我国有机固体废物(禽畜粪便,秸秆,垃圾和污泥等)产量巨大,但处理技术落后、资源利用率低,对生态环境和可持续发展带来了巨大挑战[1-2]。好氧堆肥是实现有机固体废物无害化和资源化处理最有效的途径,通过好氧堆肥,有机固体废弃物被微生物转化成富含营养元素和腐植酸类物质的稳定物质,可广泛用于土壤改良或调理[3-4]。然而普通堆肥工艺仍然因其固有缺点,如发酵温度低、无害化不彻底、二次污染严重等问题限制了其推广应用[5-6]。
近年来,学者将富含超嗜热微生物的接种剂应用于普通堆肥技术中,由此研发出超高温好氧发酵技术[7-8]。该技术在不依赖外源热量情况下,利用超嗜热微生物代谢释放的生物热,可使堆肥温度迅速提高至80℃以上,达到超高温阶段,且超高温可持续5天以上[9-10]。除接种超嗜热微生物外,超高温堆肥技术参数与普通堆肥类似,仅需配备混料、翻堆和曝气设备设施即可[7-8]。温度提升对堆肥缩短发酵周期、促进减容减重、提升无害化质量具有重要作用。超高温成因可能与其独特的群落组成密切相关,如Yu等[8]证明了超高温堆肥独特的群落组成与堆肥效率提高显著相关。此外,有学者证明超高温对氮素保留[11]、抗性基因削减[10]和微塑料去除[12]均有促进作用。超高温堆肥技术的优点均与堆肥温度提升密切相关,然而目前关于超高温堆肥群落产热相关功能的研究,尤其是超高温产生的原因的研究依然较少[12-14]。
PICRUSt(phylogenetic investigation of communities by reconstruction of unobserved states)是一款可以通过16S rDNA标志基因和其对应的参考基因组数据库来预测群落功能的生物信息学分析软件[15]。PICRUSt不仅无需宏基因组复杂的分析过程,还可以用来预测不可培养微生物的功能,且准确度较高[16]。PICRUSt已广泛应用于堆肥、污水、土壤、厌氧发酵等体系的微生物群落功能分析,如:Wang等[17]利用PICRUSt研究了牛粪堆肥过程细菌群落功能变化,发现堆肥初期脂类和碳水化合物代谢相关基因相对丰度分别增加了28.5%和22.4%,而核苷酸和氨基酸代谢相关基因相对丰度分别减少了21.6%和2.1%;Yin和Wang[18]利用PICRUSt研究了电离辐射促进微生物厌氧发酵产氢的机理,发现电离辐射可通过增强能量代谢,减少乙酰基基因,从而提高产氢能力;Zhang等[19]评估了多种生物载体对短链脂肪酸产生的影响,并通过PICRUSt分析了代谢功能的丰度,阐明了不同生物载体的工作机制;Buhlmann等[20]利用PICRUSt来观察氨对乙酸降解代谢途径的影响,并通过关键基因分析强调了氨的潜在抑制作用。因此,本研究主要基于超高温堆肥和普通过程微生物群落以及温度变化[11],采用PICRUSt对微生物群落功能潜力进行了研究,重点分析了两种堆肥方式产热相关代谢通路丰度差异及其与堆肥温度的关系,探讨了超高温堆肥微生物群落强化产热的机理。预期结果有助于进一步了解超高温堆肥体系超高温产生的机制。
1 材料与方法 1.1 实验设计与样品收集实验设计已在先前研究中报道[11]。简要的,超高温好氧堆肥处理包含10吨鸡粪,1.5吨稻壳,5吨经超高温好氧堆肥后腐熟产物,普通堆肥处理包含10吨鸡粪,1.5吨稻壳,5吨经普通好氧堆肥后腐熟产物。各自混合均匀后,送入2.5 m× 4.5 m× 3.0 m(长×宽×高)的混凝土发酵槽内开始堆肥进程,物料基本理化性质参见已发表文献[11]。每个发酵槽底部均铺设有曝气管道,采用高压鼓风机对堆体连续强制通风供氧,每立方米曝气量控制空气约0.1·t–1·h–1。根据文献研究结果,堆肥周期设置为44d,分别在堆肥过程第13和26天进行翻堆,在第0、5、13、23、37、44天采集样品。样品采集具体操作如下:在堆肥随机3个区域采集50 cm左右深处样品,将收集的样品混合均匀后再分装三份,以保证样品的代表性;一部分样品用50 mL离心管分装后,首先用液氮速冻使微生物稳定,随后取出保存于–20℃冰箱,待所有样品收集完后进行微生物分析;其余样品保存于–4℃冰箱用于理化分析。
1.2 理化性质测定采用双金属温度计(–20℃~150℃)每日10点记录堆肥温度,采用恒重法(电热鼓风恒温干燥箱)测定含水率,采用pH计和EC计分别测定堆肥样品浸提上清液(5 g堆肥+45 mL去离子水,200 r·min–1水平震荡2 h)pH和EC。本文主要研究了两种堆肥方式温度变化与微生物预测功能丰度之间的关系,具体温度数据见图 1,其他理化性质参见文献[11]。
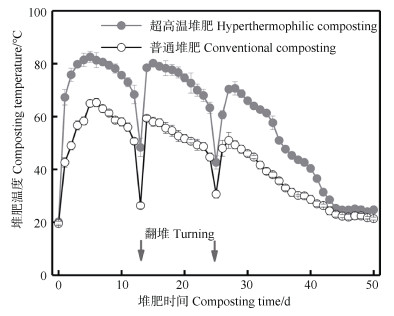
|
图 1 超高温堆肥与普通堆肥过程温度变化 Fig. 1 Temperature variation during hyperthermophilic composting and conventional composting |
