2. 中国农业科学院农业资源与农业区划研究所/耕地培育技术国家工程实验室, 北京 100081;
3. 凤阳山-百山祖国家级自然保护区凤阳山管理处, 浙江龙泉 323700
2. Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences/National Engineering Laboratory for Improving Quality of Arable Land, Beijing 100081, China;
3. Fengyang Mountain Administration of Zhejiang Fengyang Mountain-Baishanzu National Nature Reserve, Longquan, Zhejiang 323700, China
自1750年以来,大气温室气体浓度的急剧增加导致全球平均气温升高了约1℃[1]。氧化亚氮(N2O)是造成全球变暖的主要温室气体之一,在百年尺度内,其单分子的全球增温潜势是二氧化碳(CO2)的265倍[2],并且参与破坏平流层中的保护性臭氧层[3]。自2007年至2016年间,全球N2O排放量为17.0 Tg·a–1,人为N2O排放量达到7.3 Tg·a–1[4]。世界气象组织全球大气监测最新数据表明,全球平均N2O含量在2018年创下了新高,达到331.1±0.1 ppb,相比于工业革命前(1750年前)增加了23%[5]。由于N2O在大气中的滞留周期长和增温潜势强,其浓度的持续上升对温室效应贡献将会进一步增加[2]。因此,N2O排放速率和大气中N2O浓度变化将在很大程度上影响未来气候变化,减少或有效控制N2O排放对缓解全球变化、改善生态环境具有重要的现实和科学意义。
氮(N)是限制植物生长、净初级生产力和其他生态过程的重要元素[6]。氮元素可通过生物固氮、氮沉降和人工施肥向陆地生态系统输入[7-8]。在自然环境下,生物固氮是活性氮的主要来源[9],但自工业革命以来,由于化石燃料燃烧、农林业施肥和土地利用变化,人为导致的活性氮沉降已成为不容忽视的N源[10]。全球无机氮沉降在1984—2016年期间从86.6 Tg·a−1增至93.6 Tg·a−1,增加了8%,尤其是在东亚和巴西南部地区更为明显[11]。我国是全球氮沉降最高的三个地区之一[12],氮沉降在过去三十年增长了约60%,速率已达到20.4±2.6 kg·hm−2·a−1[13-14]。随着人口剧增和经济发展,东亚、欧洲、南亚和北美地区消耗了世界80%以上的合成氮肥,氮肥的用量以每年1.85 Tg的速率增长,目前已达到107.6 Tg·a–1,且呈现持续增加的趋势[15]。氮输入在满足植物养分需求的同时会给森林生态系统健康和功能带来一些负面影响。例如,过量的氮输入会导致土壤酸化[16]、化学计量比失衡、减少生物多样性[17]和加剧氮损失(N2O排放和淋溶)[18]。据估计,高氮沉降和高氮肥施用的东亚地区人为N2O排放总量最高,达到1.5 Tg·a–1[4]。
土壤表层作为陆地上最大的氮库,储存了约136 Pg的氮[19],75%的N2O排放来源于陆地土壤[20]。因此,土壤氮库微小的变化都能对N2O通量产生巨大影响,高氮输入势必会影响土壤和生态系统的氮循环,进而影响土壤N2O排放。全球数据的meta分析结果表明,N输入显著增加了土壤无机氮淋溶(461%)、铵态氮(NH4+-N,47%)和硝态氮(NO3–-N,429%)浓度,使N2O排放增加了134%[18]。森林占陆地总面积的30.7%,约为40亿公顷[21]。森林土壤作为N2O的源和汇[22],在缓解全球变暖和调解大气中N2O浓度发挥着至关重要的作用。然而,为了满足日益增长的木材和林产品需求,过度的开垦利用和不合理的经营管理(过量N输入)使16%的森林出现生产力持续下降的趋势[23],增加了森林N2O排放,从而加剧了对温室效应的贡献。因此,深入理解森林土壤N2O对N输入的响应,对可持续发展和生态文明建设具有重要的理论与实践意义。
N2O底物浓度(NH4+-N和NO3–-N)、N2O来源途径(硝化和反硝化)和N2O相关微生物是决定土壤N2O排放的三个直接因素[24-25],植物主要通过影响这三个因素调控N2O排放。N输入会增加微生物硝化和反硝化的底物浓度,而植物通过根系或菌根吸收NH4+-N和NO3–-N降低N输入引起N2O底物增加,可以有效减少N引起的森林土壤N2O排放[26-27],尤其是在N限制生态系统。N输入可能促进或抑制植物凋落物的分解,影响土壤养分归还,从而改变森林土壤N2O排放[28-29]。由于森林生态系统较高的空间异质性,N输入引起的土壤理化性质变化会不同程度的影响植物和土壤微生物。因此,N输入对森林土壤N2O排放的影响存在很大的不确定性。野外试验表明N输入可促进[30]、抑制[31]和不影响[32]森林土壤N2O排放,但这些结果主要是基于特定环境条件下土壤N2O对N输入的响应,涉及到的背后机制错综复杂,调控机理不清楚。虽然目前已有N输入对陆地生态系统中N2O排放影响的综述和meta分析,但大多侧重于N2O排放对N输入的非线性响应[33-34]、农业生态系统中N输入对N2O排放的影响[35-37]和土壤理化性质变化对N2O排放的影响[38-39],而缺乏植物和微生物调控森林N2O排放的系统性研究。氮输入对N2O通量的影响取决于微生物硝化和反硝化,而主导N2O通量的微生物过程会随环境交替变化,这使得人们理解土壤N2O通量对N输入响应的背后机制更加困难。因此,本文基于森林土壤N2O排放的主要来源(硝化和反硝化),探讨N输入如何通过植物(根系N吸收、凋落物分解和丛枝菌根)和土壤微生物(微生物生物量和群落组成)调控N2O产生的不同途径从而影响森林土壤N2O排放,为森林生态系统在持续N输入背景下,更好地发挥其生态效益和制定相应的减缓措施提供理论依据。
1 森林土壤产生N2O的过程土壤N2O排放是由产生、转化和传输三个过程共同作用决定的。土壤通过硝化(nitrification)、反硝化(denitrification)、硝化微生物反硝化(nitrifier denitrification)、硝态氮异化还原成铵(dissimilatory nitrate reduction to ammonium,DNRA)、以及化学反硝化作用产生N2O。尽管土壤N2O的形成途径复杂多样,但一般认为微生物作用下的硝化和反硝化通常是森林土壤中N2O的主要来源(图 1),造成土壤N损失。
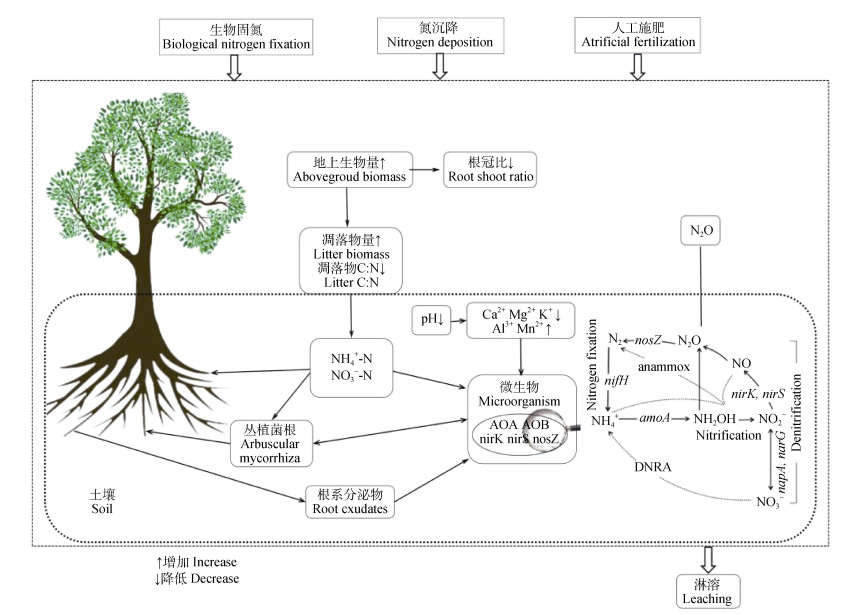
|
图 1 氮输入对森林土壤N2O排放影响的过程和机制 Fig. 1 Processes and mechanisms influencing impacts of nitrogen input on forest soil N2O emissions |
土壤硝化包括自养硝化和异养硝化。自养硝化是将铵(NH4+)或氨(NH3)氧化成硝酸盐(NO3–),并释放N2O作为副产物[40]。自养硝化的第一步是氨氧化过程也是自养硝化的限速步骤:氨氧化细菌(ammonia-oxidizing bacteria,AOB)或古菌(ammonia-oxidizing archaea,AOA)通过amoA基因编码的氨单加氧酶(AMO)将NH3或NH4+氧化成羟胺(NH2OH),NH2OH进一步在羟胺脱氢酶(HAO)催化下氧化为NO2–,至此氨氧化过程完成[20]。amoA基因由于其核苷酸序列高度保守以及在能量生成代谢中的重要作用,常用于AOA和AOB群落分子研究的标记基因[41]。自养硝化的第二步是亚硝酸氧化过程:亚硝酸氧化菌(NOB)通过亚硝酸盐氧化还原酶(NXR)将NO2–氧化为NO3–,自养硝化过程完成。异养硝化是微生物将有机氮氧化为NO3–,释放N2O的过程[42]。虽然有研究表明异养硝化在某些特定环境中对硝化的贡献可能较自养硝化更显著[43],但自养硝化作用通常是土壤中普遍存在的硝化过程。因此,本文主要讨论的是自养硝化即传统意义上的硝化,如无特殊说明,文中的硝化均指自养硝化。硝化作用主要是由好气性一类微生物进行的,在好氧条件下,森林土壤N2O排放过程以硝化为主。
