2. 中国科学院大学, 北京 100049;
3. 楚雄师范学院资源环境与化学学院, 云南楚雄 675000;
4. 北京中向利丰科技有限公司, 北京 100004
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. School of Resource, Environment and Chemistry, Chuxiong Normal University, Chuxiong, Yunnan 675000, China;
4. View Sino International Limited Company, Beijing 100004, China
土壤有机质(SOM)是土壤肥力的重要参数[1],提高SOM含量有利于维持土壤生态功能,促进作物增产[2]。土壤有机质主要由活性有机质和稳定性腐殖物质组成[3],它们分别占肥沃潮土的30%和70%左右[4];在黑土和水稻土中,惰性碳库占总有机碳的比例分别为50%~70%和65%以上[5-6]。因此,土壤肥力和作物产量的同步提升需同时考虑活性和稳定性SOM含量的提升。
秸秆还田是提升SOM含量的重要措施之一,但其存在的主要问题包括:(1)提升有机质非常缓慢。Xu等[7]研究发现即使连续10年秸秆还田,土壤有机碳含量仅提升了0.7 g·kg–1,(2)短期内增产效果不明显。秸秆投入前期由于微生物活性不够,秸秆养分释放和土壤养分循环转化慢、微生物固氮等原因导致作物可利用的速效养分降低,从而影响作物生长[8-9]。为了解决上述问题,通过添加外源生物激发材料来促进微生物繁殖、活化养分、快速转化秸秆为SOM受到广泛关注[10-11]。目前市场上的生物激发材料很多,本研究前期的筛选研究发现,三种商用激发剂配施秸秆均能显著提升水稻土的微生物生物量碳、易氧化碳和可溶性有机碳等活性有机碳(SOC)含量,同时具有激发养分转化功能微生物的潜力,从而在当季即可改善土壤理化性质和提升作物产量[10]。
木本泥炭作为一种新型土壤改良剂,主要分布在热带和温带地区,尽管在我国储量相对稀缺,但其富含腐殖酸钾和惰性有机碳等组分,是提升SOM的重要材料[11-12]。赵文慧等[13]研究发现,尽管施用木本泥炭可以在短期内快速提升SOM含量,但单一施用显著降低微生物活性和作物产量;而木本泥炭与秸秆等碳量混施对作物产量的影响与不施用有机物料处理类似。上述研究结果表示单纯提升稳定性SOM对作物产量影响小,进一步强调了在利用木本泥炭和秸秆来同步提升土壤活性/稳定性有机质和作物产量时添加外源激发剂的重要性;同时也意味着在添加激发剂时,土壤有机质和作物产量的同步提升可能受制于木本泥炭和秸秆施用量的配比,但其适宜配比及其微生物机制尚不清楚。
不同微生物在土壤中相互作用,利用网络分析可以区分土壤中微生物的生态集群(模块),即共享同一生态位的微生物的集合[14]。已有研究表明,模块特性,比如关键物种丰度、微生物群落组成变化等与土壤养分循环、有机物料降解和作物生长显著相关[15]。Shi等[16]发现华北平原典型农田土壤微生物网络的关键物种-芽单胞菌门(Gemmatimonadetes)和浮霉菌门(Planctomycetes)有助于土壤微生物C、N、P、S循环等基因的表达,驱动土壤养分循环。Bao等[17]研究发现,在低肥力土壤中添加秸秆,会形成以放线菌为关键物种的网络结构,同时其分泌的碳水化合物活性酶含量显著增加,进而促进了农田土壤碳循环。Fan等[18]通过网络分析发现土壤多营养级微生物网络模块中的关键微生物多为固氮、解磷和光合等功能微生物,通过表达与C、N、P等养分循环的功能基因,维持土壤功能潜力和作物产量。
本研究推测在水稻土中施用激发剂后,木本泥炭和秸秆不同配比施用,它们之间存在耦合效应,在该比例时,土壤有机质和作物产量同步快速提升,并与特定微生物菌群显著相关。因此,本研究以长江中下游平原发育于黄泥土的典型水稻土为研究对象,在统一施用激发剂基础上,进行了不同木本泥炭与秸秆施用比例的田间试验,其研究目的包括(1)明确木本泥炭与秸秆不同配施比例对水稻产量、土壤有机质及其组分和细菌群落组成的影响,(2)明确土壤理化性质与细菌群落组成的关系,(3)基于网络模块特性,挖掘特定细菌菌群对作物产量的潜在影响。
