2. 中国科学院大学, 北京 100049;
3. 湖泊与环境国家重点实验室(中国科学院地理与湖泊研究所), 南京 210008
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China
土壤酸化是我国面临的主要土壤退化类型之一[1-6]。土壤酸化速率的研究虽已持续了数十年,但如何准确估算仍是难题,主要是因为土壤对外源H+的复杂缓冲性能[7-10]。目前基于实际观测的流域H+平衡估算法是研究当前环境下土壤酸化速率的有效方法[11-13],但是传统的流域方法无法区分阳离子交换反应(消耗土壤盐基后引起土壤酸化)和矿物风化过程(补偿酸化消耗的盐基离子)的H+消耗量[14-17],由此获得的土壤酸化速率存在较大误差[18-20]。因此Yang等[21]首次提出利用土壤矿物风化过程中释放的盐基离子(K+、Na+、Ca2+和Mg2+)与硅的化学计量关系(BC:Si)可定量矿物风化消耗的H+量,有助于准确估算土壤酸化速率。上述研究虽然提供了一种准确估算土壤酸化速率的理论和方法,但如何准确获取土壤矿物风化过程中的BC:Si值,需要深入研究。
过去矿物风化计量关系研究主要是基于斜长石、黑云母、角闪石、橄榄石等单一的可风化矿物,或者砂岩、泥岩、花岗岩、玄武岩等新鲜岩石[22-25]。虽然可以揭示矿物风化的机理、过程和特征,但结果却难以推广至自然界的土壤,因为自然条件下发育的土壤中包含了许多风化程度各异的矿物[26]。而且自然土壤的阶段性风化一般是难以完全水解、存在中间产物的非谐溶风化[24,27],导致风化强度各异的土壤在矿物风化时具有不同的元素释放量和化学计量关系。以往矿物风化研究主要关注K、Na、Al、O、Si等元素的化学计量关系[23,27-29],未曾研究土壤风化过程中盐基离子和硅的计量关系BC:Si。
模拟酸雨淋溶实验被证明是获取土壤矿物风化过程中BC:Si值的有效方法[30-31],在亚热带地区发育于花岗岩的强风化富铁土和弱风化雏形土的BC:Si值分别为0.8~1.4[30]和1.0~1.4[31],即便是发育于同一母质,不同风化程度的土壤,BC:Si值也会不同。这些研究均集中在花岗岩发育土壤,均在风化作用强烈的亚热带地区。尚无其他气候与环境条件下不同母质发育土壤BC:Si的研究报道。为此,本研究选择地处我国温带区辽宁省发育于云母片岩、片麻岩和安山岩的土壤,通过模拟酸雨淋溶实验获取不同母质发育土壤的BC:Si,进一步揭示不同母质发育土壤的BC:Si差异及原因,为BC:Si推广至区域尺度的研究和土壤酸化速率的评价提供理论依据。
1 材料与方法 1.1 采样点信息与土样采集本次研究中三个不同母质发育的土壤剖面,均具有淡薄表层(Ah)-雏形层(Bw)-母质(C)的层次构型[32],土地利用类型为林地。云母片岩(MS)发育的土壤剖面位于辽宁省盖州市杨运镇周屯水库南(40°11′52″N,122°19′58″E,海拔125 m),土壤发生层及采样深度,Ah:0~20 cm;Bw:20~60 cm;C:60~90 cm,土壤类型为普通简育湿润雏形土。片麻岩(GN)发育的土壤剖面位于辽宁省东港市十字街镇大黑山西北(40°1′17″N,124°5′26″E,海拔95 m),土壤发生层及采样深度,Ah:0~30 cm;Bw:30~75 cm;C:75~120 cm,土壤类型为普通酸性湿润雏形土。安山岩(AN)发育的雏形土剖面位于辽宁省丹东市振安区关家岭东北(40°14′15″N,124°11'53″E,海拔17 m),Ah:0~40 cm;Bw:40~70 cm;C:70~100 cm,土壤类型为普通简育湿润雏形土。3个剖面均位于暖温带大陆性季风气候区,年均气温8.4~9.5℃,降水量600~1 200 mm[33]。
1.2 实验设计土样经室内自然风干后剔除根系、石砾等杂物,部分土样分别过2.00 mm、0.25 mm、0.150 mm和0.075 mm孔径尼龙筛用于土壤理化属性测定,用于淋溶实验的土样不研磨,以原状形式进行模拟酸雨淋溶实验。
由于土壤胶体会吸附一定量的交换性盐基离子(K+、Na+、Ca2+和Mg2+),而本研究是探讨土壤矿物风化的化学计量关系,因此必须通过盐基洗脱实验除去土壤胶体上原来吸附的交换性盐基,以消除其对土壤矿物风化过程BC:Si产生的影响。本研究采用Batch方法在常温常压下对供试土壤进行室内模拟酸雨淋溶,为此实验分为两部分,分别是土壤交换性盐基洗脱实验和模拟酸雨淋溶实验。