反硝化是NO3–通过NO2–、NO和N2O依次还原成氮气(N2)的过程(NO3–→NO2–→NO→N2O→N2)[44]。首先,硝酸还原酶(NAR & NAP)将NO3–还原为NO2–[45],由厌氧微生物完成。第二步,由nirS或nirK基因编码的两种不同NO2–还原酶(NIR)将NO2–还原为NO[46]。由于含铜的亚硝酸盐还原酶(Cu-NIR;nirK编码)和含血红素cd1亚硝酸盐还原酶(cd1-NIR;nirS编码)之间是相互排斥的[47],反硝化微生物通常只含有其中一种基因,已知分离的反硝化细菌中含nirS基因的数量占NIR的3/4[48]。第三步,NO还原酶(NOR)将NO转化为N2O,这是一定条件下的最终产物。最后一步,由nosZ基因编码的N2O还原酶(NOS)将N2O还原为N2[49]。然而,约有1/3的反硝化细菌缺少nosZ基因,无法还原N2O为N2,导致其最终产物为N2O[50]。鉴于N2O是反硝化的中间产物或最终产物,土壤可能是N2O的源或汇,这取决于N2O还原酶的相对代谢活性。与硝化作用相反,在厌氧条件下,反硝化是森林土壤N2O的主要来源。此外,硝化细菌也可进行反硝化,是自养硝化细菌直接利用NH2OH产生的NO2–作为电子受体,进行反硝化的过程,通常发生在低氧环境[51-52]。
2 氮输入影响森林土壤N2O排放的生物机制森林土壤N2O通量受生物(植物和土壤微生物)和非生物因子(土壤养分、土壤pH、土壤湿度和温度)共同调控。氮输入可通过改变森林土壤理化性质不同程度的影响植物和土壤微生物,进而影响土壤N2O通量(图 1)。
2.1 植物 2.1.1 植物N吸收对森林土壤N2O通量的影响土壤NH4+-N和NO3–-N一方面作为植物可直接吸收利用的有效氮,另一方面还分别是微生物硝化和反硝化的底物[53]。因此,植物和硝化、反硝化微生物间的竞争作用会影响森林N2O排放。通常植物对氮的竞争吸收可能会缓解氮添加对N2O排放的促进作用,但在不同的土壤条件下有所不同。
植物氮吸收对N2O通量的影响通常会取决于土壤本身的氮条件。“N饱和”是指输入到生态系统的N超出微生物和植物的需求时,生态系统就会到达N饱和状态,从而引起植物和土壤碳氮等过程发生一系列变化[54]。N饱和按顺序区分了三个N响应阶段:第一阶段,植物在土壤N有效性低的条件下吸收N;第二阶段,持续的N累积导致非生物和生物N饱和;第三阶段,增加NO3–淋溶和气体排放[55]。
在N限制的森林生态系统中,植物根系与微生物间对N的利用存在激烈竞争,外源N输入改变了这种竞争关系。如果植物根系对外源N的竞争利用处于优势,N输入则无法改善微生物对有效N的利用,外源N主要被植物根系所吸收,不足以改变土壤硝化和反硝化速率以及N矿化速率。例如,N15同位素标记表明,N输入后,植物与微生物在竞争利用N素中处于优势[56],显著促进了植物生长,N刺激的N2O排放非常有限[26]。在非N限制的森林生态系统中,如果植物对外源N的竞争处于劣势,可被硝化细菌和反硝化细菌利用的有效N增加,硝化和反硝化增强,进而增加森林土壤N2O排放。有研究表明N有效性的增加有利于促进硝化菌活性并促进森林土壤N2O排放[57]。
氮饱和理论强调在N淋溶和气体损失发生之前,从植物到土壤微生物达到N饱和的时间动态,可以有效解释N输入前期对土壤N2O通量无显著影响[58]。氮输入一方面因硝化和反硝化底物浓度的升高而增加N2O排放;另一方面促进了植物根系对无机氮的吸收,从而减少N2O排放;植物与微生物对有效氮的竞争利用受土壤“氮状态”调控,从而影响不同生态系统N2O通量对N的响应。虽然已有大量的研究证明了N饱和理论,但实验和观测数据并不支持N损失的发生顺序,也有研究表明N输入初期,N2O通量迅速增长[59],这可能是因为N输入后同时向植物吸收、土壤保持、淋溶和气态氮的排放四个途径流动,符合Lovett等提出的动力学N饱和理论[60]。
植物对不同形态N的偏好性吸收对硝化和反硝化可能产生不同的影响。NH4+和NO3–在土壤中具有不同的迁移和扩散[61],植物吸收和同化NH4+较NO3–需要的能量少,而吸收NO3–可以维持植物体内的电荷平衡[62]。因此,植物会在吸收NH4+和NO3–之间进行权衡[63],从而偏爱不同的形态氮。植物对不同形态氮的偏好性吸收会影响土壤中NH4+和NO3–的浓度,从而对硝化和反硝化产生不同的影响[64-66],进而改变森林土壤N2O排放。目前,探究植物偏好性吸收对森林土壤N2O排放的研究还很少,背后机理尚不清楚,未来可关注植物N吸收策略对土壤N2O排放的影响,尤其是在植物相对单一的人工林中。
此外,N输入可促进植物的生长,增加根系对水分的吸收和植物的蒸腾作用[67-68],从而降低土壤水分,为硝化作用创造了有利条件,从而可能增加来自于微生物硝化产生的N2O。
2.1.2 凋落物分解对森林土壤N2O通量的影响凋落物分解是森林生态系统养分循环的关键过程,在局部、区域甚至全球范围内影响土壤C、N动态[69-70]。一方面,凋落物分解产生的氮素会影响土壤N2O排放,另一方面,凋落物分解产生的次生代谢产物也会对土壤N2O排放造成一定的影响。
凋落物分解速率决定了森林生态系统中的养分释放和土壤氮的有效性[71]。氮是调节凋落物分解的重要因素。氮输入能显著增加地上凋落物的数量和质量(C/N比降低)[71],但微生物生物量的减少和相关酶活性的抑制可能很大程度上限制凋落物的分解[72],从而影响土壤养分的释放和森林土壤N2O排放。Gao等[28]发现在凋落物分解过程中,易分解物质分解阶段对土壤N2O通量的贡献最大。然而,Zheng等[73]发现N输入显著影响凋落物移除时土壤N2O排放,而不影响凋落物保留时土壤N2O排放。目前有关凋落物分解对N2O排放影响的研究主要是通过添加和移除凋落物探究凋落物养分归还对土壤N2O排放的影响,缺乏N输入影响凋落物分解速率和养分归还间接影响土壤N2O排放的研究,而这种间接影响是广泛存在的,迫切需要进一步的研究。
此外,凋落物分解过程中释放的次生代谢产物会抑制土壤微生物的生长和活性,降低森林土壤N2O通量[73]。有研究表明植物分泌的硝化抑制化合物,例如游离脂肪酸、可以阻断AMO和HAO,从而抑制土壤N2O通量[74-75]。这表明植物凋落物也可通过分解过程中产生的次生代谢产物或者分泌物影响森林土壤N2O通量,这类植物的凋落物和分泌物在未来可以开发作为自然N2O抑制剂,达到节能减排的效果。
2.1.3 丛枝菌根对森林土壤N2O通量的影响丛枝菌根真菌(arbuscular mycorrhizal fungi,AMF)是一类非常广泛的土壤真菌,能与三分之二的陆地植物根系共生形成丛枝菌根(AM)[76],通过根外菌丝吸收土壤中的N和P供给宿主植物以改善植物营养吸收,并进一步影响土壤N2O排放[77]。
菌根可直接影响土壤N2O排放。菌根通常具有减少NH4+和/或NO3–可用性以及增加水分吸收的潜力,从而降低N2O的排放。如Storer等[27]发现与无丛枝菌根定殖的植物相比,AM菌丝减少了N输入引起的N2O排放,AM菌丝主要以NH4+的形式吸收无机氮,硝化作用是N2O的主要来源;而Cavagnaro等[78]试验表明在加入硝酸盐溶液后AM的形成显著促进植物生长和养分获取,但对N2O排放的影响较小。Lazcano等[79]发现AM共生提高了植物适应土壤水分变化的能力,有助于调节高土壤水分下的N2O排放,Ernfors等[64]也发现菌根对土壤N和水分的吸收,是减少N2O通量的主要因素。