采用PowerMax Soil DNA Isolation Kit(Qiagen,USA)提取堆肥样品DNA,DNA质量采用1%琼脂糖凝胶电泳检测,最后使用NanoDrop2000测定DNA浓度和纯度[21]。采用细菌通用引物515F(5′-GT GCCAGCMGCCGCGGTAA-3′)和907R(5′-CCGTC AATTCMTTTRAGTTT-3′)扩增16S rDNA V3-V4高变区序列。在广东美格基因科技有限公司Illumina MiSeq平台上完成高通量测序。Illumina测序扩增的上游引物包括pad linker、Illumina接头和基因特异性引物,下游引物包括6 nt barcode、pad linker、Illumina接头和引物。使用Qiime2软件标准分析流程得到OTU表格,具体如下:使用UPARSE软件将高质量的核酸序列聚类到操作分类单元(operational taxonomic units,OTUs)并剔除嵌合体(97%相似度)。分类比对使用SILVA数据库得到特征表,并统计各个分类水平微生物群落组成。利用PICRUSt的Python脚本标准化特征表,然后进行功能基因预测,生成KEGG(Kyoto Encyclopedia of Genes and Genomes)同源基因(KEGG Orthologes)表,最后将同源基因表分为三个层次代谢通路表进行下游分析[18]。
1.4 数据处理与分析利用单因素方差分析(ANOVA)和图基检验显著性(Tukey’s HSD)评价超高温堆肥与普通堆肥过程温度以及微生物群落功能代谢通路丰度的显著性差异。所有数据分析及绘图使用R完成,主成分分析采用vegan包,样本Bray Curtis距离采用vegan包计算,热图(代谢通路,基因丰度)采用pheatmap包绘制,随机森林模型采用randomforest包分析。
2 结果与讨论 2.1 超高温堆肥微生物群落功能多样性特征已有研究结果表明,超高温堆肥微生物群落组成与普通堆肥显著不同(P < 0.05)[11],超高温堆肥过程微生物群落多样性显著降低,其中超嗜热微生物Thermus属相对丰度最高,超过50%,为绝对优势属,而普通堆肥过程不存在超嗜热微生物,也无明显优势菌属[8]。类似的,本研究中Thermus属在超高温阶段(第5天,堆肥温度为82.5±0.4℃)相对丰度为27.5%±4.9%,在高温阶段(第13天,堆肥温度为75.5±1.4℃)也达到22.5%±3.1%(图 2a)[11]。图 2b展示了超高温堆肥和普通堆肥基于均一化预测功能KO(KEGG ORTHOLOGY数据库)丰度组成的β多样性,基于方差分析的显著性结果表明,超高温堆肥和普通堆肥的总体功能组成β多样性无显著性差异(基于Bray-Curtis距离,P = 0.69)。超高温堆肥与普通堆肥过程之间微生物群落组成的差异与期微生物功能差异不一致,这可能是由于尽管超高温堆肥具有独特微生物群落组成,但构成其微生物群落在功能上却差别不大,因为超嗜热微生物和其他微生物一样,均含有相似的能量代谢、元素循环、环境信息处理等功能基因[22]。类似的情况也在土壤、污水处理、厌氧消化等体系中出现,例如,Louca等[22]报道相邻土壤的微生物群落组成可能存在较大变异性,但微生物群落却有着类似的代谢功能。
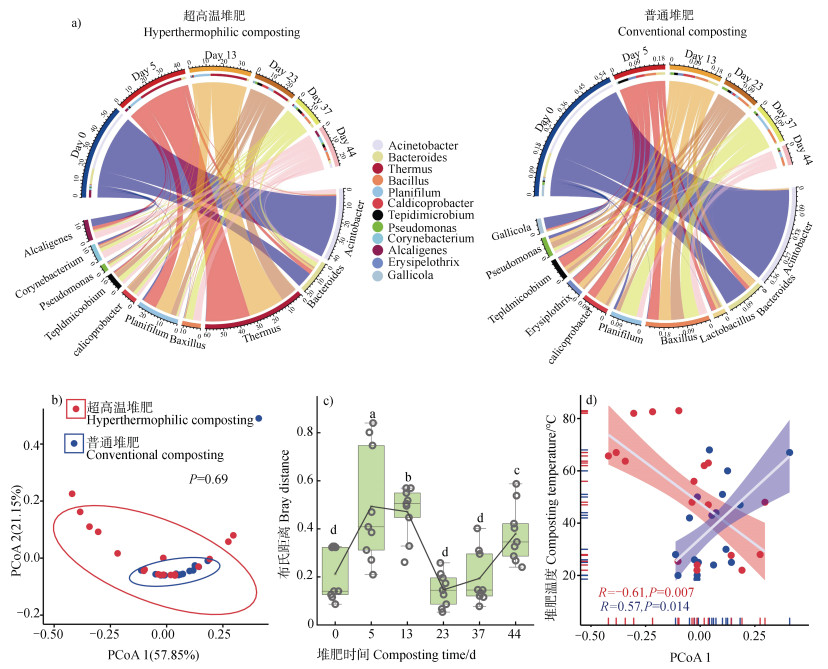
|
注:a)超高温堆肥与普通堆肥微生物群落组成;b)基于PICRUSt预测的微生物群落功能组成主坐标分析(基于Bray-Curtis距离,红色点代表超高温堆肥样本,蓝色点代表普通堆肥样本);c)超高温堆肥与普通堆肥相同天数群落功能β多样性距离变化(基于Bray-Curtis距离,显著性水平为P < 0.05);(d)PCoA1轴与温度回归分析(红色点代表超高温堆肥样本,蓝色点代表普通堆肥样本)。 Note: a)Changes in bacterial community composition in hyperthermophilic composting(hTC)and conventional composting(cTC); b)Principal coordinates analysis(PCoA)illustrating variation in microbial functional composition analysis of hTC and cTC based on PICRUSt prediction(Based on Bray-Curtis distance, red dots represent hTC samples and blue dots represent cTC samples); c)Bray-Curtis based distance between samples from hTC and cTC treatments(Based on Bray Curtis distance, significant difference level P < 0.05);(d)Correlation between the first PCoA axis and temperature in hTC and cTC.