(1)土壤交换性盐基洗脱实验。土壤原来吸附的交换性盐基离子洗脱依照文献[34]中的方法进行。洗脱实验中,每层土壤设置3个重复,每个重复称取土壤200 g置于500 mL离心瓶中并编号。先按土液比1︰1加入200 mL pH 7.0的EDTA-乙酸铵溶液(1 mol·L–1的乙酸铵和0.005 mol·L–1的EDTA),搅拌均匀并震荡后,离心10 min收集上层清液,测定其中的盐基离子含量。重复该过程,直至洗脱后上清液中的盐基离子含量连续3次不变,说明土壤中的交换性盐基离子已被完全洗脱。由于土壤矿物水解会持续释放盐基离子,因此洗脱液中一般总会含些盐基离子。实验结果表明,3种母质发育土壤经8次洗脱后,土壤中的交换性盐基离子被洗脱完全。洗脱盐基后残留在土壤中的
(2)模拟酸雨淋溶实验。模拟酸雨淋溶实验中,设计2组实验样品:一组是经过交换性盐基洗脱后的土壤,另一组则以未洗脱盐基的土壤作为对照。淋溶所用的酸雨溶液由NH4Cl和稀HCl配制而成,
土壤颗粒组成采用激光粒度仪(Beckman Coulter LS 13320)测定,通过转换公式将单位换算成g·kg–1 [35];pH采用电位计(PHS-3C)测定(土:水=1︰2.5);有机质采用重铬酸钾-硫酸消化法测定[36];交换性盐基离子K+、Na+、Ca2+和Mg2+经乙酸铵(pH 7.0)提取后,采用电感耦合等离子体发射光谱仪(PerkinElmer-Optima 8 000测定;阳离子交换量(CEC)采用乙酸铵(pH 7.0)交换法测定;矿质全量采用碳酸锂-硼酸熔融法,电感耦合等离子体发射光谱仪(PerkinElmer-Optima 8 000)测定;矿物组成采用MgCl2饱和以及甘油吸附后制成定向薄膜,通过对比X射线衍射仪(Ultima IV)获得的样品衍射特征与标准黏土矿物的衍射特征,鉴定出矿物类型并计算各自相对含量[37];盐基洗脱和模拟酸雨淋溶实验的淋出液中的K、Na、Ca、Mg和Si,用电感耦合等离子体发射光谱仪(PerkinElmer- Optima 8000)测定。BC:Si值采用以下公式计算获得。
| $ BC:Si = \frac{{{C^*}}}{{{C_{si}}}} $ |
式中,C*为四种盐基离子K+、Na+、Ca2+和Mg2+在淋溶液中的当量和(meq·kg–1),CSi为淋溶液中元素Si的摩尔浓度(mmol·kg–1)。
1.4 数据分析选择单因素方差分析法检验洗脱溶液和土壤中的盐基离子是否存在差异,以及不同母质发育土壤的BC:Si值是否存在差异。数据统计与分析均由IBM Statistics SPSS 22.0完成,图表均采用Origin 2018和Microsoft Excel 2019制作。
2 结果与讨论 2.1 土壤基本性质云母片岩、片麻岩和安山岩3种母质发育土壤的矿物组成相似,原生矿物均主要为石英和斜长石;次生矿物主要有伊利石、绿泥石、蛭石和高岭石,但矿物含量存在一定的差异(表 1)。云母片岩发育土壤的伊利石含量最高,其次是安山岩,片麻岩含量最低;而斜长石的含量则是片麻岩 > 安山岩 > 云母片岩。同一母质不同土壤发生层之间的矿物组成一致,但矿物含量存在一定的差异。斜长石含量由表层向下有增加的趋势,而高岭石含量由表层向下逐渐减少。
|
|
表 1 不同母质发育土壤及其成土母岩的矿物比例 Table 1 Mineral proportion of soils derived from different parent materials and their pedogenic rocks /% |
从土壤的颗粒组成来看,安山岩发育土壤的黏粒含量为258.8~341.9 g·kg–1,明显高于云母片岩发育土壤的125.3~206.4 g·kg–1和片麻岩发育土壤的214.2~274.4 g·kg–1(图 1)。土壤pH为4.8~6.9,为中性~偏酸性,不同母质之间的pH为:安山岩 > 云母片岩 > 片麻岩(表 2)。3种母质发育土壤的pH从Ah层至C层均呈增大趋势(表 2)。有机质含量在不同母质发育的土壤之间并无显著差异,每一个土壤剖面自表层向下均表现出降低的趋势(表 2)。土壤CEC为8.9~21.2 cmol·kg–1,有较大的变异;交换性盐基离子(K+、Na+、Ca2+和Mg2+)总量有更大的变异范围(2.73~22.8 cmol·kg–1),二者均为安山岩 > 云母片岩 > 片麻岩发育土壤(表 2)。以上这些土壤特性表明,本研究中3种母质发育土壤虽然均为雏形土,位于相近的区域,并且具有同样的土地利用类型,但在矿物学和理化性质上存在明显差异。
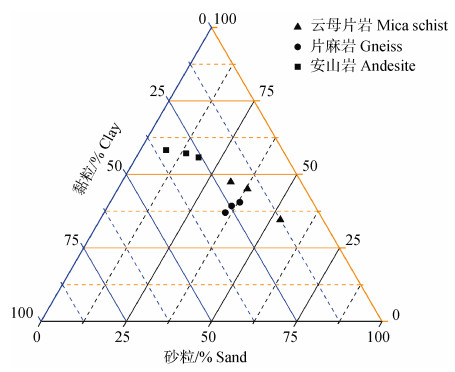
|
图 1 不同母质发育土壤的颗粒组成 Fig. 1 Particle size distribution of soils derived from different parent materials |
