壤有机质含量是决定作物暴露于土壤中除草剂有效剂量的关键因子,土壤高有机质含量会减弱作物对土壤中除草剂的吸收。除草剂的溶解性是影响作物吸收的主要因素,亲脂性越强,通常在作物的根和茎部积累越多[36],影响作物根部积累除草剂的因素还包括根部脂质组分及其含量[37]。植物能够通过不同酶系降解体内吸收的除草剂,但其反应机理比较复杂,至今尚不清楚。
水溶性较强的除草剂在土壤-水体系统中的迁移归趋,已经成为国内外关注的研究热点与前沿。目前国内外采用室内土柱淋溶试验系统研究了典型除草剂(如莠去津)在土壤-水体环境中的迁移动态规律[38-39],开发和发展了一系列能表征和预测实验室尺度下稳定流场饱和土壤中典型除草剂淋溶行为的确定性模型和随机模型[40-42]。在试验方法上,国外学者已从室内土柱试验的数值模拟逐渐发展到基于野外田间原位观测数据的数值仿真[43-44],从典型除草剂单一污染扩展到除草剂与其代谢产物的复合污染[45],并且在数学模型方面已由平衡模型发展到非平衡模型,逐渐考虑到土壤非均相属性以及土壤环境中除草剂的转化反应[46],从区域或流域尺度上较好地评估了环境中除草剂的迁移和归趋[47-48]。但国内学者目前仍更多地关注实验室尺度下典型除草剂在土壤中淋溶行为的模拟研究[49-50],而关于区域或流域尺度上除草剂在环境中的迁移归趋研究仍较为有限,尤其采用数学模型定量表征除草剂在土壤-水体中的迁移动态规律尚处起步阶段。东北黑土区具有丰富的地表水系,不同流域的土壤类型、耕作制度、种植管理方式以及环境气候条件等对除草剂在土-水系统中的迁移转化可能具有明显不同的影响[51],因此,亟需系统研究东北特定气候条件下黑土区典型耕作区土壤-地表水-地下水系统中除草剂的迁移转化过程,为黑土地土壤中除草剂的生态环境风险评估提供有力的科学依据。
除了除草剂母体化合物之外,越来越多的国外学者开始关注到除草剂的中间代谢产物在土壤环境中的迁移转化过程,尤其是环境中稳定性较强和危害性较大的降解产物[52]。目前关于典型除草剂莠去津的脱烷基代谢产物(脱乙基莠去津DEA和脱异丙基莠去津DIA)在土壤中的吸附、淋溶和降解等方面已经开展了一些研究[53-54],其在土壤中淋溶迁移导致的生态环境风险和健康风险甚至超过了母体莠去津,并且这类物质在环境中的稳定性较强,可能与母体长期共存,因此这些主要降解产物在土壤环境中的迁移转化特性研究已逐渐成为国外学者关注的研究热点,但目前国内相关研究仍相对较少。深入研究除草剂母体与其降解产物复合污染在土壤颗粒界面、土壤-作物系统、土壤-地表水-地下水系统中的迁移转化过程,对科学评估农田生态系统中除草剂的生态环境风险及其污染的防控修复具有重要的科学意义。
3 黑土地农田除草剂污染生态风险甄别生态风险评估是科学指导黑土地农田除草剂污染防控修复的重要依据。伴随着除草剂应用,其生态风险评估一直是环境毒理领域的研究热点。国内外针对除草剂对土壤生物个体水平上的生态毒性已经开展了较为系统的研究,在陆生动物/植物/微生物生长发育[55]、生理代谢[56]、基因毒性[57]、表观遗传毒性[58]、生物膜和亚细胞结构毒性[59]、氧化损伤[60]以及土壤微生物群落响应与功能酶活性变化[61-62]、毒性作用机制和重要分子生物标志物筛选[63]等基础研究上取得了一定进展。比如,除草剂会影响作物的生化组成和氮代谢。丁草胺和二氯喹啉酸等除草剂处理后,水稻叶鞘内游离氨基酸含量明显增加,蔗糖含量和总酚含量均下降。一些长期使用长残效除草剂的田块还出现了除草剂残留量累积的现象,导致后茬敏感作物生长发育受阻,产量下降,严重影响了后茬作物的轮作,形成了“癌症田”的现象。面对日趋复杂的除草剂种类形态与多变的陆生生境条件,除草剂生态毒性效应研究已从单一污染物高浓度急性暴露剂量-效应关系的室内模拟研究向多重污染物低剂量长期暴露复合剂量-效应关系的野外试验研究上转变[64],从宏观生态效应监测向宏/微观综合尺度下生物富集与生物有效性[65]、微观毒性作用机制与预测模型研究上发展[66],从简单生物个体后期生态效应向细胞、亚细胞和分子水平下早期生态效应与更高水平的种群群落(如微生物群落)响应的综合效应评估上演变[67],从生理生化响应或能量变化到分子生物标志物筛选[68],以及在分析方法上从传统的生化指标分析到宏基因组/宏蛋白组/宏转录组/宏代谢组学研究[69-71],尤其以分子生态毒理学发展迅速,其中,高通量生态毒理基因组学筛选技术或代谢组学方法在土壤生态系统分子生物标志物的应用已成为研究热点之一[72-73]。
随着组学技术的发展与应用,我国在除草剂的陆生生态毒理效应研究上不断深化,但在复合除草剂(包括不同种类除草剂复合以及除草剂与其中间代谢产物复合)对陆生生物毒性效应及作用机制和重要分子生物标志物筛选等基础研究上还相对分散,土壤生物富集与生物有效性及毒性预测模型研究仍显不足,相对缺乏中长期、多种生境因子累积的污染暴露和复合污染毒性研究,不能很好满足环境基准定量化与早期风险快速诊断的迫切需求[74]。此外,土壤实际生境中生物并不是单独存在的,而多呈复杂的网络结构,生物之间的相互作用(如捕食、竞争等)可能会改变除草剂对生物的影响[75]。因此,基于个体层次的生态毒理效应已经无法满足除草剂的实际风险评估,非常有必要建立基于食物链或食物网的生态风险评估体系[76]。