菌根也可间接影响土壤N2O排放。一些研究者认为菌根形成的根外菌丝网络和分泌的胶黏性球囊霉素会促进土壤团聚体形成[80],而土壤团聚体是控制温室气体排放的重要因素[81]。因此,AMF可以通过土壤团聚体影响森林土壤N2O排放。事实上,Okiobe等[82]已发现AM菌丝促进了土壤团聚体的形成并改变了潜在反硝化活性(potential denitrification activities,PDA)。AMF也可通过改变N2O排放相关功能基因微生物群落,进而影响土壤N2O通量。例如,Bender等[83]试验发现AMF减少了土壤N2O的排放,AMF丰度与负责N2O生产的关键基因(nirK)呈负相关而与N2O消耗基因(nosZ)正相关,表明AMF对N2O排放的调节是通过改变N2O相关功能基因群落;Teutscherova等[84]发现AMF增加了AOB丰度但抑制了N输入后的N2O排放,AMF可能改变了AOB活性,而不是丰度。菌根类型是NO3–淋溶的重要预测因子,Midgley和Phillips[85]meta分析结果表明,N输入后以AM为主的森林NO3–淋溶高于以外生菌根(ectomycorrhizal,ECM)为主的森林,这是否意味着由于缺乏微生物反硝化的底物NO3–,以AM为主的森林可能比ECM主导的森林N2O排放小,还需要进一步试验验证。可见,菌根可通过吸收有效氮和水分、促进团聚体形成以及改变N2O相关功能基因群落影响土壤N2O排放。
目前,探究菌根对N2O通量影响的试验大部分是通过盆栽或短期N输入得到的结果,缺乏N输入对菌根(菌根定殖率、根外菌丝长度密度和球囊霉素)的定量分析,具有一定的局限性。有研究表明,土壤中的根外菌丝长度密度和菌根定殖率随N的增加而显著降低[86]。这表明N输入可能会改变根系和菌根真菌的共生关系,降低AMF减少N2O排放的潜力,增加土壤N2O排放,这需要在野外进行长期N输入试验,探究N输入如何影响菌根,进而改变森林土壤N2O通量。
综上所述,植物可通过多种途径影响土壤N2O通量对N的响应,主要有:(1)根系对N2O底物的竞争性吸收;(2)植物凋落物分解过程的养分归还和次生代谢产物释放;(3)根系与真菌形成丛枝菌根(吸收有效氮和水分、促进团聚体形成以及改变N2O相关功能基因群落)。基于植物在调控土壤N2O通量的重要作用,植物对N输入的响应可以作为预测N2O通量的因子。事实上,Wolf等[87]发现N2O通量与总凋落物量和树木断面积增量密切相关,森林生产力可以作为N2O通量有效的预测因子。因此,在N输入日益增加的生态系统中,预测温室气体通量时应将植物作为解释变量纳入模型,提高模型预测准确性,尤其是在N限制的森林生态系统。
2.2 土壤微生物土壤微生物在地球生物化学循环中起着至关重要的作用,负责生态系统中土壤C、N和养分循环。微生物固氮是森林土壤中N素截留的主要机制之一。氮输入一方面会影响土壤微生物生物量,另一方面会改变微生物群落组成,从而影响土壤N2O排放。
大量的研究表明,N输入通常会降低微生物生物量[88],从而减少微生物固氮,增加N损失风险[89]。Wang等[90]发现氮输入降低了杉木人工林的微生物生物量,且与NH4+-N和NO3–-N含量呈负相关,这表明过量的N输入降低了微生物生物量。Hall和Matson [57]结果表明由于微生物的固氮作用的下降,显著增加了热带森林的N2O排放。微生物生物量的减少可能是由多种途径导致:首先,N-诱导的土壤酸化会消耗土壤中的碱基阳离子(Ca2+、Mg2+、K+、Na+)提高非碱基阳离子(Mn2+和Al3+)的浓度[91],而Al3+的毒害作用和Ca2+、Mg2+限制不利于土壤微生物生长和繁殖[92];其次,N输入增加的土壤无机氮会与土壤有机质发生反应,导致顽固性化合物的积累[93],不利于微生物生长[94];N输入改变了植物的养分分配,植物增加对地上部分的能量和养分输入而降低地下部分的输入,通常会降低根冠比,减少根系分泌物[95],而易代谢有机化合物的根系分泌物减少会造成微生物养分限制[96];最后,N输入会限制β-葡萄糖苷酶在矿质土壤中的活性和减少木质素分解氧化酶产生[97],导致微生物C限制[98]。
N输入会改变微生物群落组成,从而影响森林土壤N2O排放。细菌是N2O的主要生产者[99],部分真菌也会产生N2O[100]。细菌和真菌的平均生物量C/N比分别为5和15[101],在施N条件下,由于真菌对N的需求量比细菌低,真菌较细菌对N输入更敏感,真菌与细菌的比值可能会降低[102]。在白桦人工林中,真菌与细菌的比值和N2O间接相关[103]。Aronson和Allison[38]发现,短期N输入会增加N2O排放,但随着时间的推移,土壤微生物可能会改变组成或适应N富集,过量的N导致优势微生物的生存策略发生转变,或有利于更富营养型的微生物群落生存,从而抑制对进一步N输入增加的响应,长期N输入对森林土壤N2O排放并不会持续增加,可能会保持在一个相对稳定的状态。氮输入对微生物生长、群落组成的影响是极其复杂的,对这一过程尚不清楚,但不可否认的是过量的N输入会改变微生物群落组成[104]。尽管通过技术手段可监测微生物群落组成的变化,但无法量化因微生物群落组成变化引起的土壤N2O通量。
3 氮输入影响N2O产生的不同途径氮输入对N2O通量的影响是微生物硝化和反硝化共同作用的结果,在不同森林生态系统中主导N2O通量的微生物过程会随环境和N2O相关功能基因丰度的变化而交替变化,这使得人们理解土壤N2O通量对N输入响应的背后机制更加困难。因此,探究生态系统中氮输入引起N2O不同产生途径的变化有助于明确调节土壤N2O通量的主要因子以及制定相关减排措施。
3.1 土壤湿度和N2O底物浓度影响N2O产生的不同途径氮输入引起N2O变化的来源在不同区域有所不同。土壤水分可控制氧气有效性,进而影响微生物硝化和反硝化。Weitz等[105]发现,硝化作用随土壤含水量的增加而降低,并预计在约70%土壤充水孔隙度(water-filled pore space,WFPS)停止。Carter[106]发现在35%~45%WFPS,硝化作用的增强是N2O排放在N输入初期增加的主要来源。Mathieu等[107]结果表明在田间含水量不饱和条件下(75%),60%的N2O来自硝化作用,而在田间含水量饱和条件下(150%),反硝化作用的贡献约为85%~90%。通常在较为干旱的地区,氮输入引起的N2O排放来源于硝化[108-109],而较为湿润的地区,反硝化是其主要来源[110-111]。例如,Müller等[32]的15N示踪试验表明,反硝化是不同海拔地区热带森林N2O的主要来源;Eickenscheidt等[112]在温带红松林中用15N标记发现,N短期刺激的N2O来源于微生物硝化且以自养硝化为主。热带具有较高的降水量为微生物反硝化提供了厌氧条件,反硝化作用占主导地位,而在相对干燥的温带地区,土壤WFPS较低,硝化作用可能占主导地位。然而,也有研究表明硝化在热带地区N2O排放中占主导地位[113],反硝化是温带地区的主要来源[114]。因此,土壤湿度可以一定程度上影响N2O通量的微生物过程,产生N2O的不同微生物过程同时还受N2O底物浓度和N2O相关功能基因调控。
