2. 南京农业大学草业学院, 南京 210095
2. College of Agro-grassland Science, Nanjing Agricultural University, Nanjing 210095, China
土壤是微生物的大本营,除了有益微生物,还有能够威胁植物、动物和人体健康的病原微生物等生物污染因子。以病原细菌为例,青枯菌(Ralstonia solanacearum)、欧文氏杆菌(Erwinia chrysanthemi)等土传植物致病菌能够侵染茄科、豆科等作物,对农业造成严重的经济损失[1-2];大肠杆菌(Escherichia coli)、沙门氏菌(Salmonella enterica)和炭疽芽孢杆菌(Bacillus anthracis)等是常见的人体致病菌,能够在土壤中长期存活,通过直接或间接接触感染人畜,造成严重的健康风险[3-4]。此外,土壤病原菌携带着多种抗生素抗性基因(Antibiotic resistance genes,ARGs),尤其是在农药、除草剂等多重土壤非生物污染胁迫下,通过土壤病原菌向食物链传播ARGs、威胁人畜健康的风险日益增加[5],引起人们对一体化健康恶化的广泛关注,亟需建立生态消减土壤生物污染的技术体系。大量研究表明有益微生物在阻控土壤病原细菌生物污染中发挥重要作用[6],其中专性侵染细菌的病毒——噬菌体在环境中广泛存在(数量高达1031个[7]);因具有靶向性强、裂解速度快、对环境扰动小等优势,基于噬菌体的生态疗法引起越来越多的关注。
目前噬菌体疗法在医疗、环境和农业等领域有不少的尝试[8],但提升其在消减土壤生物污染的效果稳定性仍是当前的重大挑战。尽管已有一些影响噬菌体疗法稳定性因素的报道[9-10],但关于噬菌体消减土壤病原细菌的制约因素还缺乏梳理。基于噬菌体裂解土壤病原细菌的过程,可以将影响噬菌体疗法的因素分为三类:1)噬菌体的宿主细菌谱和种群数量,2)病原细菌的多态性,3)影响噬菌体-病原细菌互作的环境因素,包括土壤温度、pH、结构、营养物质、多重污染物等。上述因素均能够影响土壤中噬菌体的生存、迁移以及与宿主病原细菌互作,进而影响噬菌体疗法的效果和稳定性。为此,本文从噬菌体、病原细菌和环境因素三个方面系统梳理影响噬菌体靶向消减土壤病原细菌效果的因素,并提出提升噬菌体疗法稳定性的策略,为完善土壤生物污染的噬菌体疗法提供科学支撑。
1 土壤噬菌体的裂解能力和种群数量 1.1 噬菌体裂解病原细菌能力噬菌体靶向消减土壤生物污染的关键是其能特异性“猎杀”病原细菌,但不侵染裂解土壤中非宿主细菌。侵染裂解的特异性取决于噬菌体识别蛋白与细菌表面的受体分子结构的互补[11]。噬菌体的宿主范围差异较大,一般噬菌体只能侵染一种基因型或生理型的菌株,但也存在侵染多个细菌种/属的多价噬菌体。土壤噬菌体的宿主谱宽窄决定着其在土壤中的宿主可获得性和存活能力,进而影响了噬菌体疗法的稳定性。关于噬菌体的宿主范围,一般需要结合大规模的实验室测试来评估[12]。针对土壤中多种病原细菌共存的情况,筛选宿主谱不同的噬菌体组合成鸡尾酒,可以增强其在土壤中的定殖与存活。
除了宿主范围,噬菌体的裂解效率也影响其消减土壤生物污染效果及稳定性。在宿主细胞内完成复制后,烈性噬菌体需要裂解细菌的细胞壁以释放后代,其中裂解酶或穿孔蛋白能够攻击肽聚糖,干扰蛋白质的合成,从而导致宿主细菌的细胞壁错误装配或裂解[13-15]。评估噬菌体的裂解效率包括比较体系中:噬菌体滴度的增量、宿主细菌的减少量[16]、噬菌体产生的裂解酶等[17]。噬菌体裂解效率影响土壤中噬菌体-宿主病原细菌互作强度。高裂解率意味着噬菌体能够迅速侵染并破坏宿主病原细菌的细胞,释放更多的子代噬菌体到土壤中,以持续侵染裂解宿主细菌的其他细胞。
1.2 噬菌体和病原细菌的种群丰度噬菌体在土壤中大量定殖和存活是其消减土壤生物污染的基础。为了压制土壤病原菌的增殖,必须保证足够浓度/密度的噬菌体[18]。目标病原细菌周围的噬菌体数量越多,可以增加噬菌体与病原菌的接触和互作频率,侵染成功率也会随之增加[19]。噬菌体种群数量可以通过以下方式计算:单位体积内在含菌平板上形成的噬菌体空斑数量(PFU)[20]、电镜观测到的单位土壤中病毒样颗粒的数量[21]、基于高通量测序注释到的噬菌体物种丰度[22]。研究表明,噬菌体对土壤青枯菌生物污染的消减效果随着噬菌体接种剂量的增加(104→108 PFU g–1土)而提高[23]。需要注意的是,噬菌体的接种剂量并非越高越好,还需综合考虑噬菌体的最佳侵染复数(MOI)和最小抑菌浓度等因素。
此外,噬菌体消减土壤生物污染的效果和稳定性也受土壤病原细菌的丰度影响。当病原菌数量较低时,大量的噬菌体颗粒因接触不到宿主而失活或者转为溶原状态,进而降低了消减效果。当土壤中目标病原细菌的数量较多时,噬菌体侵染细菌的机会大大增加[24]。但是,当土壤病原菌的数量很高时,其种群抵御噬菌体侵染的能力会通过突变进化适应而增加,进而导致噬菌体疗法失败。因此,在实际生产应用过程中,应结合土壤病原菌的定量检测技术,基于检测结果,在适宜的病原菌种群丰度条件下施用合适滴度的噬菌体,将土壤病原菌的数量控制在对作物、生态环境和人畜健康相对安全的范围内。
2 土壤病原细菌的多态性 2.1 土壤病原细菌的多态性土壤病原菌生物污染难以消减的一个重要原因是其具有强环境适应性和高度的变异性。以土壤病原青枯菌为例,该生物污染因子可分为5个生理小种、6个生化小种、4个基因型和大量多样的序列变种[25],说明青枯菌在不同寄主植物-土壤系统中长期适应演化出丰富的基因型,表现出生理表型的多态性[26]。田间土壤中病原细菌的多态性大大制约了单一噬菌体的防控效果,所以在实际应用过程中,亟需针对特定土壤-作物系统评估病原菌的多态性,筛选出针对性的噬菌体资源,形成高效的噬菌体鸡尾酒防控策略。