|
|
表 2 不同母质发育土壤的化学性质 Table 2 Chemical properties of soils derived from different parent materials |
不同母质土壤在盐基洗脱过程中具有一致的特征:初期淋出的K+、Na+、Ca2+和Mg2+较多,随后不断降低(图 2),当洗脱溶液体积达到1 000~1 600 mL,盐基离子的淋出量不再改变,说明在土液比为1︰1的条件下,最多经过8次盐基洗脱后,土壤胶体上的交换性盐基被洗脱完全。这与已有研究中花岗岩母质发育的强风化土壤具有一致的盐基洗脱规律[30]。
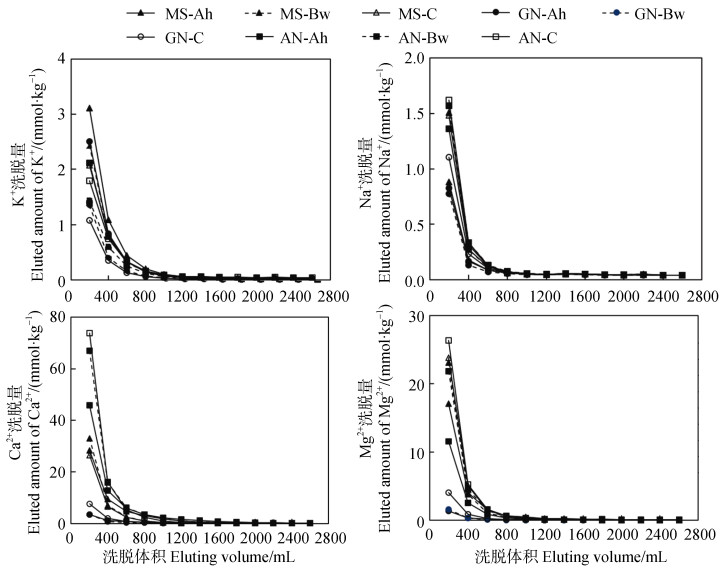
|
注:图例中MS-Ah、MS-Bw和MS-C分别表示云母片岩发育土壤的Ah、Bw和C层;GN-Ah、GN-Bw和GN-C分别表示片麻岩发育土壤的Ah、Bw和C层;AN-Ah、AN-Bw和AN-C分别表示安山岩发育土壤的Ah、Bw和C层。 Note: In the legend, MS-Ah, MS-Bw and MS-C refer to horizon Ah, Bw and C of soil derived from mica schist respectively; GN-Ah, GN-Bw and GN-C refer to horizon Ah, Bw and C of soil derived from gneiss respectively; AN-Ah, AN-Bw and AN-C refer to horizon Ah, Bw and C of soil derived from andesite respectively. 图 2 盐基洗脱过程中不同母质发育土壤的盐基离子释放特征 Fig. 2 Base cations releasing characteristics of soils derived from different parent materials during base elution |
不同母质发育土壤的交换性盐基淋出总量存在差异。安山岩发育土壤的盐基洗脱量最高,累积洗脱盐基离子257.8 mmol·kg–1,云母片岩发育土壤和片麻岩发育土壤分别为149.3 mmol·kg–1和46.9 mmol·kg–1。在被洗脱出土壤的盐基离子中,Ca2+和Mg2+的洗脱量显著高于K+和Na+(图 2),这是因为土壤交换性Ca2+和Mg2+含量显著高于交换性K+和Na+(表 2)。实验中3种母质发育土壤的K+、Na+、Ca2+和Mg2+洗脱总量与土壤中相应交换性盐基含量接近(表 3)。分别对K+、Na+、Ca2+和Mg2+的洗脱总量和土壤中含量进行方差分析,结果表明显著性系数P > 0.05,即两者无显著差异,这说明本实验已经有效地去除了土壤中的交换性盐基离子。
|
|
表 3 不同盐基离子的洗脱总量及其在土壤中含量 Table 3 Total eluting amount of different base cations and their content in the soil |
未洗脱盐基时,不同母质发育土壤在模拟酸雨淋溶下的盐基释放量呈初期高后期低的特征(图 3a、图 3c、图 3e、图 3g);洗脱盐基后,土壤释放的盐基离子较少(图 3b、图 3d、图 3f、图 3h),这与已有研究中洗脱与未洗脱盐基两种情况下的元素释放规律一致[30-31]。本研究中3种母质发育的土壤在洗脱盐基后元素释放量有小的波动(图 3b、图 3d、图 3f、图 3h),这是因为自然土壤包含了许多风化强度各异的混合矿物,在模拟实验中的矿物水解是非谐溶的[24,27],这使矿物风化反应的强度非连续性变化。

|
注:a)、c)、e)、g)为未洗脱盐基土壤,b)、d)、f)、h)为洗脱盐基土壤。 Note: a)、c)、e)、g)are base-uneluted soils, b)、d)、f)、h)are base-eluted soils. 图 3 未洗脱盐基和洗脱盐基土壤在模拟酸雨淋溶过程中的盐基离子释放特征 Fig. 3 Base cations releasing characteristics of base-uneluted and base-eluted soils under leaching with simulated acid rain |
片麻岩发育土壤按深度加权平均后的累积释放量(K+、Na+、Ca2+和Mg2+)为2.23 mmol·kg–1,云母片岩和安山岩发育土壤分别为2.99 mmol·kg–1和2.46 mmol·kg–1(表 4)。可见,这3种母质在目前的风化条件下,释放的盐基总量差异不大。