目前已建立的土壤生物对除草剂污染响应的剂量-效应关系多是基于除草剂总量表征预测的,但除草剂进入土壤后由于被土壤有机质、矿物等固相吸附、锁定而发生老化过程,甚至形成结合残留[77],导致部分除草剂生物有效性降低,难以被生物利用[78],因此,国内外学者已普遍认为以总量评价通常会高估污染物的生态风险,基于生物有效态浓度的生态风险评估已成为国际生态毒理研究领域的主要发展趋势[79]。东北黑土区土壤有机质含量高、黏质性强,土壤中除草剂的生物有效性可能更低,但目前针对黑土区土壤中除草剂生物有效性的研究相当有限,尤其是黑土区特定气候条件(如冻融循环交替等)对除草剂生物有效性的影响及机制仍不明晰,有关黑土区除草剂污染的有效剂量-效应关系研究更是少之又少,难以满足黑土地环境安全和风险管控需求。
4 黑土地农田除草剂污染高效消减与修复技术源头控制是农田除草剂污染高效消减的首要途径。研发特异性靶向高效除草剂及其精准施用技术,减少除草剂施用,已成为源头消减的重要方式,但本文对此不做详细阐述,重点综述土壤中除草剂污染的消减与修复技术。土壤中除草剂的消减过程主要包括水解、光解、氧化还原降解和生物降解,其中生物降解是最主要的转化过程[80],已研发的修复技术多是基于上述消减过程调控的强化修复技术。目前相对成熟的主要为微生物修复技术和根际强化消减技术[81]。
微生物修复是解决除草剂污染农田土壤的一种低成本绿色修复方法,具有良好的应用前景。目前,国内外已经从土壤、污水、污泥等中筛选分离到一系列典型除草剂(如莠去津、乙草胺、氯嘧磺隆等)的高效降解菌株[82-84],阐明了典型除草剂的微生物降解特性及其影响因素[85],解析了典型除草剂的微生物降解途径及其酶和基因水平上的分子机制[86-87],验证了部分除草剂污染土壤的微生物菌剂或酶制剂强化修复效果[88-89],但已开展研究多集中于实验室工作,田间实际修复研究相对缺乏,可大规模用于除草剂污染农田土壤的高效稳定修复菌剂极为有限。而且目前研发的微生物菌剂多为基于单一降解菌构建,在降解除草剂过程中通常会产生有毒的中间物质影响整体降解效率[90],而在实际污染土壤中,除草剂降解通常由微生物菌群介导,通过群落个体间代谢互补性、互营作用完成污染物去除[91]。通过构建合成微生物菌群体系降解除草剂等有机污染物已成为国内外生物技术领域的关注前沿与热点。
根际强化消减技术是实现有机污染农田土壤原位生物修复的重要技术。根际是污染物生物地球化学转化作用的重要微环境[92]。作物通过根系向土壤中分泌大量的光合产物,明显地改变了根际土壤的物理、化学和生物学性质[93-94],成倍提升了土壤中除草剂等有机污染物的转化与消减速率[95]。根际土壤中生物并不是单独存在的,在实际自然环境中多呈现复杂的网络结构[96],近年来在Nature、Science、Proceedings of the National Academy of Sciences of the United States of America(PNAS)等期刊上发表多篇论文,深入研究了根际土壤中植物、微生物、动物之间的互作规律及其对环境因子的响应机制[97-100]。但目前关于根际生物联合强化修复有机污染农田的研究仍主要集中于单一互作体系[101],对根际生态网络多界面互作的降污潜力缺乏系统性认识,从调控根际生态网络出发建立有机污染土壤原位修复技术仍相当匮乏。针对黑土区除草剂污染农田土壤,采用农艺调控措施如水肥管理、间套作、秸秆还田、碳材料等来调控根际生态网络,从而强化土壤中除草剂消减有待进一步深入研究。
农田土壤污染修复不仅需要适用性技术的创新,而且更需加强多种修复技术集成和工程应用。黑土区农田土壤有机质含量高,除草剂等有机污染物降解慢,残留期长,且有机污染分布空间异质性大,导致单一修复技术通常难以达到理想的修复效果。目前,黑土区农田除草剂污染土壤修复尚存在以下问题:(1)缺乏针对黑土区农田污染特征、土壤类型和种植体系等特征的修复技术工程参数;(2)工程示范修复模式单一,缺乏集成修复工程技术体系和系统解决方案,导致技术工程示范效果不佳,难以规模化工程应用;(3)尚未形成农田除草剂污染修复工程技术规范以及评估体系。
综上所述,从黑土区除草剂污染研究现状与发展趋势而言,针对东北黑土区土壤有机质含量高、高寒低温、冻融交替的特定生境,不同种植模式和土壤类型农田除草剂污染多介质界面过程仍不明晰,特定除草剂的生态环境风险评估工作明显滞后、生态毒性数据缺乏,特定黑土地耕作模式下农田污染消减技术体系相当匮乏。因此,亟需系统开展黑土地农田除草剂污染多介质多界面污染过程与驱动机制、生态风险效应与安全阈值、消减技术与集成示范研究,为保障我国黑土粮仓农产品质量安全和生态系统安全提供科技支撑。
5 研究展望东北黑土地各区域土壤类型、气候条件和种植制度存在明显差异,针对不同土壤类型和种植模式黑土地典型农区长残效除草剂污染特征不清、过程机制不明、风险甄别不足、高效消减技术缺乏等问题,以除草剂单一或复合条件下除草剂与其中间代谢产物(以下以除草剂代替)为研究对象,重点开展以下研究(图 1):
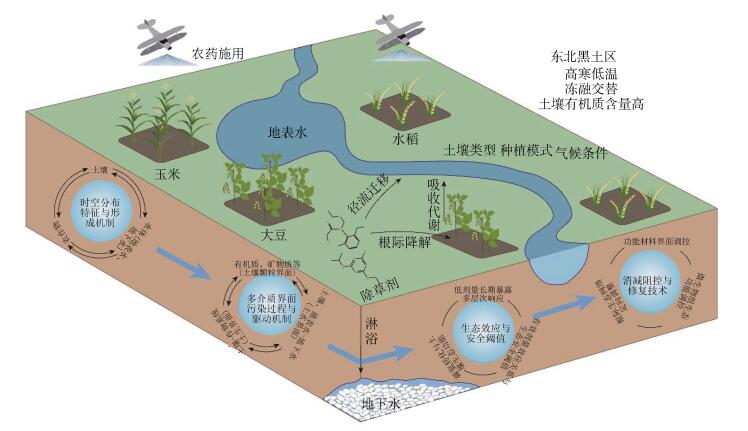
|
图 1 东北黑土地农田除草剂污染过程与消减技术研究框架体系 Fig. 1 Research framework of the pollution process and abatement technology of herbicides in black soil region in northeastern China |
系统调查不同土壤类型和种植模式黑土地农田土壤、地表水和地下水除草剂组分含量、农产品中的残留状况及其对农产品品质的影响;明确黑土地农田优先控制除草剂,解析不同农业投入品对土壤污染的贡献率,构建黑土区除草剂污染管控的源投入清单;研究典型农区除草剂的时空分布格局和演变规律,甄别黑土地农田除草剂污染形成的主控因子;研究农田土壤除草剂累积的源汇动态平衡机制,阐明黑土地农田生态系统除草剂污染的形成机制。