2.2 土壤病原细菌抗噬菌体机制的多样性土壤病原细菌在定殖致病过程中会形成生物膜或胞外多糖,通过形成“避难所”或掩蔽受体[27]阻止噬菌体对其的侵染裂解。除了这些天然的物理阻碍屏障,细菌还进化出一系列的抗噬菌体机制[28](图 1)。1)抑制吸附:细菌通过调控受体基因的表达、改变受体蛋白的空间构型或修饰受体,产生竞争性抑制子或胞外物质掩盖受体等方式阻止噬菌体对受体的识别吸附;2)Sie系统:在噬菌体突破第一重物理屏障成功吸附到细菌表面后,细菌利用Sie系统改变表面的通透性,阻止噬菌体DNA注入;3)R-M系统:噬菌体DNA进入胞内后,细菌通过启动限制/修饰防御系统(Restriction-modification system,R-M),阻断噬菌体DNA整合到染色体中;4)利用CRISPR-Cas系统靶向烈性噬菌体基因中的原型间隔序列(Proto-spacer),对入侵的dsDNA进行切割;5)化学抑制:在链霉菌属中发现了一种化学防御,宿主细菌产生一个小的抗噬菌体分子,该分子插入噬菌体DNA并抑制其复制;6)流产侵染:被侵染的细菌启动Abi系统,采取程序性自我裂解、中断噬菌体复制的“流产侵染”方式,保护其他种群免遭噬菌体的侵染。这些多样的抗噬菌体机制可以增强病原细菌对噬菌体的适应性,降低土壤生物污染的消减效果。在应用噬菌体消减土壤生物污染的过程中应加强对病原细菌抗性的监测和噬菌体鸡尾酒配方的动态更新,以积极应对病原细菌的抗性进化。
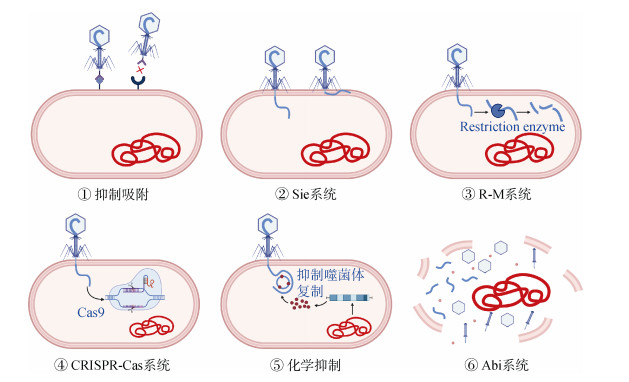
|
图 1 土壤病原细菌的抗噬菌体机制 Fig. 1 Anti-phage mechanisms act at different stages of the phage life cycle |
土壤中噬菌体的存在状态可分为两种:活跃状态和不活跃状态。噬菌体处于活跃状态时,它们能够侵染或以溶原方式存在于细菌宿主中,通过裂解释放子代噬菌体或形成溶原细菌来实现增殖。这类噬菌体维持了细菌群落的动态平衡和土壤微生物多样性。当遇到干旱、极端温度和低营养等胁迫环境时,噬菌体在土壤中可能会失去活性,暂时无法侵染细菌。此外,大部分噬菌体可能附着在土壤颗粒或有机质表面,影响了它们与细菌宿主的相互作用。土壤pH、温度、结构、营养条件等非生物因素会影响土壤中噬菌体的存在状态[29](图 2),进而影响噬菌体消减土壤生物污染的效果。

|
图 2 制约噬菌体消减土壤病原细菌效率的主要因素 Fig. 2 The main factors that limit the efficiency of bacteriophage-mediated reduction of soil pathogenic bacteria |
土壤的pH和温度不仅能够影响宿主细菌的生长、代谢活性,还能够直接影响噬菌体的存活和裂解能力[30]。研究表明,噬菌体在pH 6~8时能够长期保持较高的活性,但过酸或过碱均会影响到噬菌体的滴度[31]。Sykes等[32]试图从不同pH的土壤中分离链霉菌噬菌体,但在pH低于6.0时未检测到链霉菌噬菌体。此外,土壤pH会影响噬菌体和土壤颗粒表面的电荷和离子强度,决定了它们之间的结合力,影响土壤胶体对噬菌体的吸附,进而影响噬菌体在土壤中的迁移[33]。
土壤中噬菌体丰度随时间和季节变化而变化的特征主要是其对温度变化的差异响应。温度会通过影响噬菌体在土壤中的存活、对宿主病原细菌的裂解能力来影响其抑菌功能。噬菌体对温度的耐受范围较广,在4℃下可保存数月,且抑菌效果稳定[34];但在高温条件下(50~80℃)的抑菌效果会显著下降[35]。高温除了会影响噬菌体的存活,还可能会影响噬菌体对宿主菌的吸附能力从而降低裂解效率[36]。
3.2 土壤结构和有机质含量由于土壤结构的高度异质性,噬菌体在土壤中的分布极不均匀[37]。在范德华力的作用下,土壤黏土矿物或胶体吸附着大量噬菌体[38],限制噬菌体在土壤中的迁移以及与宿主细菌的接触和有效互作。Carlson等[39]比较了不同黏土颗粒(高岭石、蒙脱土和伊利石)对T2噬菌体的吸附作用,发现蒙脱石和伊利石对噬菌体迁移的限制更强。不同矿物吸附噬菌体的亲和力不同[40],主要取决于黏土矿物的电荷类型和阳离子交换容量(CEC)[41]。其次土壤中有机质含量也影响噬菌体的迁移能力。一方面,有机质可以改变土壤铁铝氧化物和层状硅酸盐矿物表面电荷和聚集行为[42];另一方面,有机质自身携带的电荷可以中和土壤颗粒的电荷,或与噬菌体竞争土壤颗粒表面的吸附位点来减少噬菌体的吸附固定[43-44],进而影响噬菌体的迁移能力[45]。噬菌体与宿主细菌个体差异达到几十至上千倍[46-47],除了土壤颗粒吸附对噬菌体迁移运动的影响,土壤高度异质复杂的空间结构进一步降低了噬菌体与宿主细菌接触的机率[48]。
3.3 土壤营养物质土壤中的矿质营养和有机营养可以通过影响宿主细菌的种群数量和代谢活性,进而影响噬菌体-病原细菌的互作。研究表明高营养资源条件能提高细菌对噬菌体的抗性[49-50]。对此有两个解释:1)资源丰富时,细菌对资源竞争压力小,可以将更多的成本投入到对噬菌体的抗性进化[51];2)资源可利用性的增加支撑更高的宿主种群数量,增加了抗噬菌体侵染的抗性进化突变概率[49]。相反,土壤中的养分资源相对匮乏,导致土壤病原细菌种群的减少和生长速率的降低,对噬菌体侵染产生抗性的成本增加[52],进而提升噬菌体的侵染效率。