不同形态N输入可直接影响土壤NH4+-N和NO3–-N的浓度,进而影响微生物硝化和反硝化。Storer等[27]结果表明,添加(NH4)2SO4显著促进了N2O排放,而添加KNO3未改变N2O排放,表明N输入引起的N2O排放主要来源于硝化。Xu等[115]发现添加NH4NO3、NaNO3和(NH4)2SO4均显著促进温带森林N2O排放,无法明确N2O来源的微生物过程。这可能是由于NH4+-N和NO3–-N在相互转换过程中均会伴随N2O的产生。N2O底物浓度的变化是由NH4+和NO3–的产生和消耗决定,NH4+通过自养硝化产生NO3–,有机氮通过异养硝化也可生成NO3–,而输入的NO3–可以通过DNRA转化为NH4+,随着时间的推移,土壤中NH4+和NO3–的浓度会达到相对平衡的一种状态。
3.2 N2O相关功能基因丰度影响N2O产生的不同途径N2O相关功能基因(AOB、AOA、nirK、nirS和nosZ)的丰度通常用于探索微生物硝化和反硝化对环境变化的响应。氮有效性和pH是调节森林土壤AOB和AOA相对丰度的主要因素[116]。研究表明,硝化速率通常与土壤pH呈正相关[117],当土壤pH < 5时可以显著抑制硝化作用,反硝化是好氧条件下N2O的主要来源[118],这可能是因为低pH抑制了AOA和AOB微生物群落。由此可见,反硝化主导热带地区N2O排放并不单单是因为较高的降雨量形成的厌氧环境,可能还与热带地区较低的pH有关。氮输入通过改变N有效性和土壤pH影响AOA和AOB群落的丰度和组成[119]。相比于AOB,AOA体积较小,具有更宽的表面体积比,对氨的亲和力更强,AOA的氨单加氧酶很容易被底物饱和[120]。因此,AOB较AOA更能耐受高NH4+浓度和高pH条件。由于AOB和AOA生理或代谢之间的差异可能导致在土壤中的生态位分化[121],对N输入的响应可能不同。Meta分析结果表明,N输入显著增加AOA(27%)和AOB(326%)的丰度,AOB在低中性土壤中对N输入响应最大,因为相比于高土壤pH,在低pH土壤中AOB很难吸收NH4+[122]。Di等[123]发现N输入增加了AOB的丰度而不是AOA,并且与硝化速率线性相关。Carey等[122]结果表明土壤硝化势的增加与AOB丰度显著正相关,N输入主要通过增加AOB种群大小来增加土壤硝化势。然而,有证据表明,在一些土壤中,由于某些AOA的嗜酸性[124],AOA可能主导氨氧化。在天然柏木栎林中,AOA丰度高于AOB[125],而在针叶林和落叶阔叶林土壤中AOB较AOA丰富8倍~18倍[126],表明森林生态系统负责硝化的微生物种群会因土壤环境的不同而改变。N输入后土壤pH和NH4+浓度的变化可能改变主导硝化的氨氧化微生物群落,从而影响硝化速率。反硝化基因的丰度与反硝化速率和N2O通量有关。生产N2O(nirS、nirK)和消耗N2O(nosZ)基因之间的差异可以为预测N2O排放提供一种有用的方法。在森林生态系统中,nosZ基因的丰度明显高于nirS,而在农业生态系统,nirS的丰度较nosZ高出四个数量级[127],这可能是农业生态系统中N2O排放高于森林生态系统的部分原因。Szukics等[128]结果表明,在有机碳含量较低(3.8%)的土壤中施N后迅速改变了N2O排放率,但不影响nirK基因的丰度;在高WFPS(70%)和高初始有机碳(16.0%)的土壤表现出较高的nirK基因丰度。这表明当N输入提高土壤有效氮浓度时,如果水分和土壤C水平有利于反硝化作用的发生,反硝化基因丰度增加,从而增加来源于反硝化的N2O。Kandeler等[129]发现在N限制的云杉林中N输入虽然增加了土壤中的NH4+-N但并不影响反硝化基因丰度和N2O排放,可能是反硝化受到其它因子的限制。Raut等[130]发现,N输入显著提高了酸性土壤的N2O排放速率,由于在反硝化过程中,较低的土壤pH可能导致N2O还原酶故障,从而导致较高的N2O/N2比,而在中性或者碱性土壤中,N2是较N2O更重要的反硝化产物[131]。目前,研究人员通常是用N2O排放相关功能基因丰度的变化来反映产生N2O不同的微生物途径,可N2O排放相关功能基因丰度的变化与N2O通量的变化,并不总是耦合的,可能会受到其它因素的影响,无法反映N2O来源的微生物过程。例如,Tang等[132]发现N输入增加了温带森林的土壤潜在硝化活性(potential denitrification activities,PDA),但是降低了土壤PDA,PNA和PDA的变化与NH4+-N和pH密切相关,而与硝化和反硝化功能基因丰度无关。此外,在测量N2O相关功能基因丰度的过程中,只有极少量的土壤被用来提取DNA,将极少量土壤的结果代表野外试验的结果会削弱其代表性。因此,为了提高取样的代表性,须充分混合土样,以求捕捉土壤的异质性。
4 结论氮输入对森林土壤N2O的不同影响取决于N输入后植物和土壤微生物对土壤理化性质变化的响应。植物通过根系(无机氮、水分和分泌物)、植物凋落物(养分归还和次生代谢产物)以及与真菌形成丛枝菌根(无机氮、水分、团聚体和N2O相关功能基因群落)多种途径影响土壤N2O通量对N的响应。长期的N输入不利于微生物固氮,增加了N2O排放的风险。植物与微生物对N的竞争利用受土壤氮有效性调控,进而影响森林土壤N2O排放。N诱导的森林土壤N2O排放主要来源于微生物硝化和反硝化。土壤湿度和N2O底物浓度的变化会不同程度的影响N2O不同产生途径。N输入可通过改变土壤pH和氮有效性影响N2O相关功能基因丰度,从而影响N2O不同产生途径。植物氮吸收、凋落物分解、菌根以及N2O产生途径应作为预测N2O通量的解释变量纳入模型,以提高模型预测准确性。本文主要是以微生物硝化和反硝化作为N2O的主要来源展开讨论,而忽略了其他N2O来源(硝化反硝化和DNRA)以及其他与硝化和反硝化底物相关的N途径,例如,氮矿化和淋溶。未来可通过多种技术手段结合,尽可能的剔除和量化其他N转换途径影响产生N2O的过程,揭示N输入对不同森林生态系统土壤N2O排放影响背后的机理,为全球变化背景下制订森林管理政策和制定温室气体减排措施提供科学依据。
| [1] |
IPCC. Summary for policymakers//Global warming of 1.5 ℃[M]. Geneva Switzerland: IPCC, 2018.
(  0) 0) |
| [2] |
IPCC. Climate change 2014: Synthesis report//Contribution of working groups Ⅰ, Ⅱ and Ⅲ to the fifth assessment report of the intergovernmental panel on climate change[M]. Geneva Switzerland: IPCC, 2014.
(  0) 0) |
| [3] |
Ravishankara A R, Daniel J S, Portmann R W. Nitrous oxide(N2O): The dominant ozone-depleting substance emitted in the 21st century[J]. Science, 2009, 326(5949): 123-125. DOI:10.1126/science.1176985
(  0) 0) |
| [4] |
Tian H Q, Xu R T, Canadell J G, et al. A comprehensive quantification of global nitrous oxide sources and sinks[J]. Nature, 2020, 586(7828): 248-256. DOI:10.1038/s41586-020-2780-0
(  0) 0) |
| [5] |
WMO. Statement on the state of the global climate in 2019. Geneva Switzerland, 2020.