|
|
表 4 洗脱盐基后不同母质发育土壤风化过程中的盐基离子和硅释放量 Table 4 The releasing amount of base cations and silicon of base-eluted soils derived from different parent materials in the weathering process |
硅作为矿物风化的指示性元素[38-40],在不同母质发育土壤间的释放量存在显著性差异(P < 0.05)。按深度加权平均后,3种母质发育土壤的硅释放量顺序为云母片岩 > 安山岩 > 片麻岩土壤(表 4),分别为6.06、5.40和0.81 mmol·kg–1,这说明母质类型影响了土壤矿物风化过程的强度和元素释放。由于成土母岩的差异(表 1),土壤中具有不同比例的混合矿物组成。本研究中3种母质发育土壤中均含有原生矿物(石英和斜长石)和次生矿物(伊利石、绿泥石、蛭石和高岭石),但是每种矿物的比例不同(表 1)。云母片岩发育土壤的次生矿物(伊利石、绿泥石、蛭石和高岭石)比例最高,整个土体(包括Ah、Bw和C层)达到了42%~46%,安山岩和片麻岩发育土壤的次生矿物(伊利石、绿泥石、蛭石和高岭石)分别为30%~35%和23%~26%(表 1)。土壤中次生硅酸盐矿物比例高,在酸性环境下更易发生矿物风化水解[41-42],因此云母片岩发育土壤的硅释放量高于安山岩和片麻岩发育土壤(表 4)。
2.4 矿物风化计量关系BC:Si值未洗脱盐基的情况下,根据模拟酸雨淋溶过程中盐基离子和硅的释放量,云母片岩发育土壤Ah、Bw和C层的BC:Si值分别为61.8、55.6与38.7,片麻岩发育土壤分别为19.9、11.5与11.8,安山岩发育土壤分别为55.7、32.9与47.6(表 5)。这明显不同于洗脱盐基后的土壤BC:Si值:云母片岩发育土壤Ah、Bw和C层的BC:Si分别为0.6、0.7和1.8,片麻岩发育土壤分别为3.4、4.3和5.8,安山岩发育土壤分别为0.6、0.7和1.0(表 5)。经加权平均后的洗脱盐基土壤BC:Si值顺序为片麻岩 > 云母片岩 > 安山岩发育土壤,分别为4.7±0.8、1.0±0.5和0.8±0.2,对应的未洗脱盐基时BC:Si值分别为13.7±3.0、51.4±9.2和46.4±9.6(表 5),两种处理下的BC:Si值存在极显著差异(P < 0.01)。从均值上看,两种实验方式下的BC:Si相差最大和最小的分别为安山岩土壤和片麻岩土壤,未洗脱盐基土壤较洗脱盐基土壤分别大了58倍和3倍。这说明倘若不洗脱土壤的盐基离子,将导致BC:Si值被高估3倍以上(表 5)。
|
|
表 5 洗脱盐基和未洗脱盐基时不同母质发育土壤风化过程的BC:Si值及其加权平均值 Table 5 The BC: Si values and their weighted average of soils derived from different parent materials under base elution and unelution treatments in the weathering process |
对不同母质发育土壤的BC:Si值进行方差分析,结果显示显著性系数P < 0.01,表明不同母质发育土壤的BC:Si值存在显著性差异,这说明在我国温带发育的湿润雏形土,母质类型对风化计量关系BC:Si值具有重要影响。3种母质发育土壤的BC:Si值存在较大差异的原因是土壤矿物组分含量的差异。风化过程中石英与高岭石无盐基离子释放,斜长石在片麻岩发育土壤中的比例高于云母片岩和安山岩发育土壤,但次生矿物(伊利石、绿泥石、蛭石)比例较低(表 1)。相关性分析表明,BC:Si与斜长石具有显著的正相关性(P < 0.05)(图 4a),与伊利石、绿泥石和蛭石3种次生矿物之和具有显著的负相关性(图 4b),这说明本研究中BC:Si值主要受到原生矿物斜长石与次生矿物伊利石、绿泥石和蛭石的影响。这3种次生矿物是斜长石和云母风化至高岭石过程中形成的中间产物[22,24],在3种母岩发育土壤中均存在,但是母岩类型不同,相应土壤的矿物比例会存在差异(表 1)。由于土壤矿物在模拟酸雨淋溶下的非谐溶风化,斜长石含量高的片麻岩发育土壤风化过程中硅释放的少,因此BC:Si值高于云母片岩和安山岩母质发育的土壤。这说明不同母质发育的土壤,由于具有不同比例的矿物组分,矿物风化过程中的计量关系BC:Si存在明显不同。

|