3.4 土壤非生物污染胁迫土壤中的重金属、抗生素和有机污染等非生物污染物也会影响了土壤中噬菌体的存活及其与病原细菌的相互作用[53-54]。研究表明,金属、氰化物、有机污染物等能够诱导溶原噬菌体的切离(从细菌基因组上释放,进入裂解循环)[55-56];长期暴露的铜污染土壤中检测到更高水平的活性噬菌体[57]。在抗生素存在的情况下,噬菌体的增殖速度、裂解性和爆发量大小等特征会发生变化[58]。与β-内酰胺或大环内酯类抗生素对噬菌体的负面影响相比,氟喹诺酮类药物对噬菌体抑菌效果具有改善或中性作用[59]。多种非生物复合污染胁迫如何影响噬菌体的应用效果仍有待进一步研究。
4 噬菌体疗法的提升策略 4.1 构建高效的噬菌体鸡尾酒将不同类型或不同宿主范围的噬菌体组合成“噬菌体鸡尾酒”可以一定程度提高消减土壤生物污染的效率。潜在的机制包括:1)扩宽宿主范围,能够靶标更多的病原细菌,提高噬菌体在土壤中的定殖与存活能力;2)具备不同侵染机制的噬菌体组合,能够限制病原菌抗性突变的方向、降低其突变成功率、减少交互抗性的产生[60];3)增强目标病原细菌的抗性成本,同时对所有噬菌体产生抗性需要付出巨大的适应性成本,包括毒力受损或生长减慢[61]。作者前期研究发现噬菌体鸡尾酒能够显著提升噬菌体消减土壤青枯菌生物障碍的稳定性,主要通过压制根际病原细菌的数量,即使残存下来的病原细菌对噬菌体产生抗性,但削弱了病原细菌的生长、竞争和致病能力[62]。针对田间病原细菌的高度多态性,可以通过深度学习大量的病原菌-噬菌体基因组序列,建立噬菌体-病原细菌互作的预测模型,快速筛选出针对特定病原菌的噬菌体鸡尾酒1.0配方。针对病原细菌的变异性,尤其是能够快速进化出对噬菌体的抗性,结合实验进化学方法筛选进化后的噬菌体,即能够侵染抗性菌株的噬菌体[63],获得靶向当代和进化后病原菌的进阶版噬菌体鸡尾酒2.0,限制病原菌所有可能的进化突变方向[64],从而提高噬菌体抑制病原细菌的稳定性。此外,可以结合土壤中非病原菌噬菌体资源,在解析挖掘定向增强有益菌抑菌功能的有益菌专性噬菌体的基础上,构建多重靶向病原菌的超级噬菌体鸡尾酒3.0。以土壤病原青枯菌为例,图 3展示了构建3个版本噬菌体鸡尾酒的策略,针对不同作物-土壤系统青枯菌特性和环境特点,评估不同版本的噬菌体鸡尾酒生防的稳定性,最终形成高效稳定、生态安全的根际微生态精准调控技术体系。
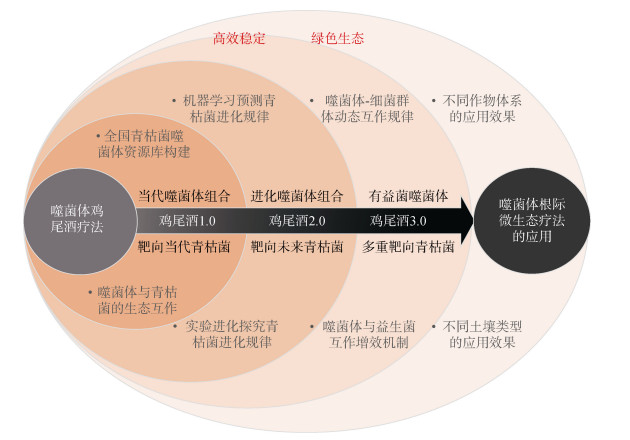
|
图 3 针对土壤青枯菌生物障碍的噬菌体鸡尾酒构建策略 Fig. 3 Construction strategy of bacteriophage cocktail targeting soil-borne pathogen Ralstonia solanacearum |
当前市场上的噬菌体产品主要有液体、包埋颗粒和固体粉末三种形式,其中液体形式的噬菌体产品的生产成本低,但运输成本高,且不耐存贮。利用纤维素、脂质体、海藻酸钠、乳清蛋白和明胶等生物材料对噬菌体进行包埋封装[65],或添加保护剂,制备噬菌体冻干粉末,能够有效提高噬菌体长期储存的稳定性[66-68]。但包埋颗粒和固体粉末形式的噬菌体生产技术和成本较高。在实际应用过程中,应根据需要选择合适的噬菌体产品和剂型,以获得最大的效益和消减效果。针对上述三种噬菌体产品,均可以在生产时添加保护剂,延长噬菌体产品的货架期,并提升噬菌体抑制病原菌的稳定性。早期研究表明抗氧化剂、氧化酶、芳香族氨基酸、活性炭、淀粉、碱性谷朊、酪蛋白等均可以较好地保护噬菌体免受外界环境影响[69-72]。多个专利也报道了一些物质,如海藻糖、甘油、壳聚糖、蔗糖、明胶等保存噬菌体过程中均有延长其活性的作用[73-74]。在实际应用的过程中,可以将噬菌体与保护剂配合,提高噬菌体在复杂土壤环境中的定殖和存活能力,进而提高噬菌体对土壤病原细菌的抑制效果。
4.3 噬菌体产品施用技术改良优化施用时间和剂量:为了降低高温和紫外线等对噬菌体的杀灭作用,噬菌体施用时间上应避开高温或强辐射时段。噬菌体施用时考虑土壤生物污染爆发的时间也至关重要,既要考虑土壤中可能预先存在病原菌,又要考虑病原菌到达根表之前实现噬菌体的精准“拦截”[75]。一般噬菌体的施用应尽量在病原菌入侵之前或移栽早期进行,以避免病原菌大规模扩散和病害严重程度的增加。为了增加噬菌体在土壤中的定殖和存活,生产过程中可以通过增加施用频次或施用浓度,强化噬菌体消减土壤生物污染的稳定性。
联合多种施用方法:农业生产过程中噬菌体的施用一般有叶面喷施、蘸根、灌根和茎部注射等方式。叶面喷施是利用喷雾装置将噬菌体均匀地喷洒在植物叶面上,主要针对一些叶部病害,如黄单胞菌(Xanthomonas oryzae pv. oryzae)引起的叶斑病[76]。蘸根是在作物幼苗移栽之前,将其根部浸泡在噬菌体溶液中[34],能够帮助植株建立早期的防御机制,并与潜在的根部病原细菌发生作用。灌根是将噬菌体制剂直接注入植物的根部土壤中,能够使噬菌体更有效地接触并控制根际区域的病原细菌[62]。茎部注射是将噬菌体制剂直接注入植物的茎部组织,一般是木质部导管中,可以针对少数土壤生物污染严重的植株做精确处理,尤其是针对像青枯菌这样在木质部导管中大量增殖的病原细菌[77]。不同的施用方式均有一定的优势和局限性,如叶面喷施噬菌体的效果会受到UV照射、雨水冲刷等因素的影响,可以通过增加噬菌体喷施的频次[78]、延长噬菌体与叶面接触的时间[79]来提高噬菌体抑菌的稳定性[80]。茎部注射法具有效率高、用量小和见效快的特点,但实际应用过程中工作量大,增加了人工成本[77]。所以需要针对特定的场景选择一种或多种施用方式联合,增强噬菌体消减土壤生物污染的稳定性。