(  0) 0) |
| [6] |
Zhang L. Camellia oleifera plantation management and nitrogen cycling (In Chinese). Beijing: Science Press, 2020. [张令. 油茶林经营与土壤氮循环[M]. 北京: 科学出版社, 2020.]
(  0) 0) |
| [7] |
Fowler D, Pyle J A, Raven J A, et al. The global nitrogen cycle in the twenty-first century: Introduction[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2013, 368(1621): 20130165. DOI:10.1098/rstb.2013.0165
(  0) 0) |
| [8] |
Wang R, Balkanski Y, Boucher O, et al. Significant contribution of combustion-related emissions to the atmospheric phosphorus budget[J]. Nature Geoscience, 2015, 8(1): 48-54. DOI:10.1038/ngeo2324
(  0) 0) |
| [9] |
Zhu X M, Zhang W, Chen H, et al. Impacts of nitrogen deposition on soil nitrogen cycle in forest ecosystems: A review[J]. Acta Ecologica Sinica, 2015, 35(3): 35-43. DOI:10.1016/j.chnaes.2015.04.004
(  0) 0) |
| [10] |
Liu D N, Guo X D, Xiao B W. What causes growth of global greenhouse gas emissions? Evidence from 40 countries[J]. Science of the Total Environment, 2019, 661: 750-766. DOI:10.1016/j.scitotenv.2019.01.197
(  0) 0) |
| [11] |
Ackerman D, Millet D B, Chen X. Global estimates of inorganic nitrogen deposition across four decades[J]. Global Biogeochemical Cycles, 2019, 33(1): 100-107. DOI:10.1029/2018GB005990
(  0) 0) |
| [12] |
Galloway J N, Cowling E B. Reactive nitrogen and the world: 200 years of change[J]. AMBIO: A Journal of the Human Environment, 2002, 31(2): 64-71. DOI:10.1579/0044-7447-31.2.64
(  0) 0) |
| [13] |
Liu X J, Zhang Y, Han W X, et al. Enhanced nitrogen deposition over China[J]. Nature, 2013, 494(7438): 459-462. DOI:10.1038/nature11917
(  0) 0) |
| [14] |
Yu G R, Jia Y L, He N P, et al. Stabilization of atmospheric nitrogen deposition in China over the past decade[J]. Nature Geoscience, 2019, 12(6): 424-429. DOI:10.1038/s41561-019-0352-4
(  0) 0) |
| [15] |
Lu C Q, Tian H Q. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance[J]. Earth System Science Data, 2017, 9(1): 181-192. DOI:10.5194/essd-9-181-2017
(  0) 0) |
| [16] |
Tian D S, Niu S L. A global analysis of soil acidification caused by nitrogen addition[J]. Environmental Research Letters, 2015, 10(2): 024019. DOI:10.1088/1748-9326/10/2/024019
(  0) 0) |
| [17] |
Ochoa-Hueso R. Nonlinear disruption of ecological interactions in response to nitrogen deposition[J]. Ecology, 2016, 97(10): 2802-2814. DOI:10.1002/ecy.1521
(  0) 0) |
| [18] |
Lu M, Yang Y H, Luo Y Q, et al. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis[J]. New Phytologist, 2011, 189(4): 1040-1050. DOI:10.1111/j.1469-8137.2010.03563.x
(  0) 0) |
| [19] |
Oertel C, Matschullat J, Zurba K, et al. Greenhouse gas emissions from soils—A review[J]. Geochemistry, 2016, 76(3): 327-352. DOI:10.1016/j.chemer.2016.04.002
(  0) 0) |
| [20] |
Kuypers M M M, Marchant H K, Kartal B. The microbial nitrogen-cycling network[J]. Nature Reviews Microbiology, 2018, 16(5): 263-276. DOI:10.1038/nrmicro.2018.9
(  0) 0) |
| [21] |
FAO. The state of the world's forests 2018-forest pathways to sustainable development: Rome 2018.
(  0) 0) |
| [22] |
Zhang K R, Zhu Q A, Liu J X, et al. Spatial and temporal variations of N2O emissions from global forest and grassland ecosystems[J]. Agricultural and Forest Meteorology, 2019, 266/267: 129-139. DOI:10.1016/j.agrformet.2018.12.011
(  0) 0) |
| [23] |
United Nations Department of Economic and Social Affairs. The Sustainable Development Goals Report 2018 . New York: United Nations, 2018.
(  0) 0) |
| [24] |
Butterbach-Bahl K, Baggs E M, Dannenmann M, et al. Nitrous oxide emissions from soils: How well do we understand the processes and their controls?[J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2013, 368(1621): 20130122. DOI:10.1098/rstb.2013.0122
(  0) 0) |
| [25] |
Deng B L, Wang S L, Xu X T, et al. Effects of biochar and dicyandiamide combination on nitrous oxide emissions from Camellia oleifera field soil[J]. Environmental Science and Pollution Research, 2019, 26(4): 4070-4077. DOI:10.1007/s11356-018-3900-3
(  0) 0) |
| [26] |
Davidson E A, Reis de Carvalho C J, Vieira I C G, et al. Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest[J]. Ecological Applications, 2004, 14(sp4): 150-163. DOI:10.1890/01-6006
(  0) 0) |
| [27] |
Storer K, Coggan A, Ineson P, et al. Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots[J]. New Phytologist, 2018, 220(4): 1285-1295. DOI:10.1111/nph.14931
(  0) 0) |
| [28] |
Gao J B, Zhou W J, Liu Y T, et al. Effects of litter inputs on N2O emissions from a tropical rainforest in southwest China[J]. Ecosystems, 2018, 21(5): 1013-1026. DOI:10.1007/s10021-017-0199-8
(  0) 0) |
| [29] |
Mo J M, Brown S, Xue J H, et al. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China[J]. Plant and Soil, 2006, 282(1/2): 135-151.
(  0) 0) |
| [30] |
Koehler B, Corre M D, Veldkamp E, et al. Immediate and long-term nitrogen oxide emissions from tropical forest soils exposed to elevated nitrogen input[J]. Global Change Biology, 2009, 15(8): 2049-2066. DOI:10.1111/j.1365-2486.2008.01826.x
(  0) 0) |
| [31] |
Steudler P A, Garcia-Montiel D C, Piccolo M C, et al. Trace gas responses of tropical forest and pasture soils to N and P fertilization[J]. Global Biogeochemical Cycles, 2002, 16(2): 7-1-7-12.
(  0) 0) |
| [32] |
Müller A K, Matson A L, Corre M D, et al. Soil N2O fluxes along an elevation gradient of tropical montane forests under experimental nitrogen and phosphorus addition[J]. Frontiers in Earth Science, 2015, 3: 66.
(  0) 0) |
| [33] |
Kim D G, Hernandez-Ramirez G, Giltrap D. Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: A meta-analysis[J]. Agriculture, Ecosystems & Environment, 2013, 168: 53-65.
(  0) 0) |
| [34] |
Shcherbak I, Millar N, Robertson G P. Global metaanalysis of the nonlinear response of soil nitrous oxide(N2O)emissions to fertilizer nitrogen[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(25): 9199-9204. DOI:10.1073/pnas.1322434111
(  0) 0) |
| [35] |
Aliyu G, Sanz-Cobena A, Müller C, et al. A meta-analysis of soil background N2O emissions from croplands in China shows variation among climatic zones[J]. Agriculture, Ecosystems & Environment, 2018, 267: 63-73.
(  0) 0) |
| [36] |
Zhou M H, Zhu B, Wang S J, et al. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis[J]. Global Change Biology, 2017, 23(10): 4068-4083. DOI:10.1111/gcb.13648
(  0) 0) |
| [37] |
He G, Li X, Zhao R T, et al. Mechanisms underlying the regulation of soil nitrous oxide emissions by arbuscular mycorrhizal fungi (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(1): 23-30. [何广, 李侠, 赵若桐, 等. 丛枝菌根真菌调控土壤氧化亚氮排放的机制[J]. 土壤学报, 2021, 58(1): 23-30.]
(  0) 0) |
| [38] |
Aronson E L, Allison S D. Meta-analysis of environmental impacts on nitrous oxide release in response to N amendment[J]. Frontiers in Microbiology, 2012, 3: 272.