(Red dots represent hTC samples and blue dots represent cTC samples). 图 2 超高温堆肥与普通堆肥微生物群落组成、功能β多样性及其与堆肥温度的关系 Fig. 2 Variation in the microbial community composition, functional composition and the correlations between β diversity and composting temperature in hyperthermophilic composting(hTC)and conventional composting(cTC) |
此外,本研究发现超高温堆肥过程微生物群落功能组成变化较普通堆肥更为剧烈,超高温堆肥样品在PCoA1轴上分布较宽,而普通堆肥样品分布相对集中。同样的,图 2c显示不同堆肥方式在相同堆肥时间的微生物功能Bray Curtis距离变化,也表明超高温阶段的微生物功能组成变化更大。结果表明,两种堆肥方式在第5天(超高温堆肥温度:82.5±0.4℃;普通堆肥温度:65.1±3.6℃)、第13天(超高温堆肥温度:75.5±1.4℃;普通堆肥温度:46.3±2.3℃)和第44天(超高温堆肥温度:25.9±2.8℃;普通堆肥温度:30.3±1.2℃)的功能组成β多样性与普通堆肥存在显著性差异(基于Bray-Curtis距离,图 1和表 1)。图 2d显示了PCoA1轴(解释了57.8%的总方差)分别与两种堆肥处理的堆肥温度均呈显著相关(超高温堆肥:P = 0.007,普通堆肥:P = 0.014),这表明两种堆肥方式的功能组成可能决定了其堆肥温度[7]。
|
|
表 1 超高温堆肥和普通堆肥不同堆肥时间功能组成β多样性差异(基于Bray-Curtis距离) Table 1 Statistical significance of functional composition β diversity between hyperthermophilic composting and conventional composting at the different composting times(based on Bray-Curtis distance) |
通过对KO数据库二级代谢途径分析发现,两种堆肥过程微生物群落较高代谢通路丰度主要包括能量代谢(Energy Metabolism)、碳水化合物代谢(Carbohydrate Metabolism)、氨基酸类物质代谢(Amino Acid Metabolism)、遗传信息复制和修复(Replication and Repair)和遗传信息翻译(Translation)等(图 3a,丰度前25类二级代谢通路)。上述结果与已有研究类似,如,Antunes利用宏基因组技术分析发现园林有机固体废物堆肥中能量代谢、碳水化合物代谢、氨基酸类物质代谢通路丰度,以及复制、修复和翻译等遗传信息处理代谢通路丰度较高[23]。两种堆肥方式微生物功能组成在第5天、第13天和第44天变化较大,而在第23天(超高温堆肥温度:60.3±3.1℃;普通堆肥::49.3±1.7℃)和第37天(超高温堆肥温度:47.7±1.5℃;普通堆肥::43.0±1.8℃)两者类似,上述结果与微生物群落功能组成分析结果互相印证。具体而言,在堆肥第5天和第13天时,分别有15类和10类二级代谢通路丰度在超高温堆肥中显著高于普通堆肥,而显著低的只有3类和2类;当堆肥进行到第23天时,2种堆肥方式功能组成趋于类似,仅有3类二级代谢通路丰度在超高温堆肥中显著性低;当堆肥到第44天时,两种堆肥方式功能组成再次明显变化,有23类二级代谢通路丰度在超高温堆肥显著降低(图 3b)。显著性分析发现,超高温堆肥过程的能量代谢、碳水化合物代谢、环境适应(Environmental Adaptation)、脂类代谢(Lipid Metabolism)、核苷酸代谢(Nucleotide Metabolism)、遗传信息翻译、膜转运(Membrane Transport)和细胞生长与死亡(Cell Growth and Death)8类代谢通路丰度与堆肥温度呈显著正相关,信号分子与相互作用(Signaling Molecules and Interaction)代谢通路与堆肥温度呈显著负相关;而普通堆肥过程中,仅运输和分解代谢(Transport and Catabolism)通路与堆肥温度呈显著正相关,碳水化合物代谢等8类二级代谢通路丰度和堆肥温度呈显著负相关(图 3c)[24-25]。