与有益菌产品配施:根际有益功能菌能够产生环状脂肽、伊枯草菌素(Iturin)和芬荠素(Fengycin)等抑菌活性物质[81]或与病原菌竞争生态位(资源和空间)[82]抑制病原菌的生长。作者前期研究发现噬菌体和解淀粉芽孢杆菌(产生拮抗物质)组合能有效降低病原菌的数量,提高土传青枯病防控效率[83]。作为预防性措施,噬菌体可能会在病原菌出现之前被施用。为了确保噬菌体的活性,可以利用活体细胞实现噬菌体的投递。Iriarte等[75]发现Xanthomonas perforans低致病力菌株可以提高噬菌体在土壤中的存活和种群数量。一般无致病力菌株能够为噬菌体提供一个良好载体,既能维持噬菌体的种群数量和抑菌活性,本身又具有一定的消减土壤生物污染的能力。将噬菌体与这类有益菌产品配合施用,能够进一步提升噬菌体消减土壤生物污染的稳定性。
5 结论与展望噬菌体在精准靶向消减土壤生物污染中具有广阔的前景,但土壤的生物和非生物因素不同程度制约了噬菌体对土壤病原细菌的消减效果。尽管本文梳理了潜在的影响因素及提升策略,但相关的研究证据有待丰富,应用效果有待进一步实践验证。针对噬菌体靶向侵染土壤病原菌的推广和应用,以下几方面研究值得关注:
(1)当前分离获得的噬菌体资源相对有限,土壤是微生物的大本营,丰富的噬菌体资源仍有待开发利用。高通量筛选鉴定噬菌体,并完善基础信息,建立噬菌体资源库是必要的。除此之外还可以利用基因工程定向改造噬菌体改造或合成新的噬菌体,以去除其携带的潜在的致病基因[84],同时扩大噬菌体的宿主范围[85],甚至可以定向合成噬菌体的裂解酶和尾霉素等抑菌物质[86-87],避免噬菌体其他内容物或溶原的风险。
(2)不同土壤-作物系统中的病原细菌的多态性还有待进一步评估,并建立具有代表性的病原菌资源库;结合通量交互测试和实验进化学探究病原细菌-噬菌体的生态互作规律及共进化动力学特征。在此基础上针对特定系统中的病原细菌能够利用机器学习从噬菌体库中快速筛选有效可用的噬菌体,获得高效的噬菌体产品。
(3)针对环境的复杂性,需要开发噬菌体产品的形式,结合室内-盆栽-大田实验,探究噬菌体产品与有益菌、生物有机肥等配合在不同作物-土壤系统的应用效果,优化施用剂量、时间和方式,建立噬菌体根际微生态疗法的应用技术体系,为不同土壤生物污染的精准靶向消减提供定制化的噬菌体调控策略。
| [1] |
Farrar J J, Nunez J J, Davis R M. Influence of soil saturation and temperature on Erwinia chrysanthemi soft rot of carrot[J]. Plant Disease, 2000, 84(6): 665-668. DOI:10.1094/PDIS.2000.84.6.665
(  0) 0) |
| [2] |
Jiang G F, Wei Z, Xu J, et al. Bacterial wilt in China: History, current status, and future perspectives[J]. Frontiers in Plant Science, 2017, 8: 1549. DOI:10.3389/fpls.2017.01549
(  0) 0) |
| [3] |
Carlson C J, Kracalik I T, Ross N, et al. The global distribution of bacillus anthracis and associated anthrax risk to humans, livestock and wildlife[J]. Nature Microbiology, 2019, 4(8): 1337-1343. DOI:10.1038/s41564-019-0435-4
(  0) 0) |
| [4] |
Rahman M T, Sobur M A, Islam M S, et al. Zoonotic diseases: Etiology, impact, and control[J]. Microorganisms, 2020, 8(9): 1405. DOI:10.3390/microorganisms8091405
(  0) 0) |
| [5] |
Zhu Y G, Gillings M, Penuelas J. Integrating biomedical, ecological, and sustainability sciences to manage emerging infectious diseases[J]. One Earth, 2020, 3(1): 23-26. DOI:10.1016/j.oneear.2020.06.004
(  0) 0) |
| [6] |
De Coninck B, Timmermans P, Vos C, et al. What lies beneath: Belowground defense strategies in plants[J]. Trends in Plant Science, 2015, 20(2): 91-101. DOI:10.1016/j.tplants.2014.09.007
(  0) 0) |
| [7] |
Pratama A A, van Elsas J D. The 'neglected' soil virome - Potential role and impact[J]. Trends in Microbiology, 2018, 26(8): 649-662. DOI:10.1016/j.tim.2017.12.004