图 4 不同母质发育土壤BC:Si与斜长石和次生矿物之和(伊利石,绿泥石,蛭石)的相关性(P < 0.05) Fig. 4 The correlation between BC: Si and plagioclase, sum of secondary minerals(illite, chlorite, vermiculite)of soils from different parent materials(a, b refer to plagioclase and sum of secondary minerals, respectively)(P < 0.05) |
同一母质发育土壤的BC:Si值在Ah层最小,并随深度变化至C层有增大的趋势(表 5)。这与皖南花岗岩发育的雏形土具有一致的BC:Si值变化趋势(A层土壤的BC:Si值低于B层和C层土壤)[31]。但是与位于亚热带南缘广东花岗岩强风化富铁土的BC:Si值在土体中的分布规律不同(A层土壤的BC:Si值高于B1和B2层土壤)[30]。这是因为强风化富铁土的整个土体中易风化矿物均较少,表层的植物和降尘对矿物风化计量关系会产生一定的影响[43-48]。可见,土壤风化程度不同,BC:Si值在整个剖面上的分布特征会出现差异。
3 结论我国温带地区不同母质发育湿润雏形土的物理、化学和矿物学性质具有明显的差异。在模拟酸雨淋溶条件下,未洗脱盐基土壤的BC:Si值为洗脱盐基后的3倍以上,因此只有洗脱了土壤胶体吸附的交换性盐基离子,才能获得来自矿物风化过程的BC:Si值。不同母质发育土壤风化过程中的计量关系BC:Si存在显著差异,在本研究中其大小顺序为片麻岩 > 云母片岩 > 安山岩。同一母质发育土壤不同发生层的BC:Si以Ah层最小,C层最大。不同母质的矿物组成和含量控制了BC:Si值的大小。
| [1] |
Fujii K, Funakawa S, Hayakawa C, et al. Contribution of different proton sources to pedogenetic soil acidification in forested ecosystems in Japan[J]. Geoderma, 2008, 144(3/4): 478-490.
(  0) 0) |
| [2] |
Li Z Y, Wang P S, Liu L, et al. High negative surface charge increases the acidification risk of purple soil in China[J]. Catena, 2021, 196: 104819. DOI:10.1016/j.catena.2020.104819
(  0) 0) |
| [3] |
Reuss J O, Johnson D W. Effect of soil processes on the acidification of water by acid deposition[J]. Journal of Environmental Quality, 1985, 14(1): 26-31.
(  0) 0) |
| [4] |
Guo J H, Liu X J, Zhang Y, et al. Significant acidification in major Chinese croplands[J]. Science, 2010, 327(5968): 1008-1010. DOI:10.1126/science.1182570
(  0) 0) |
| [5] |
van Breemen N, Driscoll C T, Mulder J. Acidic deposition and internal proton sources in acidification of soils and waters. Nature, 1984, 307(16): 599—604. .
(  0) 0) |
| [6] |
Zhang H Q, Zhao X Q, Zhang L Y, et al. Effects of liming and dicyandiamide(DCD)application on soil pH and nitrification of acidic red soil (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(1): 169-179. [张昊青, 赵学强, 张玲玉, 等. 石灰和双氰胺对红壤酸化和硝化作用的影响及其机制[J]. 土壤学报, 2021, 58(1): 169-179.]