5.2 黑土地农田多介质多界面除草剂污染过程与驱动机制研究高寒低温、冻融交替条件下黑土地典型除草剂在土壤多介质组分上的界面行为,解析土壤矿物与有机质交互作用驱动除草剂转化的多过程耦合机制;研究不同种植模式下黑土地土壤-作物系统中除草剂的迁移、转化和富集动态过程,揭示作物体内除草剂的转化机制及其关键驱动因子;研究黑土区旱作和稻田土壤-地表水-地下水系统中除草剂的多维迁移规律,阐明土水界面除草剂的迁移驱动机制;阐明黑土区土壤多介质界面典型除草剂的迁移转化机制,构建土壤微界面有机污染过程与多介质界面动态变化模型。
5.3 黑土地农田除草剂污染生态效应与安全阈值研究低剂量长期暴露条件下分子、细胞、个体、种群、群落、生态系统等不同水平上土壤生物对除草剂的响应机制;研究除草剂对农田生态系统食物链和食物网的毒理效应及作用机制;研究除草剂对土壤中微生物驱动的典型碳氮转化过程与土壤生态功能的影响机制;研究黑土地特定土壤类型、冻融交替条件下土壤中除草剂生物有效性的动态变化规律与主控因子,建立农田生态系统不同层次水平上除草剂的有效剂量-形态-效应关系,构建基于除草剂生物有效态浓度的毒性预测模型;提出针对不同类型黑土地农田土壤除草剂的生态安全阈值。
5.4 黑土地农田除草剂污染消减阻控与修复技术研发高效、低成本生物质炭基和黏土矿物基等功能复合修复材料以及高效微生物-光催化耦联协同修复材料,建立不同类型功能材料的土壤除草剂界面调控消减技术;研究黑土区农田典型种植体系和耕作方式下水肥管理、秸秆还田和碳基复合材料等对根际土壤污染物消减过程和生态网络的影响规律,构建基于根际生态网络定向调控的除草剂污染强化消减技术;构建典型有机污染物耐低温高效降解菌群,阐明功能菌群对土壤-作物系统微生物组强化消减有机污染物的作用机制,建立基于生态功能调控土壤微生物组的强化消减技术。
5.5 黑土地农田有机污染消减技术集成与示范根据东北黑土区玉米、大豆、水稻等三大主要作物种植体系除草剂污染特征,结合功能材料界面调控消减、根际生态网络协同消减、微生物组强化消减等黑土有机污染消减技术,在典型除草剂残留区域进行消减技术试验与集成,优化各项技术的生态及农艺工程参数,综合评价修复效能;构建特征除草剂修复效果和土壤生态安全及农产品安全的评价方法和指标体系;开展黑土地农田除草剂污染消减技术集成与工程示范,构建适宜高寒低温黑土区农田除草剂污染的原位综合消减集成技术体系。
通过上述重点方向研究,有望查明我国东北地区黑土地农田除草剂污染现状,揭示黑土地农田除草剂污染形成的综合驱动机制,提出黑土地农田除草剂的安全阈值,构建适宜高寒低温黑土区农田高效、安全、可推广的除草剂污染综合消减技术体系,为保障我国黑土粮仓农产品质量安全和生态系统安全提供科技支撑。
| [1] |
中华人民共和国农业村部. 东北黑土地保护规划纲要(2017—2030)[OL]. http://www.moa.gov.cn/nybgb/2017/dqq/201801/t20180103_6133926.htm. [2021–07–09]. Ministry of Agriculture and Rural Affairs of the People's Republic of China. Outline of Northeast Black Soil Protection Plan(2017—2030)[OL]. http://www.moa.gov.cn/nybgb/2017/dqq/201801/t20180103_6133926.htm. [2021–07–09]. (  0) 0) |
| [2] |
Guo L J, Wang Y. Spatiotemporal evolution of pesticide application in China (In Chinese)[J]. Jiangsu Agricultural Sciences, 2019, 47(14): 327-331. [郭利京, 王颖. 我国农药施用的时空演变[J]. 江苏农业科学, 2019, 47(14): 327-331.]
(  0) 0) |
| [3] |
Department of Rural Social and Economic Survey, National Bureau of Statistics. China rural statistical yearbook-2020 (In Chinese). Beijing: China Statistics Press, 2020. [国家统计局农村社会经济调查司. 中国农村统计年鉴-2020[M]. 北京: 中国统计出版社, 2020.]
(  0) 0) |
| [4] |
Wang W H, Wang Y H, Wang S C, et al. Residual characteristics of herbicides and organochlorine pesticides in agricultural soils in northern Liaoning Province (In Chinese)[J]. Chinese Journal of Soil Science, 2010, 41(3): 716-722. [王万红, 王颜红, 王世成, 等. 辽北农田土壤除草剂和有机氯农药残留特征[J]. 土壤通报, 2010, 41(3): 716-722.]