(  0) 0) |
| [8] |
Wagemans J, Holtappels D, Vainio E, et al. Going viral: Virus-based biological control agents for plant protection[J]. Annual Review of Phytopathology, 2022, 60: 21-42. DOI:10.1146/annurev-phyto-021621-114208
(  0) 0) |
| [9] |
Kimura M, Jia Z J, Nakayama N, et al. Ecology of viruses in soils: Past, present and future perspectives[J]. Soil Science and Plant Nutrition, 2008, 54(1): 1-32. DOI:10.1111/j.1747-0765.2007.00197.x
(  0) 0) |
| [10] |
Ye M, Sun M M, Huang D, et al. A review of bacteriophage therapy for pathogenic bacteria inactivation in the soil environment[J]. Environment International, 2019, 129: 488-496. DOI:10.1016/j.envint.2019.05.062
(  0) 0) |
| [11] |
Bull J J, Otto G, Molineux I J. In vivo growth rates are poorly correlated with phage therapy success in a mouse infection model[J]. Antimicrobial Agents and Chemotherapy, 2012, 56(2): 949-954. DOI:10.1128/AAC.05842-11
(  0) 0) |
| [12] |
Lood C, Haas P J, van Noort V, et al. Shopping for phages? Unpacking design rules for therapeutic phage cocktails[J]. Current Opinion in Virology, 2022, 52: 236-243. DOI:10.1016/j.coviro.2021.12.011
(  0) 0) |
| [13] |
Bernhardt T G, Wang I N, Struck D K, et al. A protein antibiotic in the phage q beta virion: Diversity in lysis targets[J]. Science, 2001, 292(5525): 2326-2329. DOI:10.1126/science.1058289
(  0) 0) |
| [14] |
Fischetti V A. Bacteriophage lytic enzymes: Novel anti-infectives[J]. Trends in Microbiology, 2005, 13(10): 491-496. DOI:10.1016/j.tim.2005.08.007
(  0) 0) |
| [15] |
Young R, Wang I N, Roof W D. Phages will out: Strategies of host cell lysis[J]. Trends in Microbiology, 2000, 8(3): 120-128. DOI:10.1016/S0966-842X(00)01705-4
(  0) 0) |
| [16] |
Moses S, Vagima Y, Tidhar A, et al. Characterization of Yersinia pestis phage lytic activity in human whole blood for the selection of efficient therapeutic phages[J]. Viruses, 2021, 13(1): 89. DOI:10.3390/v13010089
(  0) 0) |
| [17] |
Young R. Phage lysis: Three steps, three choices, one outcome[J]. Journal of Microbiology, 2014, 52(3): 243-258. DOI:10.1007/s12275-014-4087-z
(  0) 0) |
| [18] |
Abedon S. Phage therapy pharmacology: Calculating phage dosing[J]. Advances in Applied Microbiology, 2011, 77: 1-40.
(  0) 0) |
| [19] |
Abedon S T. Phage therapy: Eco-physiological pharmacology[J]. Scientifica(Cairo), 2014, 2014: 581639.