以上结果表明,超高温堆肥微生物群落的代谢通路丰度与堆肥温度相互促进,这种促进作用可能是由于超嗜热微生物为超高温堆肥的优势微生物群落。超嗜热微生物通常在80℃以上仍保持活性,超嗜热可能通过代谢产热以提高环境温度到接近自身最佳温度的微生物,以获得较相对非嗜热微生物更多生长优势[26]。而普通堆肥的发酵温度则对微生物代谢有抑制作用,通常这种抑制作用在堆肥温度下降后得到恢复[27]。

|
图 3 a)超高温堆肥与普通堆肥微生物群落代谢通路丰度(丰度前25);b)两种堆肥方式不同代谢通路丰度在相同天数的显著性差异;c)两种堆肥方式不同代谢通路丰度与堆肥温度的关系 Fig. 3 a)Top 25 relative abundances of level 2 functions based on KO classification during hyperthermophilic composting(hTC)and conventional composting(cTC)based on PICRUSt prediction; b)showing if a function is significantly greater in the hTC(red)or the cTC(blue)samples; c)correlation between function abundances and composting temperature during hTC and cTC |
氧化磷酸化过程合成的ATP是微生物最重要的能量来源,ATP向ADP的转化时会释放能量用于各项生命活动并产生热量,这部分热量可能是堆肥体系这类富营养环境发热的主要原因[26-28]。因此,本节重点比较和分析了两种堆肥方式氧化磷酸化过程代谢通路丰度的差异及其与堆肥温度的关系。如图 4a所示,两种堆肥过程均含有较高丰度NADH脱氢酶(EC 7.1.1.2)、琥珀酸脱氢酶(EC 1.3.5.1)、细胞色素c氧化酶(EC 7.1.1.9)和钾转运ATP酶(EC 7.2.2.19)基因。在超高温堆肥过程中,上述四种基因预测丰度在第5天和第13天丰度呈增加趋势,在第23天、第37天和第44天逐步降低,而普通堆肥整个堆肥周期上述基因丰度均无明显变化。为进一步探讨超高温堆肥超高温产生原因,本研究将氧化磷酸化过程涉及的所有同源功能基因丰度(基于KO数据库)在两种堆肥方式之间进行了比较,并将功能丰度变化与温度做了相关性分析,结果如图 4b所示。较普通堆肥,超高温堆肥分别有29和63类功能基因预测丰度在第5天和第13天上调了2倍以上,主要是NADH脱氢酶、琥珀酸脱氢酶和ATP合成酶基因。其中K03935在超高温堆肥第5天和第13天分别增加了9.5倍和8.8倍,K00235分别增加了9.6倍和7.6倍。在堆肥进行到第23天和第37天时,两种堆肥处理有显著差异丰度的功能基因数量明显减少。第44天时,超高温堆肥样品中的大多数功能基因丰度较普通堆肥要低,上述结果与图 3结果一致。Spearman显著性检验分析发现,超高温堆肥处理有27类氧化磷酸化KO功能基因丰度与堆肥温度显著正相关,其中包含15类NADH脱氢酶基因、2类琥珀酸脱氢酶基因、1类细胞色素c氧化还原酶基因、4类细胞色素c氧化酶基因和5类ATP合成酶基因;而普通堆肥仅有1类NADH脱氢酶基因(K05585)与堆肥温度显著正相关,有20类氧化磷酸化KO功能基因丰度与堆肥温度显著负相关(图 4b)。
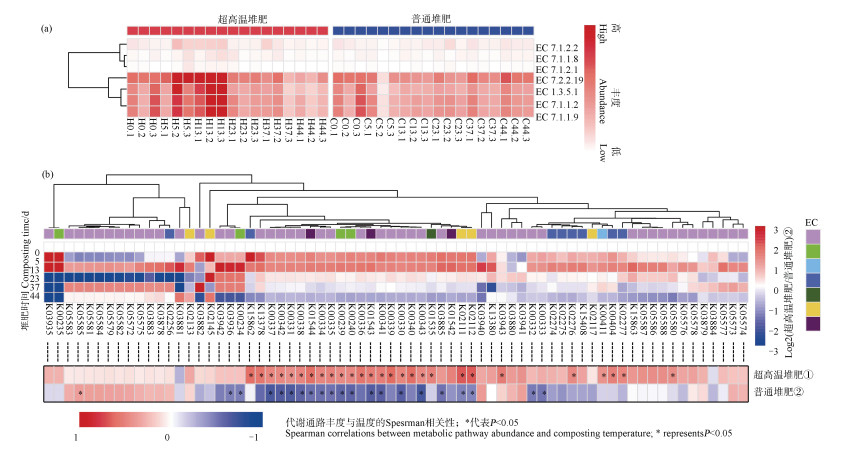
|
①Hyperthermophilic composting;②Conventional composting;③Plotted values are the logarithm transformed fold change values of each enzyme abundances in hTC compared to those of cTC samples. 注:a)H5.1,H5.2和H5.3分别代表超高温堆肥第5天的3个重复样本,其他类推;右侧EC编号为氧化磷酸化相关酶分类信息;b)(横轴编号为基于KEGG氧化磷酸化过程同源基因的分类信息),标绘值是超高温堆肥每个基因相对于普通堆肥样品的对数转换倍数变化值。与普通堆肥相比,红色和蓝色分别表示基因丰度增加和减少,以及不同功能基因丰度与堆肥温度的关系。 