(  0) 0) |
| [39] |
Fang H J, Cheng S L, Yu G R, et al. Study on the responses of nitrous oxide emission to increased nitrogen deposition in forest soils: A review (In Chinese)[J]. Acta Pedologica Sinica, 2015, 52(2): 262-271. [方华军, 程淑兰, 于贵瑞, 等. 森林土壤氧化亚氮排放对大气氮沉降增加的响应研究进展[J]. 土壤学报, 2015, 52(2): 262-271.]
(  0) 0) |
| [40] |
Wrage N, Velthof G L, van Beusichem M L, et al. Role of nitrifier denitrification in the production of nitrous oxide[J]. Soil Biology & Biochemistry, 2001, 33(12/13): 1723-1732.
(  0) 0) |
| [41] |
Norton J M, Alzerreca J J, Suwa Y, et al. Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria[J]. Archives of Microbiology, 2002, 177(2): 139-149. DOI:10.1007/s00203-001-0369-z
(  0) 0) |
| [42] |
Zhang J B, Müller C, Cai Z C. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils[J]. Soil Biology & Biochemistry, 2015, 84: 199-209.
(  0) 0) |
| [43] |
Stange C F, Spott O, Arriaga H, et al. Use of the inverse abundance approach to identify the sources of NO and N2O release from Spanish forest soils under oxic and hypoxic conditions[J]. Soil Biology & Biochemistry, 2013, 57: 451-458.
(  0) 0) |
| [44] |
Di H J, Cameron K C, Podolyan A, et al. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil[J]. Soil Biology & Biochemistry, 2014, 73: 59-68.
(  0) 0) |
| [45] |
Tavares P, Pereira A S, Moura J J G, et al. Metalloenzymes of the denitrification pathway[J]. Journal of Inorganic Biochemistry, 2006, 100(12): 2087-2100. DOI:10.1016/j.jinorgbio.2006.09.003
(  0) 0) |
| [46] |
Murugapiran S K, Huntemann M, Wei C L, et al. Thermus oshimai JL-2 and T. thermophilus JL-18 genome analysis illuminates pathways for carbon, nitrogen, and sulfur cycling[J]. Standards in Genomic Sciences, 2013, 7(3): 449-468. DOI:10.4056/sigs.3667269
(  0) 0) |
| [47] |
Hu H W, Chen D L, He J Z. Microbial regulation of terrestrial nitrous oxide formation: Understanding the biological pathways for prediction of emission rates[J]. FEMS Microbiology Reviews, 2015, 39(5): 729-749. DOI:10.1093/femsre/fuv021
(  0) 0) |
| [48] |
Bothe H, Jost G, Schloter M, et al. Molecular analysis of ammonia oxidation and denitrification in natural environments[J]. FEMS Microbiology Reviews, 2000, 24(5): 673-690. DOI:10.1111/j.1574-6976.2000.tb00566.x
(  0) 0) |
| [49] |
Henry S, Bru D, Stres B, et al. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils[J]. Applied and Environmental Microbiology, 2006, 72(8): 5181-5189. DOI:10.1128/AEM.00231-06
(  0) 0) |
| [50] |
Philippot L, Andert J, Jones C M, et al. Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil[J]. Global Change Biology, 2011, 17(3): 1497-1504. DOI:10.1111/j.1365-2486.2010.02334.x
(  0) 0) |
| [51] |
Wrage N, Velthof G L, van Beusichem M L, et al. Role of nitrifier denitrification in the production of nitrous oxide[J]. Soil Biology & Biochemistry, 2001, 33(12/13): 1723-1732.
(  0) 0) |
| [52] |
Wrage-Mönnig N, Horn M A, Well R, et al. The role of nitrifier denitrification in the production of nitrous oxide revisited[J]. Soil Biology & Biochemistry, 2018, 123: A3-A16.
(  0) 0) |
| [53] |
Robertson G P. Nitrification and denitrification in humid tropical ecosystems: potential controls on nitrogen retention[J]. Mineral Nutrients in Tropical Forest & Savanna Ecosystems, 1989, 9: 55-69.
(  0) 0) |
| [54] |
Zhang W, Mo J M, Yu G R, et al. Emissions of nitrous oxide from three tropical forests in Southern China in response to simulated nitrogen deposition[J]. Plant and Soil, 2008, 306(1/2): 221-236.
(  0) 0) |
| [55] |
Aber J D, Nadelhoffer K J, Steudler P, et al. Nitrogen saturation in northern forest ecosystems: Excess nitrogen from fossil fuel combustion may stress the biosphere[J]. BioScience, 1989, 39(6): 378-386. DOI:10.2307/1311067
(  0) 0) |
| [56] |
Kaye J P, Hart S C. Competition for nitrogen between plants and soil microorganisms[J]. Trends in Ecology & Evolution, 1997, 12(4): 139-143.
(  0) 0) |
| [57] |
Hall S J, Matson P A. Nutrient status of tropical rain forests influences soil N dynamics after N additions[J]. Ecological Monographs, 2003, 73(1): 107-129. DOI:10.1890/0012-9615(2003)073[0107:NSOTRF]2.0.CO;2
(  0) 0) |
| [58] |
Borken W, Beese F. Control of nitrous oxide emissions in European beech, Norway spruce and Scots pine forests[J]. Biogeochemistry, 2005, 76(1): 141-159. DOI:10.1007/s10533-005-2901-8
(  0) 0) |
| [59] |
Erickson H, Keller M, Davidson E A. Nitrogen oxide fluxes and nitrogen cycling during postagricultural succession and forest fertilization in the humid tropics[J]. Ecosystems, 2001, 4(1): 67-84. DOI:10.1007/s100210000060
(  0) 0) |
| [60] |
Lovett G M, Goodale C L. A new conceptual model of nitrogen saturation based on experimental nitrogen addition to an oak forest[J]. Ecosystems, 2011, 14(4): 615-631. DOI:10.1007/s10021-011-9432-z
(  0) 0) |
| [61] |
Brady N C, Weil R R. Elements of the nature and properties of soils . 3rd ed[M]. New Jersey: Pearson Prentice Hall, 2010.
(  0) 0) |
| [62] |
Boudsocq S, Niboyet A, Lata J C, et al. Plant preference for ammonium versus nitrate: A neglected determinant of ecosystem functioning?[J]. The American Naturalist, 2012, 180(1): 60-69. DOI:10.1086/665997
(  0) 0) |
| [63] |
Maire V, Gross N, da Silveira Pontes L, et al. Trade-off between root nitrogen acquisition and shoot nitrogen utilization across 13 co-occurring pasture grass species[J]. Functional Ecology, 2009, 23(4): 668-679. DOI:10.1111/j.1365-2435.2009.01557.x
(  0) 0) |
| [64] |
Ernfors M, Rütting T, Klemedtsson L. Increased nitrous oxide emissions from a drained organic forest soil after exclusion of ectomycorrhizal mycelia[J]. Plant and Soil, 2011, 343(1/2): 161-170.
(  0) 0) |
| [65] |
Hawkes C V, Wren I F, Herman D J, et al. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community[J]. Ecology Letters, 2005, 8(9): 976-985. DOI:10.1111/j.1461-0248.2005.00802.x
(  0) 0) |
| [66] |
Lata J C, Guillaume K, Degrange V, et al. Relationships between root density of the African grass Hyparrhenia diplandra and nitrification at the decimetric scale: An inhibition-stimulation balance hypothesis[J]. Proceedings Biological Sciences, 2000, 267(1443): 595-600. DOI:10.1098/rspb.2000.1043
(  0) 0) |
| [67] |
Lu X K, Vitousek P M, Mao Q G, et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(20): 5187-5192. DOI:10.1073/pnas.1720777115
(  0) 0) |
| [68] |
Jiang J, Wang Y P, Yang Y H, et al. Interactive effects of nitrogen and phosphorus additions on plant growth vary with ecosystem type[J]. Plant and Soil, 2019, 440(1/2): 523-537.
(  0) 0) |
| [69] |
Liu J, Wu N N, Wang H, et al. Nitrogen addition affects chemical compositions of plant tissues, litter and soil organic matter[J]. Ecology, 2016, 97(7): 1796-1806. DOI:10.1890/15-1683.1
(  0) 0) |
| [70] |
Porre R J, van der Werf W, de Deyn G B, et al. Is litter decomposition enhanced in species mixtures? A meta-analysis[J]. Soil Biology & Biochemistry, 2020, 145: 107791.