(  0) 0) |
| [20] |
Anderson B, Rashid M H, Carter C, et al. Enumeration of bacteriophage particles: Comparative analysis of the traditional plaque assay and real-time qPCR- and nanosight-based assays[J]. Bacteriophage, 2011, 1(2): 86-93. DOI:10.4161/bact.1.2.15456
(  0) 0) |
| [21] |
Swanson M M, Fraser G, Daniell T J, et al. Viruses in soils: Morphological diversity and abundance in the rhizosphere[J]. Annals of Applied Biology, 2009, 155(1): 51-60. DOI:10.1111/j.1744-7348.2009.00319.x
(  0) 0) |
| [22] |
Johansen J, Plichta D R, Nissen J N, et al. Genome binning of viral entities from bulk metagenomics data[J]. Nature Communications, 2022, 13(1): 965. DOI:10.1038/s41467-022-28581-5
(  0) 0) |
| [23] |
Zhang S M. Study on preparation and application method optimization of phage for preventing and controlling soil-borne bacterial wilt[D]. Nanjing: Nanjing Agricultural University, 2021.[张淑敏. 防控番茄土传青枯病的噬菌体制备及施用方法优化[D]. 南京: 南京农业大学, 2021.]
(  0) 0) |
| [24] |
Levin B R, Bull J J. Population and evolutionary dynamics of phage therapy[J]. Nature Reviews Microbiology, 2004, 2(2): 166-173. DOI:10.1038/nrmicro822
(  0) 0) |
| [25] |
Genin S, Denny T P. Pathogenomics of the Ralstonia solanacearum species complex[J]. Annual Review of Phytopathology, 2012, 50: 67-89. DOI:10.1146/annurev-phyto-081211-173000
(  0) 0) |
| [26] |
Wang X F. Effects and eco-evolutionary mechanisms of bacteriophages on suppression of soil-borne bacterial[D]. Nanjing: Nanjing Agricultural University, 2019.[王孝芳. 噬菌体抑制土传青枯菌入侵番茄根际的效果及进化生态学机制研究[D]. 南京: 南京农业大学, 2019.]
(  0) 0) |
| [27] |
Bull J J, Christensen K A, Scott C, et al. Phage-bacterial dynamics with spatial structure: Self organization around phage sinks can promote increased cell densities[J]. Antibiotics(Basel), 2018, 7(1): 8.
(  0) 0) |
| [28] |
Labrie S J, Samson J E, Moineau S. Bacteriophage resistance mechanisms[J]. Nature Reviews Microbiology, 2010, 8(5): 317-327. DOI:10.1038/nrmicro2315
(  0) 0) |
| [29] |
Wang G H, Liu J J, Zhu D, et al. A review of researches on viruses in soil – Advancement and challenges (In Chinese)[J]. Acta Pedologica Sinica, 2020, 57(6): 1319-1332. [王光华, 刘俊杰, 朱冬, 等. 土壤病毒的研究进展与挑战[J]. 土壤学报, 2020, 57(6): 1319-1332.]
(  0) 0) |
| [30] |
Taj M K, Ling J X, Bing L L, et al. Effect of dilution, temperature and pH on the lysis activity of t4 phage against E. coli bl21[J]. Journal of Animal and Plant Sciences, 2014, 24(4): 1252-1255.
(  0) 0) |
| [31] |
Ly-Chatain M H. The factors affecting effectiveness of treatment in phages therapy[J]. Frontiers in Microbiology, 2014, 5: 51.
(  0) 0) |
| [32] |
Sykes I, Lanning S, Williams S. The effect of pH on soil actinophage[J]. Microbiology and Molecular Biology Reviews, 1981, 122(2): 271-280.
(  0) 0) |
| [33] |
Kapuscinski R B, Mitchell R. Processes controlling virus inactivation in coastal waters[J]. Water Research, 1980, 14(4): 363-371. DOI:10.1016/0043-1354(80)90084-6
(  0) 0) |
| [34] |
Fujiwara A, Fujisawa M, Hamasaki R, et al. Biocontrol of Ralstonia solanacearum by treatment with lytic bacteriophages[J]. Applied and Environmental Microbiology, 2011, 77(12): 4155-4162. DOI:10.1128/AEM.02847-10
(  0) 0) |
| [35] |
Ma Y Y, Li E N, Qi Z Z, et al. Isolation and molecular characterisation of Achromobacter phage phiAxp-3, an N4-like bacteriophage[J]. Scientific Reports, 2016, 6: 24776. DOI:10.1038/srep24776
(  0) 0) |
| [36] |
Tokman J I, Kent D J, Wiedmann M, et al. Temperature significantly affects the plaquing and adsorption efficiencies of listeria phages[J]. Frontiers in Microbiology, 2016, 7: 631.
(  0) 0) |
| [37] |
Williamson K E, Fuhrmann J J, Wommack K E, et al. Viruses in soil ecosystems: An unknown quantity within an unexplored territory[J]. Annual Review of Virology, 2017, 4: 201-219. DOI:10.1146/annurev-virology-101416-041639
(  0) 0) |
| [38] |
Chattopadhyay S, Puls R W. Forces dictating colloidal interactions between viruses and soil[J]. Chemosphere, 2000, 41(8): 1279-1286. DOI:10.1016/S0045-6535(99)00519-6
(  0) 0) |
| [39] |
Carlson G F Jr, Woodard F E, Wentworth D F, et al. Virus inactivation on clay particles in natural waters[J]. Journal Water Pollution Control Federation, 1968, 40(2): R89-106.