Note:a)H5.1,H5.2 and H5.3 represent three repeated samples on day 5 in hyperthermophilic composting(hTC). The EC number on the right is the classification information of oxidative phosphorylation related enzymes;b)Plotted values are the logarithm transformed fold change values of each gene in hTC samples compared to those of conventional composting(cTC)samples(The horizontal axis represents the classification information of homologous genes related to the oxidative phosphorylation process based on KEGG database). Red and blue indicate increased and decreased gene abundances,respectively. The below colors represent correlation between function gene abundances and composting temperature during hTC and cTC. 图 4 a)超高温堆肥和普通堆肥氧化磷酸化相关酶代谢通路预测丰度变化;b)两种堆肥方式功能基因丰度变化热图 Fig. 4 a)Changes in predicted pathway abundances of oxidative phosphorylation related enzymes during hyperthermophilic composting(hTC)and conventional composting(cTC), b)Heatmaps showing the function gene abundances dynamics during hTC and cTC |
图 5展示了随机森林回归模型评估的不同功能基因丰度对超高温堆肥温度变化的重要程度。如图 5a所示,采用的氧化磷酸化同源功能基因丰度均可较好的预测两种堆肥温度(超高温堆肥:R = 0.96,P < 0.001;普通堆肥:R = 0.97,P < 0.001)。图 5b和图 5c分别展示了模型确定的超高温堆肥和普通堆肥中重要性前10与温度变化密切关联的同源基因。超高温堆肥中,重要性排在前3的功能基因分别是K03943(NDUFV2;NADH脱氢酶-泛醌-黄素蛋白)、K15862(细胞色素c氧化酶cbb3型亚基)和K05580(ndhI,NADH -醌氧化还原酶亚基I),这3类功能基因也都在超高温堆肥中被富集,而且丰度变化均与堆肥温度呈显著正相关。上述氧化磷酸化功能基因的富集可能使微生物代谢更多有机物,从而提高ATP合成速率,进而产生更多热量[29]。而普通堆肥中,重要性排在前3的功能基因分别是K00337(nuoH,NADH醌氧化还原酶亚基H)、K00342(nuoM,NADH醌氧化还原酶亚基M)和K00343(nuoN,NADH醌氧化还原酶亚基N),这3类功能基因在普通堆肥中显著减少,而且丰度变化均与堆肥温度呈显著负相关。

|
图 5 a)基于随机森林回归分析和实际值的预测性能指标,该模型是基于18个超高温堆肥样品(黑色)或18个普通堆肥样品(灰色)各自的70类能量代谢KO功能基因丰度建立的;b)超高温堆肥中根据随机森林预测重要性排序前10的KO功能基因(均方误差降序);c)普通堆肥中根据随机森林预测重要性排序前10的KO功能基因(均方误差降序)。 Fig. 5 a)Predicted composting temperature values based on random forest regression analyses versus actual values, models were based on 70 KEGG ORTHOLOGY functional gene abundances involved in oxidative phosphorylation of 18 samples from hyperthermophilic composting(hTC, in black)and conventional composting(cTC, in gray), respectively; b)Random forest predictions of the effects of important functional genes(top 10, descending order in mean square errors)on composting temperature in hTC; c)Random forest predictions of the effects of important functional genes(top 10, descending order in mean square errors)on composting temperature in cTC. |