1 材料与方法 1.1 田间试验田间试验位于江苏省江阴市东支新村(31°53′19″ N,120°6′34″ E),地处亚热带季风气候区,年均温16.7℃,年降水量1 000 mm。土壤类型为潴育水稻土亚类黄泥土田土属。试验前3年(2016—2019)试验地处于撂荒状态,试验开始前土壤基本性质如下:pH为5.21;有机质(SOM)含量为9.78 g·kg–1;全氮(TN)含量为1.07 g·kg–1。
试验于2019年晚稻季开始,共设5个处理:(1)CK:不施用任何有机物料对照,(2)RJ:秸秆(R)配施商用激发剂(J),秸秆用量为3 000 kg·hm–2,激发剂(J)用量为1 500 kg·hm–2;(3)RJM1:RJ基础上施用低量木本泥炭(M1),用量为6 000 kg·hm–2;(4)RJM2:RJ基础上施用中量木本泥炭(M2),用量为9 000 kg·hm–2;(5)RJM3:RJ基础上施用高量木本泥炭(M3),用量为12 000 kg·hm–2。RJM1、RJM2、RJM3处理木本泥炭和秸秆的施用比例分别为2:1、3:1、4:1(表 1)。
|
|
表 1 不同处理中有机物料用量 Table 1 Amount of organic materials in different treatments |
1)秸秆和激发剂配施Crop straw and activator;2)秸秆和激发剂配施基础上施用低量木本泥炭Crop straw,activator,and woody peat at a low rate;3)秸秆和激发剂配施基础上施用中量木本泥炭Crop residue,activator,and woody peat at a middle rate;4)秸秆和激发剂配施基础上施用高量木本泥炭Crop residue,activator,and woody peat at a high rate.下同,The same as below.
每个处理3个重复,共15个小区,均随机设计,每个小区面积为600 m2,长30 m,宽20 m。秸秆为南京三美农业发展有限公司生产的腐熟秸秆其有机碳含量为121.23 g·kg–1,TN、全磷(TP)和全钾(TK)含量分别为0.28 g·kg–1、10.53 g·kg–1、16.03 g·kg–1;激发剂和木本泥炭均购买自北京中向利丰科技有限公司。商用激发剂有机碳含量为337.11 g·kg–1,TN、TP和TK含量为1.56 g·kg–1、0.08 g·kg–1、49.36 g·kg–1;木本泥炭有机碳含量为428.62 g·kg–1,TN、TP和TK含量为0.08 g·kg–1、0.04 g·kg–1、0.39 g·kg–1。所有有机物料均在水稻插秧前施入土壤,同时所有处理在插秧前以碳铵形式施入等量基肥,N施用量为64 kg·hm–2,上述物料与表层土壤充分混匀,然后稻田整地打浆,静置过夜后插秧;插秧7d后追施尿素,N施用量为69 kg·hm–2。插秧时间为2019年6月22日,机插,行距30 cm,株距12 cm,密度为27.8万穴·hm–2,水稻品种为粳稻(Oryza saliva subsp. keng,南粳5055)。
1.2 样品采集与土壤理化性质测定水稻于2019年10月28日收获,每个小区内5点取样法测产,计算求得各小区平均产量。每个小区内多点法采集耕层土壤(0~20 cm)混匀为一个土样。每个土壤样品分为3份,一份风干、过筛,用于测定土壤pH及全量和速效养分;一份鲜样置于–20℃冰箱保存,用于测定土壤速效氮,可溶性有机碳/氮、易氧化态有机碳以及微生物生物量碳/氮,一份鲜样置于–80℃冰箱保存,用于提取土壤DNA。
土壤pH、SOM、TN、TP、TK、硝态氮(NO3–-N)、铵态氮(NH4+-N)、有效磷(AP)含量、速效钾(AK)含量采用常规方法测定[19]。土壤可溶性有机碳(DOC)/氮(DON)采用Jones和Willett的方法测定[20];易氧化态有机碳(ROC)采用高锰酸钾氧化法测定[21]。土壤微生物生物量碳(MBC)/氮(MBN)采用氯仿熏蒸-K2SO4浸提法测定,以熏蒸和未熏蒸土壤的有机碳、氮之差除以转换系数0.38和0.45得到[19]。