(  0) 0) |
| [40] |
Schiffenbauer M, Stotzky G. Adsorption of coliphages T1 and T7 to host and non-host microbes and to clay minerals[J]. Current Microbiology, 1983, 8(4): 245-249. DOI:10.1007/BF01579554
(  0) 0) |
| [41] |
Lipson S M, Stotzky G. Adsorption of reovirus to clay minerals: Effects of cation-exchange capacity, cation saturation, and surface area[J]. Applied and Environmental Microbiology, 1983, 46(3): 673-682. DOI:10.1128/aem.46.3.673-682.1983
(  0) 0) |
| [42] |
Zhuang J, Yu G R. Effects of surface coatings on electrochemical properties and contaminant sorption of clay minerals[J]. Chemosphere, 2002, 49(6): 619-628. DOI:10.1016/S0045-6535(02)00332-6
(  0) 0) |
| [43] |
Gerba C P. Applied and theoretical aspects of virus adsorption to surfaces[J]. Advances in Applied Microbiology, 1984, 30: 133-168.
(  0) 0) |
| [44] |
Powelson D K, Simpson J R, Gerba C P. Effects of organic-matter on virus transport in unsaturated flow[J]. Applied and Environmental Microbiology, 1991, 57(8): 2192-2196. DOI:10.1128/aem.57.8.2192-2196.1991
(  0) 0) |
| [45] |
Zhuang J, Jin Y. Virus retention and transport as influenced by different forms of soil organic matter[J]. Journal of Environmental Quality, 2003, 32(3): 816-823. DOI:10.2134/jeq2003.8160
(  0) 0) |
| [46] |
Kuzyakov Y, Mason-Jones K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions[J]. Soil Biology & Biochemistry, 2018, 127: 305-317.
(  0) 0) |
| [47] |
Mendes R, Garbeva P, Raaijmakers J M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms[J]. FEMS Microbiology Reviews, 2013, 37(5): 634-663. DOI:10.1111/1574-6976.12028
(  0) 0) |
| [48] |
Chevallereau A, Pons B J, van Houte S, et al. Interactions between bacterial and phage communities in natural environments[J]. Nature Reviews Microbiology, 2022, 20(1): 49-62. DOI:10.1038/s41579-021-00602-y
(  0) 0) |
| [49] |
Gomez P, Bennie J, Gaston K J, et al. The impact of resource availability on bacterial resistance to phages in soil[J]. PLoS One, 2015, 10(4): e0123752. DOI:10.1371/journal.pone.0123752
(  0) 0) |
| [50] |
Lopez Pascua L, Hall A R, Best A, et al. Higher resources decrease fluctuating selection during host–parasite coevolution[J]. Ecology Letters, 2014, 17(11): 1380-1388. DOI:10.1111/ele.12337
(  0) 0) |
| [51] |
Kraaijeveld A R, Godfray H C J. Trade-off between parasitoid resistance and larval competitive ability in drosophila melanogaster[J]. Nature, 1997, 389(6648): 278-280. DOI:10.1038/38483
(  0) 0) |
| [52] |
Gomez P, Buckling A. Bacteria-phage antagonistic coevolution in soil[J]. Science, 2011, 332(6025): 106-109. DOI:10.1126/science.1198767
(  0) 0) |
| [53] |
Denes T, Wiedmann M. Environmental responses and phage susceptibility in foodborne pathogens: Implications for improving applications in food safety[J]. Current Opinion in Biotechnology, 2014, 26: 45-49. DOI:10.1016/j.copbio.2013.09.001
(  0) 0) |
| [54] |
Fister S, Robben C, Witte A K, et al. Influence of environmental factors on phage-bacteria interaction and on the efficacy and infectivity of phage P100[J]. Frontiers in Microbiology, 2016, 7: 1152.
(  0) 0) |
| [55] |
Choi J D, Kotay S M, Goel R. Various physico-chemical stress factors cause prophage induction in Nitrosospira multiformis 25196-an ammonia oxidizing bacteria[J]. Water Research, 2010, 44(15): 4550-4558. DOI:10.1016/j.watres.2010.04.040
(  0) 0) |
| [56] |
Motlagh A M, Bhattacharjee A S, Goel R. Microbiological study of bacteriophage induction in the presence of chemical stress factors in enhanced biological phosphorus removal(EBPR)[J]. Water Research, 2015, 81: 1-14. DOI:10.1016/j.watres.2015.04.023
(  0) 0) |
| [57] |
Jacquiod S, Nunes I, Brejnrod A, et al. Long-term soil metal exposure impaired temporal variation in microbial metatranscriptomes and enriched active phages[J]. Microbiome, 2018, 6(1): 223. DOI:10.1186/s40168-018-0606-1
(  0) 0) |
| [58] |
Cairns J, Becks L, Jalasvuori M, et al. Sublethal streptomycin concentrations and lytic bacteriophage together promote resistance evolution[J]. Philosophical Transactions of the Royal Society B-Biological Sciences, 2017, 372: 20160040. DOI:10.1098/rstb.2016.0040
(  0) 0) |
| [59] |
Torres-Barcelo C, Gurney J, Gougat-Barbera C, et al. Transient negative effects of antibiotics on phages do not jeopardise the advantages of combination therapies[J]. FEMS Microbiology Ecology, 2018, 94(8): fiy107.
(  0) 0) |
| [60] |
van Houte S, Buckling A, Westra E R. Evolutionary ecology of prokaryotic immune mechanisms[J]. Microbiology and Molecular Biology Reviews, 2016, 80(3): 745-763.
(  0) 0) |
| [61] |
Das M, Bhowmick T S, Ahern S J, et al. Control of pierce's disease by phage[J]. PLoS One, 2015, 10(6): e0128902. DOI:10.1371/journal.pone.0128902
(  0) 0) |
| [62] |
Wang X F, Wei Z, Yang K M, et al. Phage combination therapies for bacterial wilt disease in tomato[J]. Nature Biotechnology, 2019, 37(12): 1513-1520. DOI:10.1038/s41587-019-0328-3
(  0) 0) |
| [63] |
Yu L, Wang S, Guo Z M, et al. A guard-killer phage cocktail effectively lyses the host and inhibits the development of phage-resistant strains of Escherichia coli[J]. Applied Microbiology and Biotechnology, 2018, 102: 971-983.