尽管原核生物可用种类繁多的化学物质作为电子传递反应底物(如甲酸、氢气、乳酸等)[30-31],这类底物在堆肥体系中含量极少,生化氧化呼吸链仍然是微生物群落的主要能量来源。然而关于超嗜热微生物能量代谢的研究主要集中于化能无机自养代谢,因为超嗜热微生物主要分布的热泉、火山口、海底等有机物匮乏的超高温环境,但该环境通常富含硫化氢、氢气、亚铁等无机电子供体[32-33]。因此,关于超嗜热微生物在有机物丰富环境下的能量代谢特征比较欠缺。在超高温堆肥中,优势微生物群落为异常球菌-栖热菌门(Deinococcus-Thermus)和放线菌门(Actinobacteria),而普通堆肥则以厚壁菌门(Firmicutes)和变形菌门(Proteobacteria)为主。超嗜热微生物通常具有较高的最适生长温度(> 80℃),在超高温环境下仍具有较高活性,并同时拥有耐碱、耐高压、耐盐等其他耐受外界环境胁迫的特征[34]。超高温堆肥中,上述代谢通路丰度显著的提高与超嗜热微生物的特征密切相关。然而,在普通堆肥过程中,由于没有超嗜热微生物存在,发酵温度一般不超过80℃,并且高温会较大程度抑制微生物活性。即便利用微生物易分解碳源组分更高的鸡粪、酒糟、厨余垃圾等有机固体废物作为堆肥原料,或通过调节含水率、供氧、翻堆等工艺参数,普通堆肥技术堆肥温度最高依然不超过80℃。此外,超高温堆肥已被证明可成功应用于污泥、畜禽粪便、抗生素药渣等多种有机固体废物好氧发酵处理,相似的微生物群落以及功能组成可能是超高温产生及维持的核心因素。更重要的是,超嗜热微生物更强的耐热性能和更高的最适生长温度更有可能利用发热来作为其生态位竞争武器,使其在碳源充足的堆肥环境中保持优势地位。这种竞争性发热也可使超嗜热微生物利用产生的热量作为武器抵御噬菌体入侵,进一步提高其存活几率[26]。
3 结论本研究采用PICRUSt功能预测对超高温堆肥技术超高温产生的原因进行了初步探讨。结果表明,超高温堆肥的超高温阶段微生物群落功能组成β多样性显著区别与普通堆肥,能量代谢等与产热相关的代谢通路预测丰度显著高于普通堆肥,且与温度显著正相关(P < 0.05)。超高温堆肥可能通过富集氧化磷酸化功能基因使微生物更迅速代谢有机物,从而提高ATP合成速率,进而产生更多热量。高温成因还需结合宏转录组和蛋白组学技术进一步探索。
| [1] |
Jiang J S, Yu D, Wang Y, et al. Use of additives in composting informed by experience from agriculture: Effects of nitrogen fertilizer synergists on gaseous nitrogen emissions and corresponding genes(AmoA and nirS)[J]. Bioresource Technology, 2021, 319: 124127. DOI:10.1016/j.biortech.2020.124127
(  0) 0) |
| [2] |
Wan L B, Wang X T, Cong C, et al. Effect of inoculating microorganisms in chicken manure composting with maize straw[J]. Bioresource Technology, 2020, 301: 122730. DOI:10.1016/j.biortech.2019.122730
(  0) 0) |
| [3] |
Preble C V, Chen S S, Hotchi T, et al. Air pollutant emission rates for dry anaerobic digestion and composting of organic municipal solid waste[J]. Environmental Science & Technology, 2020, 54(24): 16097-16107.