1.3 高通量测序分析土壤DNA采用Fast®DNA Spin Kit for Soil(MP Biomedicals,Santa Ana,CA,USA)试剂盒按照说明书操作从0.5 g鲜土中提取。使用NanodropTM2000分光光度计(Nanodrop Technologies,Wilmington,DE,USA)测量DNA纯度(A260/A280范围为1.85~1.92)和浓度(112.89~157.71 ng·μL–1)。选取细菌16S rRNA V4~V5区进行高通量测序。PCR扩增采用特异性引物515F(5′-GTGCCAGCMGCCGCCGC GG-3′)/907R(5′-CCGTCAATTCMTTTRAGTTT-3′)。PCR扩增条件包括95℃ 3 min,(95℃ 30 s,55℃ 30 s,72℃ 40 s),27个循环,之后72℃ 10 min。反应产物采用QIA quick PCR Purification kit(Qiagen)纯化。将不同样品的PCR扩增产物等摩尔混合后,采用Illumin公司MiSeq测序仪完成序列分析。
所得序列在QIIME(1.91)软件中采用以下步骤进行分析:(1)采用FLASH软件进行序列双端合并;(2)使用Cutadapt软件切除引物;(3)细菌质控;(4)高质量序列采用Uparse软件,以97%相似度进行OTU划分,采用Blast方法以Greengeens 13.8数据库对细菌进行注释。基于Bray-Curtis距离对处理间细菌群落进行的主坐标分析(Principal Co-ordinates Analysis,PCoA)和冗余分析(Redundancy Analysis,RDA)在QIIME中完成。原始序列已上传至NCBI平台,编号为PRJNA739050。
1.4 细菌群落共现网络分析使用R 3.6.3中“Hmisc、igraph”程序包,计算细菌物种(OTUs)两两之间的“Spearman”相关系数,并采用BH(Bonferroni)方法校正,选择相关系数|r| > 0.8且P < 0.05的显著相关关系构建细菌网络,并进行网络模块化分析,利用交互平台Gephi 0.9.2实现网络可视化[22]。模块内各处理的相对丰度用其包含的OTU累积丰度占网络中所有OTU总丰度得到,根据模块网络节点数从大到小对模块进行排序,选择排序前3的模块作为微生物集群用于后续分析。基于OTU水平将模块1中物种的丰度占所有物种总丰度的1%以上的OTU标记为优势微生物。
1.5 数据统计分析使用SPSS 25.0软件进行单因素方差分析(ANOVA),Duncan多重比较分析不同处理间水稻产量和土壤理化性质的差异。利用R 3.6.3对土壤pH、有机质、及养分含量进行主成分分析(Principal Component Analysis,PCA)。置换多元方差分析(Permutational multivariate analysis of variance,PERMANOVA)判断不同处理间土壤理化性质和细菌群落结构的差异。偏最小二乘路径模型(Partial Least Squares Path Modeling,PLS-PM)用于评估土壤理化性质与细菌集群(以模块丰度和模块PCoA 1为表征变量)两个因子间的相互作用及其对水稻产量的影响;每个因子中的指标变量均有显著作用。PERMANOVA和PLS-PM分析分别在R 3.6.3“vegan”和“plspm”包中进行。模块中优势物种的差异分析在R 3.6.3“DESeq2”包中进行,增加的(Enriched)OTUs用log2 fold change > 1.0鉴定,减少的(Deleted)OTUs用log2 fold change < 1.0鉴定。微生物丰度与水稻产量的相关性用“Spearman”相关系数表示。
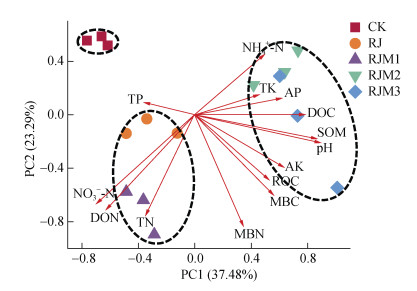
2 结果 2.1 土壤有机质、养分含量和pH基于土壤pH、有机质、养分含量的PCA分析处理可以分为三组:CK、RJ+RJM1和RJM2+RJM3(图 1),PERMANOVA表明三组之间两两差异显著(P < 0.01)。土壤pH、SOM、DOC均随着木本泥炭施用量的增加而增加,RJM2+RJM3达最高水平,较其他组分别高3.68%~13.78%、26.11%~58.53%和17.19%~29.01%(图 1,表 2)。尽管有机物料施用显著提升ROC和MBC含量,但它们在有机物料处理间变化不显著。土壤养分在不同处理间变化主要表现为RJ+RJM1积累氮素,其中NO3–-N和DON含量分别较其他组高20.00%~121.82%和24.67%~92.18%;而RJM2+RJM3则主要表现在AP和AK等元素含量有增加趋势,尤其是AP含量较其他组高5.33%~25.93%(表 2,图 1)。