(  0) 0) |
| [5] |
Zhang K X, Zhang J Y, Wang Y F. The spatial distribution of herbicide residues in soybean fields in Mishan area (In Chinese)[J]. Agrochemicals, 2020, 59(1): 56-59. DOI:10.3969/j.issn.1002-5480.2020.01.015 [张可鑫, 张金艳, 王亚飞. 密山地区大豆田除草剂残留的空间分布[J]. 农药, 2020, 59(1): 56-59.]
(  0) 0) |
| [6] |
蔡霖. 东北农业区土壤中农药残留特征及风险识别[D]. 辽宁大连: 大连理工大学, 2017. Cai L. Residues and risk identification of pesticides in soil in northeast agricultural region of China[D]. Dalian, Liaoning: Dalian University of Technology, 2017. (  0) 0) |
| [7] |
Barrios R E, Akbariyeh S, Liu C, et al. Climate change impacts the subsurface transport of atrazine and estrone originating from agricultural production activities[J]. Environmental Pollution, 2020, 265: 115024. DOI:10.1016/j.envpol.2020.115024
(  0) 0) |
| [8] |
Willkommen S, Lange J, Ulrich U, et al. Field insights into leaching and transformation of pesticides and fluorescent tracers in agricultural soil[J]. Science of the Total Environment, 2021, 751: 141658. DOI:10.1016/j.scitotenv.2020.141658
(  0) 0) |
| [9] |
Bexfield L M, Belitz K, Lindsey B D, et al. Pesticides and pesticide degradates in groundwater used for public supply across the United States: Occurrence and human-health context[J]. Environmental Science & Technology, 2021, 55(1): 362-372.
(  0) 0) |
| [10] |
Fisher I J, Phillips P J, Bayraktar B N, et al. Pesticides and their degradates in groundwater reflect past use and current management strategies, Long Island, New York, USA[J]. Science of the Total Environment, 2021, 752: 141895. DOI:10.1016/j.scitotenv.2020.141895
(  0) 0) |
| [11] |
Ghirardelli A, Otto S, Masin R, et al. Thirty-year monitoring of s-triazine herbicide contamination in the aquifer north of Vicenza(north-east Italy)[J]. Science of the Total Environment, 2021, 752: 141647. DOI:10.1016/j.scitotenv.2020.141647
(  0) 0) |
| [12] |
Montiel-León J M, Vo Duy S, Munoz G, et al. Quality survey and spatiotemporal variations of atrazine and desethylatrazine in drinking water in Quebec, Canada[J]. Science of the Total Environment, 2019, 671: 578-585. DOI:10.1016/j.scitotenv.2019.03.228
(  0) 0) |
| [13] |
Yan D H, He Y, Wang H. Environmental characteristics of the atrazine in the waters in east Liaohe River basin (In Chinese)[J]. Environmental Science, 2005, 26(3): 203-208. DOI:10.3321/j.issn:0250-3301.2005.03.041 [严登华, 何岩, 王浩. 东辽河流域地表水体中atrazine的环境特征[J]. 环境科学, 2005, 26(3): 203-208.]
(  0) 0) |
| [14] |
Caceres-Jensen L, Rodriguez-Becerra J, Escudey M, et al. Nicosulfuron sorption kinetics and sorption/desorption on volcanic ash-derived soils: Proposal of sorption and transport mechanisms[J]. Journal of Hazardous Materials, 2020, 385: 121576. DOI:10.1016/j.jhazmat.2019.121576
(  0) 0) |
| [15] |
Mendes K F, Wei M C F, Furtado I F, et al. Spatial distribution of sorption and desorption process of 14C-radiolabelled hexazinone and tebuthiuron in tropical soil[J]. Chemosphere, 2021, 264(1): 128494.
(  0) 0) |
| [16] |
Guo F Y, Zhou M, Xu J C, et al. Glyphosate adsorption onto kaolinite and kaolinite-humic acid composites: Experimental and molecular dynamics studies[J]. Chemosphere, 2021, 263: 127979. DOI:10.1016/j.chemosphere.2020.127979
(  0) 0) |
| [17] |
Kersten M, Tunega D, Georgieva I, et al. Adsorption of the herbicide 4-chloro-2-methylphenoxyacetic acid(MCPA) by goethite[J]. Environmental Science & Technology, 2014, 48(20): 11803-11810.
(  0) 0) |
| [18] |
Ren W J, Wang M E, Zhou Q X. Adsorption characteristics and influencing factors of chlorimuron-ethyl in two typical chinese soils[J]. Soil Science Society of America Journal, 2011, 75(4): 1394-1401. DOI:10.2136/sssaj2010.0228
(  0) 0) |
| [19] |
Huang Y F, Liu Z Z, Li Y L, et al. Effects of humic acids and minerals on adsorption-desorption of atrazine in soil (In Chinese)[J]. Acta Pedologica Sinica, 2016, 53(1): 155-165. [黄玉芬, 刘忠珍, 李衍亮, 等. 土壤矿物和胡敏酸对阿特拉津的吸附-解吸作用研究[J]. 土壤学报, 2016, 53(1): 155-165.]
(  0) 0) |
| [20] |
Huang Y F, Liu Z Z, We L, et al. Effects of amorphous fe oxides on adsorption-desorption of atrazine in soil (In Chinese)[J]. Acta Pedologica Sinica, 2018, 55(1): 148-158. [黄玉芬, 刘忠珍, 魏岚, 等. 无定型氧化铁对土壤中阿特拉津吸附-解吸的影响[J]. 土壤学报, 2018, 55(1): 148-158.]