(  0) 0) |
| [71] |
Zhang T A, Luo Y Q, Chen H Y H, et al. Responses of litter decomposition and nutrient release to N addition: A meta-analysis of terrestrial ecosystems[J]. Applied Soil Ecology, 2018, 128: 35-42. DOI:10.1016/j.apsoil.2018.04.004
(  0) 0) |
| [72] |
Xiao W, Chen X, Jing X, et al. A meta-analysis of soil extracellular enzyme activities in response to global change[J]. Soil Biology & Biochemistry, 2018, 123: 21-32.
(  0) 0) |
| [73] |
Zheng X, Liu Q, Zheng L Y, et al. Litter removal enhances soil N2O emissions: Implications for management of leaf-harvesting Cinnamomum camphora plantations[J]. Forest Ecology and Management, 2020, 466: 118121. DOI:10.1016/j.foreco.2020.118121
(  0) 0) |
| [74] |
Subbarao G V, Nakahara K, Ishikawa T, et al. Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification[J]. Plant and Soil, 2008, 313(1/2): 89-99.
(  0) 0) |
| [75] |
Zakir H A K M, Subbarao G V, Pearse S J, et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl)propionate, responsible for biological nitrification inhibition by sorghum(Sorghum bicolor)[J]. New Phytologist, 2008, 180(2): 442-451. DOI:10.1111/j.1469-8137.2008.02576.x
(  0) 0) |
| [76] |
Smith S E, Smith F A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales[J]. Annual Review of Plant Biology, 2011, 62(1): 227-250. DOI:10.1146/annurev-arplant-042110-103846
(  0) 0) |
| [77] |
Hodge A, Storer K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems[J]. Plant and Soil, 2015, 386(1/2): 1-19.
(  0) 0) |
| [78] |
Cavagnaro T R, Barrios-Masias F H, Jackson L E. Arbuscular mycorrhizas and their role in plant growth, nitrogen interception and soil gas efflux in an organic production system[J]. Plant and Soil, 2012, 353(1/2): 181-194.
(  0) 0) |
| [79] |
Lazcano C, Barrios-Masias F H, Jackson L E. Arbuscular mycorrhizal effects on plant water relations and soil greenhouse gas emissions under changing moisture regimes[J]. Soil Biology & Biochemistry, 2014, 74: 184-192.
(  0) 0) |
| [80] |
Zhang J, Tang X L, He X H, et al. Glomalin-related soil protein responses to elevated CO2 and nitrogen addition in a subtropical forest: Potential consequences for soil carbon accumulation[J]. Soil Biology & Biochemistry, 2015, 83: 142-149.
(  0) 0) |
| [81] |
Chen Z J, Setälä H, Geng S C, et al. Nitrogen addition impacts on the emissions of greenhouse gases depending on the forest type: A case study in Changbai Mountain, Northeast China[J]. Journal of Soils and Sediments, 2017, 17(1): 23-34. DOI:10.1007/s11368-016-1481-7
(  0) 0) |
| [82] |
Okiobe S T, Augustin J, Mansour I, et al. Disentangling direct and indirect effects of mycorrhiza on nitrous oxide activity and denitrification[J]. Soil Biology & Biochemistry, 2019, 134: 142-151.
(  0) 0) |
| [83] |
Bender S F, Plantenga F, Neftel A, et al. Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil[J]. The ISME Journal, 2014, 8(6): 1336-1345. DOI:10.1038/ismej.2013.224
(  0) 0) |
| [84] |
Teutscherova N, Vazquez E, Arango J, et al. Native arbuscular mycorrhizal fungi increase the abundance of ammonia-oxidizing bacteria, but suppress nitrous oxide emissions shortly after urea application[J]. Geoderma, 2019, 338: 493-501. DOI:10.1016/j.geoderma.2018.09.023
(  0) 0) |
| [85] |
Midgley M G, Phillips R P. Mycorrhizal associations of dominant trees influence nitrate leaching responses to N deposition[J]. Biogeochemistry, 2014, 117(2/3): 241-253.
(  0) 0) |
| [86] |
Li L, McCormack M L, Chen F S, et al. Different responses of absorptive roots and arbuscular mycorrhizal fungi to fertilization provide diverse nutrient acquisition strategies in Chinese fir[J]. Forest Ecology and Management, 2019, 433: 64-72. DOI:10.1016/j.foreco.2018.10.055
(  0) 0) |
| [87] |
Wolf K, Veldkamp E, Homeier J, et al. Nitrogen availability links forest productivity, soil nitrous oxide and nitric oxide fluxes of a tropical montane forest in southern Ecuador[J]. Global Biogeochemical Cycles, 2011, 25(4): GB4009.
(  0) 0) |
| [88] |
Zhang T A, Chen H Y H, Ruan H H. Global negative effects of nitrogen deposition on soil microbes[J]. The ISME Journal, 2018, 12(7): 1817-1825. DOI:10.1038/s41396-018-0096-y
(  0) 0) |
| [89] |
Baldos A P, Corre M D, Veldkamp E. Response of N cycling to nutrient inputs in forest soils across a 1000-3000 m elevation gradient in the Ecuadorian Andes[J]. Ecology, 2015, 96(3): 749-761. DOI:10.1890/14-0295.1
(  0) 0) |
| [90] |
Wang Q, Wang J L, Li Y Z, et al. Influence of nitrogen and phosphorus additions on N2-fixation activity, abundance, and composition of diazotrophic communities in a Chinese fir plantation[J]. Science of the Total Environment, 2018, 619/620: 1530-1537. DOI:10.1016/j.scitotenv.2017.10.064
(  0) 0) |
| [91] |
Bowman W D, Cleveland C C, Halada Ĺ, et al. Negative impact of nitrogen deposition on soil buffering capacity[J]. Nature Geoscience, 2008, 1(11): 767-770. DOI:10.1038/ngeo339
(  0) 0) |
| [92] |
Treseder K K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies[J]. Ecology Letters, 2008, 11(10): 1111-1120. DOI:10.1111/j.1461-0248.2008.01230.x
(  0) 0) |
| [93] |
Fog K. The effect of added nitrogen on the rate of decomposition of organic matter[J]. Biological Reviews, 1988, 63(3): 433-462. DOI:10.1111/j.1469-185X.1988.tb00725.x
(  0) 0) |
| [94] |
Janssens I A, Dieleman W, Luyssaert S, et al. Reduction of forest soil respiration in response to nitrogen deposition[J]. Nature Geoscience, 2010, 3(5): 315-322. DOI:10.1038/ngeo844
(  0) 0) |
| [95] |
Lu M, Zhou X H, Luo Y Q, et al. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis[J]. Agriculture, Ecosystems and Environment, 2011, 140(1/2): 234-244.
(  0) 0) |
| [96] |
Bardgett R D, Mommer L, de Vries F T. Going underground: Root traits as drivers of ecosystem processes[J]. Trends in Ecology and Evolution, 2014, 29(12): 692-699. DOI:10.1016/j.tree.2014.10.006
(  0) 0) |
| [97] |
Jian S Y, Li J W, Chen J, et al. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis[J]. Soil Biology & Biochemistry, 2016, 101: 32-43.
(  0) 0) |
| [98] |
DeForest J L, Zak D R, Pregitzer K S, et al. Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests[J]. Soil Science Society of America Journal, 2004, 68(1): 132-138. DOI:10.2136/sssaj2004.1320
(  0) 0) |
| [99] |
Bru D, Ramette A, Saby N P A, et al. Determinants of the distribution of nitrogen-cycling microbial communities at the landscape scale[J]. The ISME Journal, 2011, 5(3): 532-542. DOI:10.1038/ismej.2010.130
(  0) 0) |
| [100] |
Shoun H, Kim D H, Uchiyama H, et al. Denitrification by fungi[J]. FEMS Microbiology Letters, 1992, 94(3): 277-281. DOI:10.1111/j.1574-6968.1992.tb05331.x
(  0) 0) |
| [101] |
Strickland M S, Rousk J. Considering fungal: Bacterial dominance in soils - Methods, controls, and ecosystem implications[J]. Soil Biology & Biochemistry, 2010, 42(9): 1385-1395.