(  0) 0) |
| [64] |
Bernheim A, Sorek R. The pan-immune system of bacteria: Antiviral defence as a community resource[J]. Nature Reviews Microbiology, 2020, 18(2): 113-119.
(  0) 0) |
| [65] |
Colom J, Cano-Sarabia M, Otero J, et al. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy[J]. Scientific Reports, 2017, 7: 41441.
(  0) 0) |
| [66] |
Ackermann H W, Tremblay D, Moineau S. Long-term bacteriophage preservation[J]. World Federation for Culture Collections Newsletter, 2004, 38: 35-40.
(  0) 0) |
| [67] |
Clark W A. Comparison of several methods for preserving bacteriophages[J]. Applied Microbiology, 1962, 10(5): 466-471.
(  0) 0) |
| [68] |
Manohar P, Ramesh N. Improved lyophilization conditions for long-term storage of bacteriophages[J]. Scientific Reports, 2019, 9: 15242.
(  0) 0) |
| [69] |
Balogh B, Jones J B, Momol M T, et al. Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato[J]. Plant Disease, 2003, 87(8): 949-954.
(  0) 0) |
| [70] |
Ignoffo C M, Garcia C. Antioxidant and oxidative enzyme effects on the inactivation of inclusion-bodies of the heliothis baculovirus by simulated sunlight-UV[J]. Environmental Entomology, 1994, 23(4): 1025-1029.
(  0) 0) |
| [71] |
Ignoffo C M, Garcia C, Saathoff S G. Sunlight stability and rain-fastness of formulations of baculovirus heliothis[J]. Environmental Entomology, 1997, 26(6): 1470-1474.
(  0) 0) |
| [72] |
Trong H L, Beier N, Sonnenburg W K, et al. Amino-acid-sequence of the cyclic-gmp stimulated cyclic-nucleotide phosphodiesterase from bovine heart[J]. Biochemistry, 1990, 29(44): 10280-10288.
(  0) 0) |
| [73] |
Liu X Q, Wang G P, Qian X Q. A bacteriophage protectant and its application(CN201910647890.8)[P]. 2019-11-15.[刘小琴, 王贵平, 钱雪桥. 一种噬菌体保护剂及其应用(CN201910647890.8)[P]. 2019-11-15.]
(  0) 0) |
| [74] |
Xu W S, Song Z F, Chen B. A novel bacteriological phage preservation protective agent and its preparation method and application(CN201610416037.1)[P]. 2016-10-12.[许维素, 宋增福, 陈彪. 一种新型噬菌体保存保护剂及其制备方法和应用(CN201610416037.1)[P]. 2016-10-12.]
(  0) 0) |
| [75] |
Iriarte F B, Obradovic A, Wernsing M H, et al. Soil-based systemic delivery and phyllosphere in vivo propagation of bacteriophages: Two possible strategies for improving bacteriophage persistence for plant disease control[J]. Bacteriophage, 2012, 2(4): 215-224.
(  0) 0) |
| [76] |
Chae J C, Hung N B, Yu S M, et al. Diversity of bacteriophages infecting Xanthomonas oryzae pv. oryzae in paddy fields and its potential to control bacterial leaf blight of rice[J]. Journal of Microbiology and Biotechnology, 2014, 24(6): 740-747.
(  0) 0) |
| [77] |
Hou Y G. Selection of Ralstonia solanacearum- specific bacteriophages and their effects on control of tomato bacterial wilt[D]. Nanjing: Nanjing Agricultural University, 2018.[侯玉刚. 青枯菌专性噬菌体的筛选及其防控番茄土传青枯病的效果研究[D]. 南京: 南京农业大学, 2018.]
(  0) 0) |
| [78] |
Lang J M, Gent D H, Schwartz H F. Management of Xanthomonas leaf blight of onion with bacteriophages and a plant activator[J]. Plant Disease, 2007, 91(7): 871-878.
(  0) 0) |
| [79] |
Gasic K, Kuzmanovic N, Ivanovic M, et al. Complete genome of the Xanthomonas euvesicatoria specific bacteriophage KΦ1, its survival and potential in control of pepper bacterial spot[J]. Frontiers in Microbiology, 2018, 9: 02021.
(  0) 0) |
| [80] |
Nakayinga R, Makumi A, Tumuhaise V, et al. Xanthomonas bacteriophages: A review of their biology and biocontrol applications in agriculture[J]. BMC Microbiology, 2021, 21(1): 291.
(  0) 0) |
| [81] |
Ongena M, Jacques P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol[J]. Trends in Microbiology, 2008, 16(3): 115-125.
(  0) 0) |
| [82] |
Wei Z, Yang T, Friman V-P, et al. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health[J]. Nature Communications, 2015, 6(1): 1-9.
(  0) 0) |
| [83] |
Wang X F, Wei Z, Li M, et al. Parasites and competitors suppress bacterial pathogen synergistically due to evolutionary trade-offs[J]. Evolution, 2017, 71(3): 733-746.
(  0) 0) |
| [84] |
Altamirano F L G, Barr J J. Phage therapy in the postantibiotic era[J]. Clinical Microbiology Reviews, 2019, 32(2): e00066-18.
(  0) 0) |
| [85] |
Hesse S, Adhya S. Phage therapy in the twenty-first century: Facing the decline of the antibiotic era; is it finally time for the age of the phage?[J]. Annual Review of Microbiology, 2019, 73: 155-174.
(  0) 0) |
| [86] |
Sao-Jose C. Engineering of phage-derived lytic enzymes: Improving their potential as antimicrobials[J]. Antibiotics(Basel), 2018, 7(2): 29.
(  0) 0) |
| [87] |
Young R, Gill J J. Phage therapy redux—What is to be done?[J]. Science, 2015, 350(6265): 1163-1164.
(  0) 0) |
 2023, Vol. 60
2023, Vol. 60