(  0) 0) |
| [21] |
Wang Y F, Zhang X Y, Zhang X, et al. Characterization of spectral responses of dissolved organic matter(DOM) for atrazine binding during the sorption process onto black soil[J]. Chemosphere, 2017, 180: 531-539. DOI:10.1016/j.chemosphere.2017.04.063
(  0) 0) |
| [22] |
Yue L, Ge C J, Feng D, et al. Adsorption-desorption behavior of atrazine on agricultural soils in China[J]. Journal of Environmental Sciences, 2017, 57: 180-189. DOI:10.1016/j.jes.2016.11.002
(  0) 0) |
| [23] |
Liu Y X, Lonappan L, Brar S K, et al. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review[J]. Science of the Total Environment, 2018, 645: 60-70. DOI:10.1016/j.scitotenv.2018.07.099
(  0) 0) |
| [24] |
Muskus A M, Krauss M, Miltner A, et al. Degradation of glyphosate in a Colombian soil is influenced by temperature, total organic carbon content and pH[J]. Environmental Pollution, 2020, 259: 113767. DOI:10.1016/j.envpol.2019.113767
(  0) 0) |
| [25] |
Ren W J, Wang M E, Zhou Q X. Effect of soil pH and organic matter on desorption hysteresis of chlorimuron-ethyl in two typical Chinese soils[J]. Journal of Soils and Sediments, 2011, 11(4): 552-561. DOI:10.1007/s11368-011-0337-4
(  0) 0) |
| [26] |
Ren W J, Zhou Q X, Wang M E, et al. Interactive effects of chlorimuron-ethyl and copper(Ⅱ) on their sorption and desorption on two typical chinese soils[J]. European Journal of Soil Science, 2011, 62(6): 882-890. DOI:10.1111/j.1365-2389.2011.01396.x
(  0) 0) |
| [27] |
Namiki S, Otani T, Motoki Y, et al. Differential uptake and translocation of organic chemicals by several plant species from soil[J]. Journal of Pesticide Science, 2018, 43(2): 96-107. DOI:10.1584/jpestics.D17-088
(  0) 0) |
| [28] |
Pang N N, Cui Y, Hu J Y. Weather dependent dynamics of the herbicides florasulam, carfentrazone-ethyl, fluroxypyr-meptyl and fluroxypyr in wheat fields through field studies and computational simulation[J]. Chemosphere, 2016, 165: 320-328. DOI:10.1016/j.chemosphere.2016.09.026
(  0) 0) |
| [29] |
Chen J J, Li M R, Zhang K, et al. Uptake and removal efficiency of atrazine in soil by several weeds (In Chinese)[J]. Journal of Agro-Environment Science, 2014, 33(12): 2368-2373. DOI:10.11654/jaes.2014.12.013 [陈建军, 李明锐, 张坤, 等. 几种植物对土壤中阿特拉津的吸收富集特征及去除效率研究[J]. 农业环境科学学报, 2014, 33(12): 2368-2373.]
(  0) 0) |
| [30] |
Ju C, Zhang H C, Wu R L, et al. Upward translocation of acetochlor and atrazine in wheat plants depends on their distribution in roots[J]. Science of the Total Environment, 2020, 703: 135636. DOI:10.1016/j.scitotenv.2019.135636
(  0) 0) |
| [31] |
Zhang J J, Gao S, Xu J Y, et al. Degrading and phytoextracting atrazine residues in rice(Oryza sativa) and growth media intensified by a phase Ⅱ mechanism modulator[J]. Environmental Science & Technology, 2017, 51(19): 11258-11268.
(  0) 0) |
| [32] |
Xia J, Lin J, Zhu S Y, et al. Lycopene protects against atrazine-induced hepatotoxicity through modifications of cytochrome P450 enzyme system in microsomes[J]. Experimental and Toxicologic Pathology, 2016, 68(4): 223-231. DOI:10.1016/j.etp.2015.12.004
(  0) 0) |
| [33] |
Chen Z L, Schmidt B, Schäffer A. Uptake and decomposition of the herbicide propanil in the plant Bidens pilosa L. dominating in the Yangtze Three Gorges Reservoir(TGR), China[J]. Environmental Science and Pollution Research, 2017, 24(12): 11141-11153. DOI:10.1007/s11356-016-6068-8
(  0) 0) |
| [34] |
张静静. 阿特拉津在紫花苜蓿和水稻中解毒代谢机制的研究[D]. 南京: 南京农业大学, 2017. Zhang J J. Detoxification and metabolism mechanism of atrazine in Medicago sativa and Oryza sativa[D]. Nanjing: Nanjing Agricultural University, 2017. (  0) 0) |
| [35] |
Singh S, Kumar V, Datta S, et al. Glyphosate uptake, translocation, resistance emergence in crops, analytical monitoring, toxicity and degradation: A review[J]. Environmental Chemistry Letters, 2020, 18(3): 663-702. DOI:10.1007/s10311-020-00969-z
(  0) 0) |
| [36] |
Szpyrka E, Słowik-Borowiec M, Książek P, et al. The difference in dissipation of clomazone and metazachlor in soil under field and laboratory conditions and their uptake by plants[J]. Scientific Reports, 2020, 10(1): 3747. DOI:10.1038/s41598-020-60720-0
(  0) 0) |
| [37] |
Ju C, Li X, He S H, et al. Root uptake of imidacloprid and propiconazole is affected by root composition and soil characteristics[J]. Journal of Agricultural and Food Chemistry, 2020, 68(52): 15381-15389. DOI:10.1021/acs.jafc.0c02170
(  0) 0) |
| [38] |
Barba V, Marín-Benito J M, Sánchez-Martín M J, et al. Transport of 14C-prosulfocarb through soil columns under different amendment, herbicide incubation and irrigation regimes[J]. Science of the Total Environment, 2020, 701: 134542. DOI:10.1016/j.scitotenv.2019.134542
(  0) 0) |
| [39] |
Haddad K, Gheid A, Haddad D, et al. Experimental and numerical study on the leaching of pesticides into the groundwater through a porous medium: Effects of transport parameters[J]. Environmental Technology & Innovation, 2019, 13: 244-256.
(  0) 0) |
| [40] |
Farlin J, Galle T, Bayerle M, et al. Breakthrough dynamics of S-metolachlor metabolites in drinking water wells: Transport pathways and time to trend reversal[J]. Journal of Contaminant Hydrology, 2018, 213: 62-72. DOI:10.1016/j.jconhyd.2018.05.002
(  0) 0) |
| [41] |
Lammoglia S K, Brun F, Quemar T, et al. Modelling pesticides leaching in cropping systems: Effect of uncertainties in climate, agricultural practices, soil and pesticide properties[J]. Environmental Modelling & Software, 2018, 109: 342-352.