(  0) 0) |
| [102] |
Zechmeister-Boltenstern S, Keiblinger K M, Mooshammer M, et al. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations[J]. Ecological Monographs, 2015, 85(2): 133-155. DOI:10.1890/14-0777.1
(  0) 0) |
| [103] |
Rütting T, Huygens D, Boeckx P, et al. Increased fungal dominance in N2O emission hotspots along a natural pH gradient in organic forest soil[J]. Biology and Fertility of Soils, 2013, 49(6): 715-721. DOI:10.1007/s00374-012-0762-6
(  0) 0) |
| [104] |
Wang C, Liu D W, Bai E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition[J]. Soil Biology & Biochemistry, 2018, 120: 126-133.
(  0) 0) |
| [105] |
Weitz A M, Linder E, Frolking S, et al. N2O emissions from humid tropical agricultural soils: Effects of soil moisture, texture and nitrogen availability[J]. Soil Biology & Biochemistry, 2001, 33(7/8): 1077-1093.
(  0) 0) |
| [106] |
Carter M S. Contribution of nitrification and denitrification to N2O emissions from urine patches[J]. Soil Biology & Biochemistry, 2007, 39(8): 2091-2102.
(  0) 0) |
| [107] |
Mathieu O, Hénault C, Lévêque J, et al. Quantifying the contribution of nitrification and denitrification to the nitrous oxide flux using 15N tracers[J]. Environmental Pollution, 2006, 144(3): 933-940. DOI:10.1016/j.envpol.2006.02.005
(  0) 0) |
| [108] |
Song L, Tian P, Zhang J B, et al. Effects of three years of simulated nitrogen deposition on soil nitrogen dynamics and greenhouse gas emissions in a Korean pine plantation of northeast China[J]. Science of the Total Environment, 2017, 609: 1303-1311. DOI:10.1016/j.scitotenv.2017.08.017
(  0) 0) |
| [109] |
Yan Y L, Ganjurjav H, Hu G Z, et al. Nitrogen deposition induced significant increase of N2O emissions in an dry alpine meadow on the central Qinghai-Tibetan Plateau[J]. Agriculture, Ecosystems & Environment, 2018, 265: 45-53.
(  0) 0) |
| [110] |
Zheng X, Liu Q, Ji X F, et al. How do natural soil NH4+, NO3− and N2O interact in response to nitrogen input in different climatic zones? A global meta-analysis[J]. European Journal of Soil Science, 2021, 72(5): 2231-2245. DOI:10.1111/ejss.13131
(  0) 0) |
| [111] |
Xie D N, Si G Y, Zhang T, et al. Nitrogen deposition increases N2O emission from an N-saturated subtropical forest in southwest China[J]. Environmental Pollution, 2018, 243: 1818-1824. DOI:10.1016/j.envpol.2018.09.113
(  0) 0) |
| [112] |
Eickenscheidt N, Brumme R, Veldkamp E. Direct contribution of nitrogen deposition to nitrous oxide emissions in a temperate beech and spruce forest – A 15N tracer study[J]. Biogeosciences, 2011, 8(3): 621-635. DOI:10.5194/bg-8-621-2011
(  0) 0) |
| [113] |
Xu Y B, Xu Z H, Cai Z C, et al. Review of denitrification in tropical and subtropical soils of terrestrial ecosystems[J]. Journal of Soils and Sediments, 2013, 13(4): 699-710. DOI:10.1007/s11368-013-0650-1
(  0) 0) |
| [114] |
Opdyke M R, Ostrom N E, Ostrom P H. Evidence for the predominance of denitrification as a source of N2O in temperate agricultural soils based on isotopologue measurements[J]. Global Biogeochemical Cycles, 2009, 23(4): GB4018.
(  0) 0) |
| [115] |
Xu K, Wang C M, Yang X T. Five-year study of the effects of simulated nitrogen deposition levels and forms on soil nitrous oxide emissions from a temperate forest in Northern China[J]. PLoS One, 2017, 12(12): e0189831. DOI:10.1371/journal.pone.0189831
(  0) 0) |
| [116] |
Levy-Booth D J, Prescott C E, Grayston S J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems[J]. Soil Biology & Biochemistry, 2014, 75: 11-25.
(  0) 0) |
| [117] |
Zhang J B, Cai Z C, Zhu T B, et al. Mechanisms for the retention of inorganic N in acidic forest soils of Southern China[J]. Scientific Reports, 2013, 3(1): 1-8.
(  0) 0) |
| [118] |
Cheng Y, Zhang J B, Wang J, et al. Soil pH is a good predictor of the dominating N2O production processes under aerobic conditions[J]. Journal of Plant Nutrition and Soil Science, 2015, 178(3): 370-373. DOI:10.1002/jpln.201400577
(  0) 0) |
| [119] |
Hallin S, Jones C M, Schloter M, et al. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment[J]. The ISME Journal, 2009, 3(5): 597-605. DOI:10.1038/ismej.2008.128
(  0) 0) |
| [120] |
Martens-Habbena W, Berube P M, Urakawa H, et al. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria[J]. Nature, 2009, 461(7266): 976-979. DOI:10.1038/nature08465
(  0) 0) |
| [121] |
Prosser J I, Nicol G W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation[J]. Trends in Microbiology, 2012, 20(11): 523-531. DOI:10.1016/j.tim.2012.08.001
(  0) 0) |
| [122] |
Carey C J, Dove N C, Beman J M, et al. Meta-analysis reveals ammonia-oxidizing bacteria respond more strongly to nitrogen addition than ammonia-oxidizing archaea[J]. Soil Biology & Biochemistry, 2016, 99: 158-166.
(  0) 0) |
| [123] |
Di H J, Cameron K C, Shen J P, et al. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions[J]. FEMS Microbiology Ecology, 2010, 72(3): 386-394. DOI:10.1111/j.1574-6941.2010.00861.x
(  0) 0) |
| [124] |
Lehtovirta L E, Prosser J I, Nicol G W. Soil pH regulates the abundance and diversity of Group 1.1c Crenarchaeota[J]. FEMS Microbiology Ecology, 2009, 70(3): 367-376. DOI:10.1111/j.1574-6941.2009.00748.x
(  0) 0) |
| [125] |
Onodera Y, Nakagawa T, Takahashi R, et al. Seasonal change in vertical distribution of ammonia-oxidizing Archaea and bacteria and their nitrification in temperate forest soil[J]. Microbes and Environments, 2010, 25(1): 28-35. DOI:10.1264/jsme2.ME09179
(  0) 0) |
| [126] |
[126] Petersen D G, Blazewicz S J, Firestone M, et al. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska[J]. Environmental Microbiology, 2012, 14(4): 993-1008. DOI:10.1111/j.1462-2920.2011.02679.x
(  0) 0) |
| [127] |
Morales S E, Cosart T, Holben W E. Bacterial gene abundances as indicators of greenhouse gas emission in soils[J]. The ISME Journal, 2010, 4(6): 799-808. DOI:10.1038/ismej.2010.8
(  0) 0) |
| [128] |
Szukics U, Hackl E, Zechmeister-Boltenstern S, et al. Contrasting response of two forest soils to nitrogen input: Rapidly altered NO and N2O emissions and nirK abundance[J]. Biology and Fertility of Soils, 2009, 45(8): 855-863. DOI:10.1007/s00374-009-0396-5
(  0) 0) |
| [129] |
Kandeler E, Brune T, Enowashu E, et al. Response of total and nitrate-dissimilating bacteria to reduced N deposition in a spruce forest soil profile[J]. FEMS Microbiology Ecology, 2009, 67(3): 444-454. DOI:10.1111/j.1574-6941.2008.00632.x
(  0) 0) |
| [130] |
Raut N, Dörsch P, Sitaula B K, et al. Soil acidification by intensified crop production in South Asia results in higher N2O/(N2 + N2O)product ratios of denitrification[J]. Soil Biology & Biochemistry, 2012, 55: 104-112.
(  0) 0) |
| [131] |
Šimek M, Jı́šová L, Hopkins D W. What is the so-called optimum pH for denitrification in soil?[J]. Soil Biology & Biochemistry, 2002, 34(9): 1227-1234.
(  0) 0) |
| [132] |
Tang Y Q, Yu G R, Zhang X Y, et al. Environmental variables better explain changes in potential nitrification and denitrification activities than microbial properties in fertilized forest soils[J]. Science of the Total Environment, 2019, 647: 653-662. DOI:10.1016/j.scitotenv.2018.07.437
(  0) 0) |
 2022, Vol. 59
2022, Vol. 59