|
图 1 基于土壤pH、有机质、养分含量的PCA分析 Fig. 1 Principal component analysis (PCA) depicting the Bray-Curtis distance of soil physicochemical properties as affected by combined application of crop straw with different amounts of woody peat |
|
|
表 2 秸秆配施不同用量木本泥炭对水稻土理化性质的影响 Table 2 Effects of combined application of crop straw with different amounts of woody peat on soil physicochemical properties |
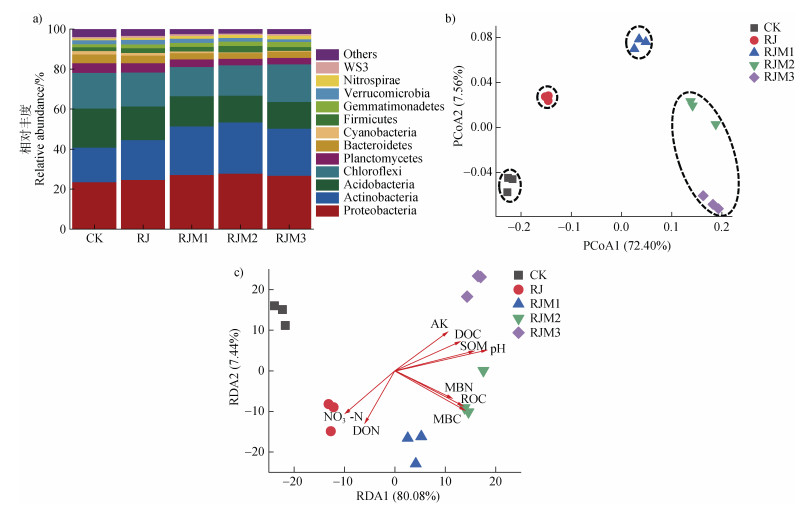
图 2a表明,五个处理门水平的细菌优势菌群均为变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、酸杆菌门(Acidobacteria)和绿弯菌门(Chloroflexi),占细菌总丰度的78.13%~82.40%。对不同处理细菌群落组成进行PCoA分析(图 2b),发现主坐标前两轴共解释了群落79.96%的变异,五个处理可分为四组,分别为CK、RJ、RJM1、RJM2+RJM3。PERMANOVA表明四组之间两两差异显著(P < 0.01)。土壤理化性质与细菌群落的RDA分析表明(图 2c),pH、SOM和MBC是主导细菌群落变化的最主要环境因子(P < 0.01),同时细菌群落组成还受ROC、DOC、NO3–-N、DON、AK和MBN等指标的影响(P < 0.05),其中RJM2+RJM3的细菌群落组成主要受制于pH、SOM、DOC的变化。
|
注:(a)土壤细菌在门水平上的组成及相对丰度;(b)不同处理的细菌群落PCoA分析;(c)土壤细菌群落组成和理化性质的RDA分析。 Note: (a) Relative abundance of the major bacterial phyla, (b) principal co-ordinate analysis (PCoA) plots OTU-based Bray-Curtis distance between 15 soil samples, (c) redundancy analysis (RDA) of bacterial communities based on 16S rRNA genes under five treatments. 图 2 秸秆配施不同用量木本泥炭对土壤细菌群落组成的影响 Fig. 2 Effects of combined application of crop straw with different amounts of woody peat on soil bacterial community composition |
为了明确细菌群落对有机物料的响应,利用“Spearman”相关关系构建细菌共现网络(图 3)。网络主要由3个模块组成,3个模块包含的节点数占网络中所有节点数的86.20%。其中,模块1占比最大(37.63%)(图 3a);模块2和模块3占比分别为33.52%和15.05%。模块1中细菌相对丰度随着木本泥炭施用量的增加而增加,但在RJM3处理中丰度有所降低;RJM2处理的模块2相对丰度最高;有机物料施用显著降低了模块3中细菌相对丰度,其中RJM2和RJM3处理最低(图 3b)。对不同模块中的OTU进行PCoA分析(图 3c),发现对于模块1和模块2的细菌群落,处理均可分为四组,即CK、RJ、RJM1、RJM2+RJM3,该分组模式与总体细菌群落的分组模式(图 2b)相同;而对于模块3,处理分为三组,即CK、RJ+RJM1、RJM2+RJM3(图 3c)。PERMANOVA结果表明每个模块的组间两两差异显著(P < 0.01)。