(  0) 0) |
| [7] |
Liao B H, Dai Z H. Soil buffering capacity to acid precipitation and weathering characteristics of soil minerals (In Chinese)[J]. Acta Scientiae Circumstantiae, 1991, 11(4): 425-431. [廖柏寒, 戴昭华. 土壤对酸沉降的缓冲能力与土壤矿物的风化特征[J]. 环境科学学报, 1991, 11(4): 425-431.]
(  0) 0) |
| [8] |
Hao J M, Duan L, Xie S D. Mapping the relative sensitivity of soils to acid deposition in China (In Chinese)[J]. Chinese Journal of Enviromental Science, 1999, 20(4): 1-5. [郝吉明, 段雷, 谢绍东. 中国土壤对酸沉降的相对敏感性区划[J]. 环境科学, 1999, 20(4): 1-5.]
(  0) 0) |
| [9] |
Wang S Q, Zheng Z C, Li T X, et al. Effects of age of tea plantations on distribution of exchangeable base cations in soil aggregates (In Chinese)[J]. Acta Pedologica Sinica, 2013, 50(5): 1013-1020. [王晟强, 郑子成, 李廷轩, 等. 植茶年限对土壤团聚体中交换性盐基离子分布的影响[J]. 土壤学报, 2013, 50(5): 1013-1020.]
(  0) 0) |
| [10] |
Sverdrup H, Vries W. Calculating critical loads for acidity with the simple mass balance method[J]. Water, Air, and Soil Pollution, 1994, 72(1/2/3/4): 143-162.
(  0) 0) |
| [11] |
Moody P W, Aitken R L. Soil acidification under some tropical agricultural systems. 1. Rates of acidification and contributing factors[J]. Soil Research, 1997, 35(1): 163. DOI:10.1071/S96069
(  0) 0) |
| [12] |
Vries W, Breeuwsma A. The relation between soil acidification and element cycling[J]. Water, Air, and Soil Pollution, 1987, 35(3/4): 293-310.
(  0) 0) |
| [13] |
Forsius M, Kleemola S, Starr M, et al. Ion mass budgets for small forested catchments in Finland[J]. Water, Air, and Soil Pollution, 1995, 79(1/2/3/4): 19-38.
(  0) 0) |
| [14] |
Zhou B Q, Qiu L X, Zhang L M, et al. Study on driving factors of soil acidification based on grey correlation- structure equation model (In Chinese)[J]. Acta Pedologica Sinica, 2018, 55(5): 1233-1242. [周碧青, 邱龙霞, 张黎明, 等. 基于灰色关联-结构方程模型的土壤酸化驱动因子研究[J]. 土壤学报, 2018, 55(5): 1233-1242.]
(  0) 0) |
| [15] |
Bonten L T C, Reinds G J, Posch M. A model to calculate effects of atmospheric deposition on soil acidification, eutrophication and carbon sequestration[J]. Environmental Modelling & Software, 2016, 79: 75-84.
(  0) 0) |
| [16] |
Cosby B J, Ferrier R C, Jenkins A, et al. Modelling the effects of acid deposition: Refinements, adjustments and inclusion of nitrogen dynamics in the MAGIC model[J]. Hydrology and Earth System Sciences, 2001, 5(3): 499-518. DOI:10.5194/hess-5-499-2001
(  0) 0) |
| [17] |
Reinds G J, Posch M, Leemans R. Modelling recovery from soil acidification in European forests under climate change[J]. Science of the Total Environment, 2009, 407(21): 5663-5673. DOI:10.1016/j.scitotenv.2009.07.013
(  0) 0) |
| [18] |
Koïta M, Yonli H, Soro D, et al. Taking into account the role of the weathering profile in determining hydrodynamic properties of hard rock aquifers[J]. Geosciences, 2017, 7(3): 89. DOI:10.3390/geosciences7030089
(  0) 0) |
| [19] |
Blake L, Goulding K W T, Mott C J B, et al. Changes in soil chemistry accompanying acidification over more than 100 years under woodland and grass at Rothamsted Experimental Station, UK[J]. European Journal of Soil Science, 1999, 50(3): 401-412. DOI:10.1046/j.1365-2389.1999.00253.x
(  0) 0) |
| [20] |
Keskinen R, Ketoja E, Heikkinen J, et al. 35-year trends of acidity and soluble nutrients in cultivated soils of Finland[J]. Geoderma Regional, 2016, 7(4): 376-387. DOI:10.1016/j.geodrs.2016.11.005
(  0) 0) |
| [21] |
Yang J L, Zhang G L, Huang L M, et al. Estimating soil acidification rate at watershed scale based on the stoichiometric relations between silicon and base cations[J]. Chemical Geology, 2013, 337/338: 30-37. DOI:10.1016/j.chemgeo.2012.11.009
(  0) 0) |
| [22] |
Kalinowski B E, Schweda P. Kinetics of muscovite, phlogopite, and biotite dissolution and alteration at pH 1-4, room temperature[J]. Geochimica et Cosmochimica Acta, 1996, 60(3): 367-385. DOI:10.1016/0016-7037(95)00411-4
(  0) 0) |
| [23] |
Chittleborough D J. Indices of weathering for soils and palaeosols formed on silicate rocks[J]. Australian Journal of Earth Sciences, 1991, 38(1): 115-120. DOI:10.1080/08120099108727959
(  0) 0) |
| [24] |
White A F, Brantley S L. The effect of time on the weathering of silicate minerals: Why do weathering rates differ in the laboratory and field?[J]. Chemical Geology, 2003, 202(3/4): 479-506.