(  0) 0) |
| [42] |
Noshadi M, Foroutani A, Sepaskhah A. Evaluation of HYDRUS-1D and modified PRZM-3 models for tribenuron methyl herbicide transport in soil profile under vetiver cultivation[J]. International Journal of Phytoremediation, 2019, 21(9): 878-891. DOI:10.1080/15226514.2019.1583632
(  0) 0) |
| [43] |
Bannwarth M A, Sangchan W, Hugenschmidt C, et al. Pesticide transport simulation in a tropical catchment by SWAT[J]. Environmental Pollution, 2014, 191: 70-79. DOI:10.1016/j.envpol.2014.04.011
(  0) 0) |
| [44] |
Pérez D J, Okada E, De Gerónimo E, et al. Spatial and temporal trends and flow dynamics of glyphosate and other pesticides within an agricultural watershed in Argentina[J]. Environmental Toxicology and Chemistry, 2017, 36(12): 3206-3216. DOI:10.1002/etc.3897
(  0) 0) |
| [45] |
Salazar-Ledesma M, Prado B, Zamora O, et al. Mobility of atrazine in soils of a wastewater irrigated maize field[J]. Agriculture Ecosystems & Environment, 2018, 255: 73-83.
(  0) 0) |
| [46] |
Xie S, Wen Z, Jakada H. A new model approach for reactive solute transport in dual-permeability media with depth-dependent reaction coefficients[J]. Journal of Hydrology, 2019, 577: 123946. DOI:10.1016/j.jhydrol.2019.123946
(  0) 0) |
| [47] |
Chen H, Luo Y, Potter C, et al. Modeling pesticide diuron loading from the San Joaquin watershed into the Sacramento-San Joaquin Delta using SWAT[J]. Water Research, 2017, 121: 374-385. DOI:10.1016/j.watres.2017.05.032
(  0) 0) |
| [48] |
Ouyang W, Cai G Q, Tysklind M, et al. Temporal-spatial patterns of three types of pesticide loadings in a middle-high latitude agricultural watershed[J]. Water Research, 2017, 122: 377-386. DOI:10.1016/j.watres.2017.06.023
(  0) 0) |
| [49] |
He L W, Shi L L, Kong D Y, et al. Leaching of carbofuran and atrazine in soil columns and its affecting factors (In Chinese)[J]. Journal of Ecology and Rural Environment, 2006, 22(2): 71-74. DOI:10.3969/j.issn.1673-4831.2006.02.015 [何利文, 石利利, 孔德洋, 等. 呋喃丹和阿特拉津在土柱中的淋溶及其影响因素[J]. 生态与农村环境学报, 2006, 22(2): 71-74.]
(  0) 0) |
| [50] |
Wang Y J, Li W P, Zhang Z, et al. Study of atrazine eluviation regularity in soil column (In Chinese)[J]. Journal of Soil and Water Conservation, 2011, 25(3): 254-256. [王玉军, 李文朋, 张铎, 等. 阿特拉津在土柱中的淋溶规律研究[J]. 水土保持学报, 2011, 25(3): 254-256.]
(  0) 0) |
| [51] |
Willkommen S, Pfannerstill M, Ulrich U, et al. How weather conditions and physico-chemical properties control the leaching of flufenacet, diflufenican, and pendimethalin in a tile-drained landscape[J]. Agriculture Ecosystems & Environment, 2019, 278: 107-116.
(  0) 0) |
| [52] |
Sidoli P, Devau N, Jaramillo R A, et al. Reactivity of vadose-zone solids to S-metolachlor and its two main metabolites: Case of a glaciofluvial aquifer[J]. Environmental Science and Pollution Research, 2020, 27(18): 22865-22877. DOI:10.1007/s11356-020-08579-6
(  0) 0) |
| [53] |
Krutz L J, Shaner D L, Zablotowicz R M. Enhanced degradation and soil depth effects on the fate of atrazine and major metabolites in Colorado and Mississippi soils[J]. Journal of Environmental Quality, 2010, 39(4): 1369-1377. DOI:10.2134/jeq2009.0197
(  0) 0) |
| [54] |
Xue Y, Zhang Z M, Zhang R R, et al. Aquaculture-derived distribution, partitioning, migration, and transformation of atrazine and its metabolites in seawater, sediment, and organisms from a typical semi-closed mariculture bay[J]. Environmental Pollution, 2021, 271: 116362. DOI:10.1016/j.envpol.2020.116362
(  0) 0) |
| [55] |
Jalal A, de Oliveira Junior J C, Ribeiro J S, et al. Hormesis in plants: Physiological and biochemical responses[J]. Ecotoxicology and Environmental Safety, 2021, 207: 111225. DOI:10.1016/j.ecoenv.2020.111225
(  0) 0) |
| [56] |
Owagboriaye F, Dedeke G, Bamidele J, et al. Biochemical response and vermiremediation assessment of three earthworm species(Alma millsoni, Eudrilus eugeniae and Libyodrilus violaceus) in soil contaminated with a glyphosate-based herbicide[J]. Ecological Indicators, 2020, 108: 105678. DOI:10.1016/j.ecolind.2019.105678
(  0) 0) |
| [57] |
Roustan A, Aye M, De Meo M, et al. Genotoxicity of mixtures of glyphosate and atrazine and their environmental transformation products before and after photoactivation[J]. Chemosphere, 2014, 108: 93-100. DOI:10.1016/j.chemosphere.2014.02.079
(  0) 0) |
| [58] |
Boutin C, Strandberg B, Carpenter D, et al. Herbicide impact on non-target plant reproduction: What are the toxicological and ecological implications?[J]. Environmental Pollution, 2014, 185: 295-306. DOI:10.1016/j.envpol.2013.10.009
(  0) 0) |
| [59] |
Lackmann C, Velki M, Bjedov D, et al. Commercial preparations of pesticides exert higher toxicity and cause changes at subcellular level in earthworm Eisenia andrei[J]. Environmental Sciences Europe, 2021, 33(1): 15. DOI:10.1186/s12302-021-00460-8
(  0) 0) |
| [60] |
Zhang Y, Jiang D, Yang C, et al. The oxidative stress caused by atrazine in root exudation of Pennisetum americanum(L.) K. Schum[J]. Ecotoxicology and Environmental Safety, 2021, 211: 111943. DOI:10.1016/j.ecoenv.2021.111943
(  0) 0) |
| [61] |
Wang M, Faber J H, Chen W. Application of stress index in evaluating toxicological response of soil microbial community to contaminants in soils[J]. Ecological Indicators, 2017, 75: 118-125. DOI:10.1016/j.ecolind.2016.12.002
(  0) 0) |
| [62] |
Wu X H, Zhang Y, Du P Q, et al. Impact of fomesafen on the soil microbial communities in soybean fields in Northeastern China[J]. Ecotoxicology and Environmental Safety, 2018, 148: 169-176. DOI:10.1016/j.ecoenv.2017.10.003
(  0) 0) |
| [63] |
Lackmann C, Velki M, Seiler T B, et al. Herbicides diuron and fluazifop-p-butyl affect avoidance response and multixenobiotic resistance activity in earthworm Eisenia andrei[J]. Chemosphere, 2018, 210: 110-119. DOI:10.1016/j.chemosphere.2018.07.008
(  0) 0) |
| [64] |
Jimmo A, Isbister K M, Lamb E G, et al. Linking herbicide dissipation to soil ecological risk along transmission rights-of-way in the Yukon territory, Canada[J]. Journal of Environmental Quality, 2018, 47(6): 1356-1364. DOI:10.2134/jeq2018.01.0053
(  0) 0) |
| [65] |
Ye X Q, Xiong K, Liu J. Comparative toxicity and bioaccumulation of fenvalerate and esfenvalerate to earthworm Eisenia fetida[J]. Journal of Hazardous Materials, 2016, 310: 82-88. DOI:10.1016/j.jhazmat.2016.02.010
(  0) 0) |
| [66] |
Awuah K F, Jegede O, Hale B, et al. Introducing the adverse ecosystem service pathway as a tool in ecological risk assessment[J]. Environmental Science & Technology, 2020, 54(13): 8144-8157.