(  0) 0) |
| [4] |
Wang X J, Bei Q C, Liu G, et al. Microbial abundance and community composition in different types of paddy soils in China and their affecting factors (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(3): 767-776. [王晓洁, 卑其成, 刘钢, 等. 不同类型水稻土微生物群落结构特征及其影响因素[J]. 土壤学报, 2021, 58(3): 767-776.]
(  0) 0) |
| [5] |
Nigussie A, Kuyper T W, Bruun S, et al. Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting[J]. Journal of Cleaner Production, 2016, 139: 429-439. DOI:10.1016/j.jclepro.2016.08.058
(  0) 0) |
| [6] |
Robledo-Mahón T, Martín M A, Gutiérrez M C, et al. Sewage sludge composting under semi-permeable film at full-scale: Evaluation of odour emissions and relationships between microbiological activities and physico-chemical variables[J]. Environmental Research, 2019, 177: 108624. DOI:10.1016/j.envres.2019.108624
(  0) 0) |
| [7] |
Yu Z, Liu X M, Zhao M H, et al. Hyperthermophilic composting accelerates the humification process of sewage sludge: Molecular characterization of dissolved organic matter using EEM-PARAFAC and two-dimensional correlation spectroscopy[J]. Bioresource Technology, 2019, 274: 198-206. DOI:10.1016/j.biortech.2018.11.084
(  0) 0) |
| [8] |
Yu Z, Tang J, Liao H P, et al. The distinctive microbial community improves composting efficiency in a full-scale hyperthermophilic composting plant[J]. Bioresource Technology, 2018, 265: 146-154. DOI:10.1016/j.biortech.2018.06.011
(  0) 0) |
| [9] |
Cui P, Chen Z, Zhao Q, et al. Hyperthermophilic composting significantly decreases N2O emissions by regulating N2O-related functional genes[J]. Bioresource Technology, 2019, 272: 433-441. DOI:10.1016/j.biortech.2018.10.044
(  0) 0) |
| [10] |
Liao H P, Lu X M, Rensing C, et al. Hyperthermophilic composting accelerates the removal of antibiotic resistance genes and mobile genetic elements in sewage sludge[J]. Environmental Science & Technology, 2018, 52(1): 266-276.
(  0) 0) |
| [11] |
Cui P, Liao H P, Bai Y D, et al. Hyperthermophilic composting reduces nitrogen loss via inhibiting ammonifiers and enhancing nitrogenous humic substance formation[J]. Science of the Total Environment, 2019, 692: 98-106. DOI:10.1016/j.scitotenv.2019.07.239
(  0) 0) |
| [12] |
Chen Z, Zhao W Q, Xing R Z, et al. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology[J]. Journal of Hazardous Materials, 2020, 384: 121271. DOI:10.1016/j.jhazmat.2019.121271
(  0) 0) |
| [13] |
Liu X M, Hou Y, Li Z, et al. Hyperthermophilic composting of sewage sludge accelerates humic acid formation: Elemental and spectroscopic evidence[J]. Waste Management, 2020, 103: 342-351. DOI:10.1016/j.wasman.2019.12.053
(  0) 0) |
| [14] |
Wang C, Dong D, Strong P J, et al. Microbial phylogeny determines transcriptional response of resistome to dynamic composting processes[J]. Microbiome, 2017, 5(1): 1-15. DOI:10.1186/s40168-016-0209-7
(  0) 0) |
| [15] |
Langille M G I, Zaneveld J, Caporaso J G, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences[J]. Nature Biotechnology, 2013, 31(9): 814-821. DOI:10.1038/nbt.2676
(  0) 0) |
| [16] |
Cui Y X, Biswal B K, van Loosdrecht M C M, et al. Long term performance and dynamics of microbial biofilm communities performing sulfur-oxidizing autotrophic denitrification in a moving-bed biofilm reactor[J]. Water Research, 2019, 166: 115038. DOI:10.1016/j.watres.2019.115038
(  0) 0) |
| [17] |
Wang K, Chu C, Li X K, et al. Succession of bacterial community function in cow manure composing[J]. Bioresource Technology, 2018, 267: 63-70. DOI:10.1016/j.biortech.2018.06.028
(  0) 0) |
| [18] |
Yin Y N, Wang J L. Predictive functional profiling of microbial communities in fermentative hydrogen production system using PICRUSt[J]. International Journal of Hydrogen Energy, 2021, 46(5): 3716-3725. DOI:10.1016/j.ijhydene.2020.10.246
(  0) 0) |
| [19] |
Zhang Q Q, Zhao X Y, Li W J, et al. Responses of short-chain fatty acids production to the addition of various biocarriers to sludge anaerobic fermentation[J]. Bioresource Technology, 2020, 304: 122989. DOI:10.1016/j.biortech.2020.122989
(  0) 0) |
| [20] |
Buhlmann C H, Mickan B S, Jenkins S N, et al. Ammonia stress on a resilient mesophilic anaerobic inoculum: Methane production, microbial community, and putative metabolic pathways[J]. Bioresource Technology, 2019, 275: 70-77. DOI:10.1016/j.biortech.2018.12.012
(  0) 0) |
| [21] |
Zhang B B, Wan X H, Yang J Q, et al. Effects of litters different in quality on soil microbial community structure in Cunninghamia lanceolata plantation (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(4): 1040-1049. [张冰冰, 万晓华, 杨军钱, 等. 不同凋落物质量对杉木人工林土壤微生物群落结构的影响[J]. 土壤学报, 2021, 58(4): 1040-1049.]