(  0) 0) |
| [25] |
Gudbrandsson S, Wolff-Boenisch D, Gislason S R, et al. Experimental determination of plagioclase dissolution rates as a function of its composition and pH at 22 ℃[J]. Geochimica et Cosmochimica Acta, 2014, 139: 154-172. DOI:10.1016/j.gca.2014.04.028
(  0) 0) |
| [26] |
Agnelli A, Cocco S, Massaccesi L, et al. Features of skeleton water-extractable fines from different acidic soils[J]. Geoderma, 2017, 289: 82-96. DOI:10.1016/j.geoderma.2016.11.026
(  0) 0) |
| [27] |
Brantley S L, Olsen A A. Reaction kinetics of primary rock-forming minerals under ambient conditions[M]// Treatise on Geochemistry. Amsterdam: Elsevier, 2014: 69—113. .
(  0) 0) |
| [28] |
Alekseyev A B, Liebermann H P, Hirsch G, et al. Relativistic configuration interaction calculations of the potential curves and radiative lifetimes of the low-lying states of bismuth nitride[J]. Chemical Physics, 1997, 225(1/2/3): 247-258.
(  0) 0) |
| [29] |
West A J. Thickness of the chemical weathering zone and implications for erosional and climatic drivers of weathering and for carbon-cycle feedbacks[J]. Geology, 2012, 40(9): 811-814.
(  0) 0) |
| [30] |
Zhao Y, Yang J L, Dong Y, et al. Stoichiometry of soil mineral weathering in intensity weathered soil as leached by simulated acid rain (In Chinese)[J]. Acta Pedologica Sinica, 2019, 56(2): 310-319. [赵越, 杨金玲, 董岳, 等. 模拟酸雨淋溶下强风化土壤矿物风化计量关系研究[J]. 土壤学报, 2019, 56(2): 310-319.]
(  0) 0) |
| [31] |
Zhuang X Y, Yang J L, Zhang G L, et al. Release characteristics of base cations during soil mineral weathering in subtropical granite area (In Chinese)[J]. Soils, 2016, 48(2): 374-380. [庄翔宇, 杨金玲, 张甘霖, 等. 亚热带花岗岩地区土壤矿物风化过程中盐基离子的释放特征[J]. 土壤, 2016, 48(2): 374-380.]
(  0) 0) |
| [32] |
Chinese Soil Taxonomy Research Group, Institute of Soil Science, Chinese Academy of Sciences, Cooperative Research Group on Chinese Soil Taxonomy. Keys to Chinese Soil Taxonomy (In Chinese). 3rd ed[M]. Hefei: Press of University of Science and Technology of China, 2001. [中国科学院南京土壤研究所土壤系统分类课题组, 中国土壤系统分类课题研究协作组. 中国土壤系统分类检索(第三版)[M]. 合肥: 中国科学技术大学出版社, 2001.]
(  0) 0) |
| [33] |
Liaoning Bureau of Statistic. Liaoning province statistic yearbook in 2020 (In Chinese). Beijing: China Statistics Press, 2020. [辽宁省统计局. 辽宁省2020年统计年鉴[M]. 北京: 中国统计出版社, 2020.]
(  0) 0) |
| [34] |
Yang J L, Zhang G L, Song X D, et al. A method for estimating the stoichiometric relationship between base cations and silicon weathering of soil mixed minerals[P]. Patent No: ZL201610137790.7. Issuing unit: State Intellectual Property Office of the People's Republic of China.[杨金玲, 张甘霖, 宋效东, 等. 一种土壤混合矿物的盐基离子和硅风化计量关系估算方法[P]. 专利号: ZL201610137790.7. 发证单位: 中华人民共和国国家知识产权局. .]