|
注:a)细菌共现网络;b)不同处理的模块丰度;c)单一模块内细菌的PCoA分析。 Note: a) co-occurrence network of bacterial; b) relative abundance of modularity under five treatments; and c) for principal co-ordinates analysis of bacterial in a single muddle. 图 3 秸秆配施不同用量木本泥炭的土壤细菌生态集群组成及其丰度 Fig. 3 Effects of combined application of crop straw with different amounts of woody peat on soil bacterial clusters composition and their relative abundance |
图 4表明,与不添加任何有机物料的对照(CK)相比,施用有机物料能显著提升水稻产量,其中秸秆与木本泥炭联合施用处理的增产效果更明显,但木本泥炭施用量之间没有显著差异。与CK相比,RJ处理的水稻产量显著提高了13%,而RJM1、RJM2、RJM3的产量平均增幅为31%。

|
注:不同小写字母表示处理间的差异显著性(Duncan,P < 0.05)。 Note: Different letters indicate significant differences between treatments (Duncan method, P < 0.05). 图 4 秸秆配施不同用量木本泥炭对水稻产量的影响 Fig. 4 Effects of combined application of crop straw with different amounts of woody peat on rice yield |
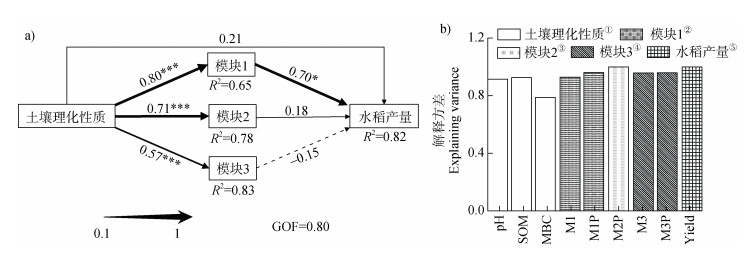
通过构建偏最小二乘路径模型,探究土壤理化性质和微生物集群性质对水稻产量的影响(图 5)。结果表示,微生物集群中模块1对水稻产量具有直接显著正效应,其中土壤理化性质通过直接显著影响模块1性质而间接影响作物产量(图 5a),并且产量变化主要通过模块1的相对丰度(M1)和群落组成(PCoA)变化来调控(图 5b);而模块2和模块3与水稻产量均没有显著效应(图 5a)。因此,决定模块1相对丰度变化的优势物种,及模块1不同处理间的差异物种均可能对水稻产量产生显著影响。
|
①Soil physicochemical properties,②Module 1,③Module 2,④Module 3,⑤Rice yield. 注:a)中箭头表示因果关系,黑色数字表示直接效应系数,实线和虚线实线分别表示正效应和负效应;*表示P ≤ 0.05水平上显著,**表示P≤ 0.01水平上显著,***表示P≤0.001水平上显著;GOF:该模型的拟合优度。b)因子的解释变量:M1,M3:模块1,模块3的相对丰度,M1P,M2P,M3P:基于模块1、模块2、模块3 OTU的PCoA 1。路径系数执行自主抽样1 000次。 Note: a) The line with arrows indicates the causal relationships, black numbers represent direct effect path coefficient, and solid and dashed lines indicate positive and negative relationships, respectively; Coefficients differ significantly from 0 are indicated by * P ≤ 0.05, ** P ≤ 0.01, ***P ≤0.001. GOF: The Goodness of Fit assesses the model. b) the amount of variability explained by the variables. M1 and M3:the relative abundance of Module 1 and Module 3;M1P, M2P, and M3P: PCoA 1 of Module 1, Module 2, and Module 3. Path coefficients are calculated after 1000 bootstraps. 