(  0) 0) |
| [22] |
Louca S, Polz M F, Mazel F, et al. Function and functional redundancy in microbial systems[J]. Nature Ecology & Evolution, 2018, 2(6): 936-943.
(  0) 0) |
| [23] |
Antunes L P, Martins L F, Pereira R V, et al. Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics[J]. Scientific Reports, 2016, 6: 38915. DOI:10.1038/srep38915
(  0) 0) |
| [24] |
Chng K R, Ghosh T S, Tan Y H, et al. Metagenome-wide association analysis identifies microbial determinants of post-antibiotic ecological recovery in the gut[J]. Nature Ecology & Evolution, 2020, 4(9): 1256-1267.
(  0) 0) |
| [25] |
Guo J H, Li J, Chen H, et al. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements[J]. Water Research, 2017, 123: 468-478. DOI:10.1016/j.watres.2017.07.002
(  0) 0) |
| [26] |
Frank S A. Metabolic heat in microbial conflict and cooperation[J]. Frontiers in Ecology and Evolution, 2020, 8: 275. DOI:10.3389/fevo.2020.00275
(  0) 0) |
| [27] |
Sadef Y, Poulsen T G, Bester K. Impact of compost process temperature on organic micro-pollutant degradation[J]. Science of the Total Environment, 2014, 494/495: 306-312. DOI:10.1016/j.scitotenv.2014.07.003
(  0) 0) |
| [28] |
Robador A, LaRowe D E, Finkel S E, et al. Changes in microbial energy metabolism measured by nanocalorimetry during growth phase transitions[J]. Frontiers in Microbiology, 2018, 9: 109. DOI:10.3389/fmicb.2018.00109
(  0) 0) |
| [29] |
Tabata K, Hida F, Kiriyama T, et al. Measurement of soil bacterial colony temperatures and isolation of a high heat-producing bacterium[J]. BMC Microbiology, 2013, 13: 56. DOI:10.1186/1471-2180-13-56
(  0) 0) |
| [30] |
Liu X, Jing X Y, Ye Y, et al. Bacterial vesicles mediate extracellular electron transfer[J]. Environmental Science & Technology Letters, 2020, 7(1): 27-34.
(  0) 0) |
| [31] |
Liu X, Zhuo S Y, Rensing C, et al. Syntrophic growth with direct interspecies electron transfer between pili-free Geobacter species[J]. The ISME Journal, 2018, 12(9): 2142-2151. DOI:10.1038/s41396-018-0193-y
(  0) 0) |
| [32] |
Atomi H. Recent progress towards the application of hyperthermophiles and their enzymes[J]. Current Opinion in Chemical Biology, 2005, 9(2): 166-173. DOI:10.1016/j.cbpa.2005.02.013
(  0) 0) |
| [33] |
Stetter K O. A brief history of the discovery of hyperthermophilic life[J]. Biochemical Society Transactions, 2013, 41(1): 416-420. DOI:10.1042/BST20120284
(  0) 0) |
| [34] |
Atomi H, Sato T, Kanai T. Application of hyperthermophiles and their enzymes[J]. Current Opinion in Biotechnology, 2011, 22(5): 618-626. DOI:10.1016/j.copbio.2011.06.010
(  0) 0) |
 2022, Vol. 59
2022, Vol. 59