(  0) 0) |
| [67] |
Jiang R, Wang M E, Chen W P, et al. Ecological risk of combined pollution on soil ecosystem functions: Insight from the functional sensitivity and stability[J]. Environmental Pollution, 2019, 255(1): 113184.
(  0) 0) |
| [68] |
Xu N H, Qu Q, Zhang Z Y, et al. Effects of residual S-metolachlor in soil on the phyllosphere microbial communities of wheat(Triticum aestivum L.)[J]. Science of the Total Environment, 2020, 748: 141342. DOI:10.1016/j.scitotenv.2020.141342
(  0) 0) |
| [69] |
Benndorf D, Balcke G U, Harms H, et al. Functional metaproteome analysis of protein extracts from contaminated soil and groundwater[J]. The ISME Journal, 2007, 1(3): 224-234. DOI:10.1038/ismej.2007.39
(  0) 0) |
| [70] |
Kostopoulou S, Ntatsi G, Arapis G, et al. Assessment of the effects of metribuzin, glyphosate, and their mixtures on the metabolism of the model plant Lemna minor L. applying metabolomics[J]. Chemosphere, 2020, 239: 124582. DOI:10.1016/j.chemosphere.2019.124582
(  0) 0) |
| [71] |
Low-Décarie E, Kolber M, Homme P, et al. Community rescue in experimental metacommunities[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(46): 14307-14312. DOI:10.1073/pnas.1513125112
(  0) 0) |
| [72] |
Matich E K, Chavez Soria N G, Aga D S, et al. Applications of metabolomics in assessing ecological effects of emerging contaminants and pollutants on plants[J]. Journal of Hazardous Materials, 2019, 373: 527-535. DOI:10.1016/j.jhazmat.2019.02.084
(  0) 0) |
| [73] |
Romdhane S, Devers-Lamrani M, Beguet J, et al. Assessment of the ecotoxicological impact of natural and synthetic β-triketone herbicides on the diversity and activity of the soil bacterial community using omic approaches[J]. Science of the Total Environment, 2019, 651(1): 241-249.
(  0) 0) |
| [74] |
Levine S L, Borgert C J. Review and recommendations on criteria to evaluate the relevance of pesticide interaction data for ecological risk assessments[J]. Chemosphere, 2018, 209: 124-136. DOI:10.1016/j.chemosphere.2018.06.081
(  0) 0) |
| [75] |
Zhu D, Ke X, Wu L H, et al. Ecotoxicity of cadmium in a soil collembolan-predatory mite food chain: Can we use the 15N labeled litter addition method to assess soil functional change?[J]. Environmental Pollution, 2016, 219: 37-46. DOI:10.1016/j.envpol.2016.09.051
(  0) 0) |
| [76] |
Sharma A, Jha P, Reddy G V P. Multidimensional relationships of herbicides with insect-crop food webs[J]. Science of the Total Environment, 2018, 643: 1522-1532. DOI:10.1016/j.scitotenv.2018.06.312
(  0) 0) |
| [77] |
Luks A K, Zegarski T, Nowak K M, et al. Fate of pendimethalin in soil and characterization of non-extractable residues(NER)[J]. Science of the Total Environment, 2021, 753: 141870. DOI:10.1016/j.scitotenv.2020.141870
(  0) 0) |
| [78] |
Rubio-Bellido M, Morillo E, Villaverde J. Assessment of soil diuron bioavailability to plants and microorganisms through non-exhaustive chemical extractions of the herbicide[J]. Geoderma, 2018, 312: 130-138. DOI:10.1016/j.geoderma.2017.09.031
(  0) 0) |
| [79] |
Critto A, Torresan S, Semenzin E, et al. Development of a site-specific ecological risk assessment for contaminated sites: Part Ⅰ. A multi-criteria based system for the selection of ecotoxicological tests and ecological observations[J]. Science of the Total Environment, 2007, 379(1): 16-33. DOI:10.1016/j.scitotenv.2007.02.035
(  0) 0) |
| [80] |
Cycon M, Mrozik A, Piotrowska-Seget Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review[J]. Chemosphere, 2017, 172: 52-71. DOI:10.1016/j.chemosphere.2016.12.129
(  0) 0) |
| [81] |
Eevers N, White J C, Vangronsveld J, et al. Bio-and phytoremediation of pesticide-contaminated environments: A review[J]. Phytoremediation, 2017, 83: 277-318.