图 5 路径分析评估土壤理化性质和微生物集群对作物产量的影响 Fig. 5 Directed graph of the partial least squares path model(PLS-PM)evaluated the effects between soil physicochemical properties, microbial clusters, and rice yield |
图 6表示,导致模块1中不同处理间物种相对丰度变化的优势物种包括:盖勒氏菌(Gaiellaceae unidentified)、类诺卡氏菌(Nocardioidaceae unidentified)、土壤球菌(Terracoccus)、从毛单胞菌(Comamonadaceae unidentified)和黄色土源菌(Flavisolibacter)。与CK相比,有机物料施用均有提升盖勒氏菌、类诺卡氏菌、土壤球菌、从毛单胞菌丰度的趋势,而秸秆与木本泥炭联合施用(即RJM1、RJM2、RJM3处理)的提升效果均达显著水平,其中盖勒氏菌、类诺卡氏菌、土壤球菌丰度在RJM2处理中最高。与CK相比,RJ处理显著提升黄色土源菌相对丰度,但RJM1、RJM2、RJM3处理中其丰度则没有显著变化。

|
图 6 不同处理模块1中细菌优势物种的丰度(丰度占所有物种总丰度的1%以上的物种) Fig. 6 Relative abundance (%) of dominant species (their sum accounts for more than 1% of the total species abundance) in module 1 of treatments |
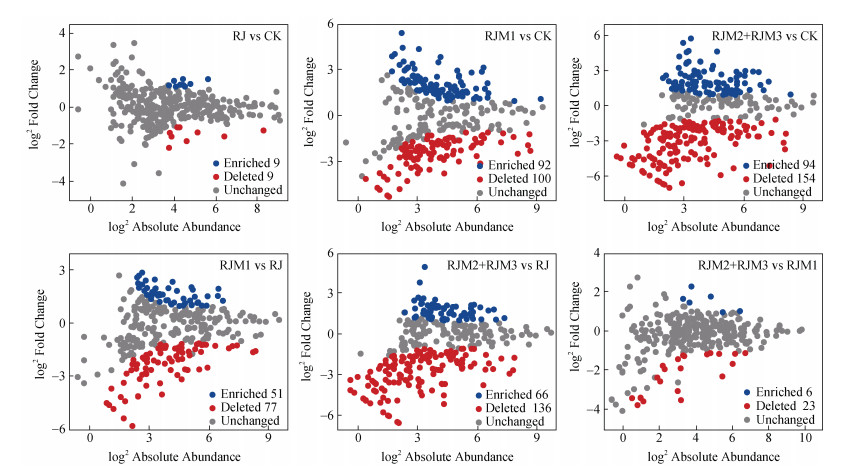
基于火山灰图对四组处理(即CK、RJ、RJM1、RJM2+RJM3)模块1的的差异物种分析结果表明(图 7),RJ中SC-I-84 unidentified丰度显著增加,而红螺菌(Rhodospirillaceae unidentified)丰度显著降低;RJM1WD2101 unidentified丰度显著增加,而厌氧蝇菌(Anaerolinaceae unidentified)丰度显著降低;RJM2+RJM3中WD2101 unidentified和鞘脂杆菌(Sphingobacteriales unidentified)丰度显著增加。
|
注:每一个节点代表一个OTU,依次为模块1中RJ处理与CK对比;RJM1处理与CK对比;RJM2处理与CK对比;RJM3处理与CK对比。灰色部分代表丰度差异不显著的OTU。 Note: Each point represents an individual OTU; the plot depicts a comparison between CK and RJ in module 1;a comparison between CK and RJM1 in module 1;a comparison between CK and RJM2 in module 1;comparison between CK and RJM3 in module 1, respectively. The grey part represents no significant difference. 图 7 模块1中处理物种丰度显著增加(蓝色)或降低(红色)的火山灰图分析 Fig. 7 Effects of the treatments on the abundance of OTUs in module 1 as depicted in the volcano of significantly enriched (blue) and depleted (red) among different treatments |