(  0) 0) |
| [35] |
Yang J L, Zhang G L, Li D C, et al. Relationships of soil particle size distribution between sieve-pipette and laser diffraction method (In Chinese)[J]. Acta Pedologica Sinica, 2009, 46(5): 772-780. [杨金玲, 张甘霖, 李德成, 等. 激光法与湿筛-吸管法测定土壤颗粒组成的转换及质地确定[J]. 土壤学报, 2009, 46(5): 772-780.]
(  0) 0) |
| [36] |
Lu R K. Analytical methods for soil and agro-chemical analysis (In Chinese). Beijing: China Agricultural Science and technology Press, 2000. [鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000.]
(  0) 0) |
| [37] |
Zhang G L, Gong Z T. Soil survey laboratory methods (In Chinese). Beijing: Science Press, 2012. [张甘霖, 龚子同. 土壤调查实验室分析方法[M]. 北京: 科学出版社, 2012.]
(  0) 0) |
| [38] |
Nesbitt H W, Wilson R E. Recent chemical weathering of basalts[J]. American Journal of Science, 1992, 292(10): 740-777.
(  0) 0) |
| [39] |
White A F, Blum A E. Effects of climate on chemical weathering in watersheds[J]. Geochimica et Cosmochimica Acta, 1995, 59(9): 1729-1747.
(  0) 0) |
| [40] |
Hartmann J, Moosdorf N. Chemical weathering rates of silicate-dominated lithological classes and associated liberation rates of phosphorus on the Japanese Archipelago—Implications for global scale analysis[J]. Chemical Geology, 2011, 287(3/4): 125-157.
(  0) 0) |
| [41] |
Cui J Y, Ren L, Wang J G, et al. Effect of organic acids on mobilization of K from K-bearing minerals and stochastic simulation of dynamic K release (In Chinese)[J]. Acta Pedologica Sinica, 2002, 39(3): 341-350. [崔建宇, 任理, 王敬国, 等. 有机酸影响矿物钾释放的室内试验与数学模拟[J]. 土壤学报, 2002, 39(3): 341-350.]
(  0) 0) |
| [42] |
Huang W H, Keller W D. Dissolution of rock-forming silicate minerals in organic acids: Simulated first-stage weathering of fresh mineral surfaces[J]. American Mineralogist, 1970, 55(11/12): 2076-2094.
(  0) 0) |
| [43] |
Ren Y C, Ruan L, Li X, et al. Atmospheric dust input to soils in Xin-Sheng Basin, Zhejiang Province based on grain size analysis (In Chinese)[J]. Soils, 2021, 53(4): 865-873. [任玉成, 阮丽, 李欣, 等. 基于粒度方法的大气降尘对浙江省新嵊盆地土壤的物源输入研究[J]. 土壤, 2021, 53(4): 865-873.]
(  0) 0) |
| [44] |
Qiao Q Q, Huang B C, Zhang C X. Magnetic properties and pollution sources in dustfall and topsoil of North China (In Chinese)[J]. Chinese Science Bulletin, 2014, 59(18): 1748-1760. [乔庆庆, 黄宝春, 张春霞. 华北地区大气降尘和地表土壤磁学特征及污染来源[J]. 科学通报, 2014, 59(18): 1748-1760.]
(  0) 0) |
| [45] |
Ni L J, Zhang G L, Yang J L, et al. Effects of atmospheric dust on soils in industrial zones (In Chinese)[J]. Acta Pedologica Sinica, 2007, 44(4): 637-642. [倪刘建, 张甘霖, 杨金玲, 等. 钢铁工业区降尘对周边土壤的影响[J]. 土壤学报, 2007, 44(4): 637-642.]
(  0) 0) |
| [46] |
Liu J, Luo L Q. Advances in research on the mechanisms of plant-driven mineral weathering (In Chinese)[J]. Chinese Journal of Applied and Environmental Biology, 2019, 25(6): 1503-1511. [柳检, 罗立强. 植物对矿物的风化作用机制研究进展[J]. 应用与环境生物学报, 2019, 25(6): 1503-1511.]
(  0) 0) |
| [47] |
Cai Z C. The role of soil in the formation of plant biodiversity and its research significance (In Chinese)[J]. Acta Pedologica Sinica, 2022, 59(1): 1-9. [蔡祖聪. 土壤在植物多样性形成中的作用及其研究意义[J]. 土壤学报, 2022, 59(1): 1-9.]
(  0) 0) |
| [48] |
Zhu Y G, Duan G L, Chen B D, et al. Mineral weathering and element cycling in soil microbial plant system (In Chinese)[J]. Scientia Sinica(Terrae), 2014, 44(6): 1107-1116. [朱永官, 段桂兰, 陈保冬, 等. 土壤-微生物-植物系统中矿物风化与元素循环[J]. 中国科学(地球科学), 2014, 44(6): 1107-1116.]
(  0) 0) |
 2023, Vol. 60
2023, Vol. 60