(  0) 0) |
| [82] |
Cao D T, He S H, Li X, et al. Characterization, genome functional analysis, and detoxification of atrazine by Arthrobacter sp. C2[J]. Chemosphere, 2021, 264(2): 128514.
(  0) 0) |
| [83] |
Duc H D, Oanh N T. Biodegradation of acetochlor and 2-methyl-6-ethylaniline by Bacillus subtilis and Pseudomonas fluorescens[J]. Microbiology, 2019, 88(6): 729-738. DOI:10.1134/S0026261719060031
(  0) 0) |
| [84] |
Zhang S S, Zhang C, Sun F J, et al. Glutathione-S-transferase (GST) catalyzes the degradation of Chlorimuron-ethyl by Klebsiella jilinsis 2N3[J]. Science of the Total Environment, 2020, 729: 139075. DOI:10.1016/j.scitotenv.2020.139075
(  0) 0) |
| [85] |
Li Y, Chen Q, Wang C H, et al. Degradation of acetochlor by consortium of two bacterial strains and cloning of a novel amidase gene involved in acetochlor-degrading pathway[J]. Bioresource Technology, 2013, 148: 628-631. DOI:10.1016/j.biortech.2013.09.038
(  0) 0) |
| [86] |
Xiong M H, Hu Z G, Zhang Y, et al. Survival of GFP-tagged Rhodococcus sp. D310-1 in chlorimuron-ethyl-contaminated soil and its effects on the indigenous microbial community[J]. Journal of Hazardous Materials, 2013, 252: 347-354.
(  0) 0) |
| [87] |
Zang H L, Wang H L, Miao L, et al. Carboxylesterase, a de-esterification enzyme, catalyzes the degradation of chlorimuron-ethyl in Rhodococcus erythropolis D310-1[J]. Journal of Hazardous Materials, 2020, 387: 121684. DOI:10.1016/j.jhazmat.2019.121684
(  0) 0) |
| [88] |
Chen Y K, Jiang Z, Wu D, et al. Development of a novel bio-organic fertilizer for the removal of atrazine in soil[J]. Journal of Environmental Management, 2019, 233: 553-560. DOI:10.1016/j.jenvman.2018.12.086
(  0) 0) |
| [89] |
Zhang Y, Wang X, Jiang Z, et al. Effect of atrazine-contaminated soil by immobilization enzyme and dynamic variation of soil microbial (In Chinese)[J]. Journal of Northeast Agricultural University, 2013, 44(11): 1-6. DOI:10.3969/j.issn.1005-9369.2013.11.001 [张颖, 王溪, 姜昭, 等. 固定化酶修复阿特拉津污染土壤效果及土壤细菌多样性动态变化分析[J]. 东北农业大学学报, 2013, 44(11): 1-6.]
(  0) 0) |
| [90] |
Kolekar P D, Patil S M, Suryavanshi M V, et al. Microcosm study of atrazine bioremediation by indigenous microorganisms and cytotoxicity of biodegraded metabolites[J]. Journal of Hazardous Materials, 2019, 374: 66-73. DOI:10.1016/j.jhazmat.2019.01.023
(  0) 0) |
| [91] |
Xu X H, Zarecki R, Medina S, et al. Modeling microbial communities from atrazine contaminated soils promotes the development of biostimulation solutions[J]. The ISME Journal, 2019, 13(2): 494-508. DOI:10.1038/s41396-018-0288-5
(  0) 0) |
| [92] |
Xu J M, He Y. Micro-ecological processes in root-soil interface and their impacts on environmental behavior of organic pollutants (In Chinese)[J]. Soils, 2006, 38(4): 353-358. DOI:10.3321/j.issn:0253-9829.2006.04.001 [徐建明, 何艳. 根-土界面的微生态过程与有机污染物的环境行为研究[J]. 土壤, 2006, 38(4): 353-358.]
(  0) 0) |
| [93] |
Preece C, Peñuelas J. A return to the wild: Root exudates and food security[J]. Trends in Plant Science, 2020, 25(1): 14-21. DOI:10.1016/j.tplants.2019.09.010
(  0) 0) |
| [94] |
Zhang X C, Kuzyakov Y, Zang H D, et al. Rhizosphere hotspots: Root hairs and warming control microbial efficiency, carbon utilization and energy production[J]. Soil Biology & Biochemistry, 2020, 148: 107872.
(  0) 0) |
| [95] |
Hand L H, Gougoulias C, Bramke I, et al. Evaluation of the rhizosphere contribution to the environmental fate of the herbicide prometryn[J]. Environmental Toxicology and Chemistry, 2020, 39(2): 450-457. DOI:10.1002/etc.4604
(  0) 0) |
| [96] |
Zhalnina K, Louie K B, Hao Z, et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly[J]. Nature Microbiology, 2018, 3(4): 470-480. DOI:10.1038/s41564-018-0129-3
(  0) 0) |
| [97] |
Castrillo G, Teixeira P J P L, Paredes S H, et al. Root microbiota drive direct integration of phosphate stress and immunity[J]. Nature, 2017, 543(7646): 513-518. DOI:10.1038/nature21417
(  0) 0) |
| [98] |
de Vries F T, Griffiths R I, Knight C G, et al. Harnessing rhizosphere microbiomes for drought-resilient crop production[J]. Science, 2020, 368(6488): 270-274. DOI:10.1126/science.aaz5192
(  0) 0) |
| [99] |
Korenblum E, Dong Y H, Szymanski J, et al. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(7): 3874-3883. DOI:10.1073/pnas.1912130117
(  0) 0) |
| [100] |
Trivedi P, Leach J E, Tringe S G, et al. Plant-microbiome interactions: From community assembly to plant health[J]. Nature Reviews Microbiology, 2020, 18(11): 607-621. DOI:10.1038/s41579-020-0412-1
(  0) 0) |
| [101] |
Zheng T Y, Liu R, Chen J J, et al. Fire phoenix plant mediated microbial degradation of pyrene: Increased expression of functional genes and diminishing of degraded products[J]. Chemical Engineering Journal, 2021, 407: 126343. DOI:10.1016/j.cej.2020.126343
(  0) 0) |
 2022, Vol. 59
2022, Vol. 59