为了进一步明确特定微生物物种丰度与水稻产量的潜在关系,对模块1中导致丰度变化的优势物种和不同处理间的主要差异物种丰度与水稻产量进行了相关分析。结果表明,水稻产量与盖勒氏菌、类诺卡氏菌、土壤球菌、从毛单胞菌、SC-I-84 unidentified、WD2101 unidentified和鞘脂杆菌丰度呈显著正相关,与黄色土源菌和红螺菌丰度呈显著负相关,与厌氧蝇菌丰度相关性不显著(表 3)。
|
|
表 3 模块1中优势物种和差异物种与产量相关性 Table 3 Spearman correlations among the relative abundance of module 1 dominant species, differential species, and rice yield |
不同有机物料施用导致土壤理化性质的变化不同,5个处理表现为3组(即,CK、RJ+RJM1、RJM2+RJM3)不同特征的理化性质(图 1),其中RJM2+RJM3的主要特征为显著提升了土壤SOM、DOC、pH、AK、AP含量,RJ+RJM1则显著增加了NO3–-N和DON含量(表 2)。有研究表明,土壤耕层SOM含量增加10 g·kg–1(1%),需要一年两熟的农田秸秆连续全量还田10~20年,而每公顷土壤施用约23 t木本泥炭增加的腐殖质量,即可达到耕层SOM含量增加1%的目标;木本泥炭和秸秆配施,同时添加生物激发剂,可以促进团聚体的形成和腐殖质向颗粒态、闭蓄态和有机无机结合态转变[11]。同时,付威等[23]的研究表明,木本泥炭配施腐熟秸秆和生物激发剂可快速提高土壤有机质含量,增加土壤团聚体数量及稳定性,有利于土壤有机质的累积。
RJM2+RJM3的SOM含量最高,因为其木本泥炭的施用量高。大量研究已经发现SOM含量与外源有机物料的施用量呈显著正相关[24-25];本研究所用的木本泥炭难分解,主要用来提高SOM中的惰性有机碳含量[25];秸秆作为易分解的有机物料,提供的碳源一方面促进土壤微生物的生长,同时微生物分解的有机物质又作为土壤活性碳如DOC等储存起来[26]。土壤pH由CK的5.88显著提升至RJM2+ RJM3的6.48和6.69(表 2),这可能是由于木本泥炭含有大量羟基等官能团[27],可以中和土壤溶液中的氢离子,从而使土壤由酸性逐渐趋于中性。另一方面,RJM2+RJM3的AP和AK含量比较高(图 1,表 2),表明这两个处理木本泥炭和秸秆的施用比例有活化养分的功效,尤其是活化P的效果更为明显。这可能与RJM2+RJM3中土壤球菌和从毛单胞菌相对丰度显著增加有关(图 6),土壤球菌多数种类具有溶钾功能,能显著提高土壤AK的含量[28];而从毛单胞菌具有溶磷的功能,能显著提高土壤中AP的含量[29]。
RJ+RJM1的NO3–-N、DON含量显著增加(图 1,表 2),表示其有积累和固定氮素的趋势,这可能是由于添加外源有机物料后,土壤微生物群落组成发生了改变。同时,RJ的NO3–-N、DON含量增加,作物产量较RJM1显著降低,可能也与作物氮素携出量低有关。比如,RJ处理黄色土源菌相对丰度显著升高(图 6),其有利于土壤硝化反应的进行,进而促进NO3–-N的积累[30]。RJM1处理类诺卡氏菌和土壤球菌相对丰度显著升高(图 6),二者均属于放线菌,其具有降解秸秆的能力,降解过程中产生大量DON[31-33]。
3.2 土壤理化性质对细菌群落组成的影响本研究中pH、SOM和MBC是主导细菌群落变化的最主要环境因子(P < 0.01),同时细菌群落组成还受ROC、DOC、NO3–-N、DON、AK和MBN等指标影响(P < 0.05),其中RJM2+RJM3的细菌群落组成主要受制于pH、SOM、DOC的变化(图 1)。
施用有机物料可显著改变细菌群落组成[18,34]。本研究表明,根据细菌群落组成特征,处理可分成4组,即CK,RJ,RJM1,RJM2+RJM3(图 2b),主要与土壤pH、SOM和MBC变化有关,其中RJM2+RJM3的细菌群落组成主要与pH、SOM和DOC变化有关(图 2c)。大量研究表示,土壤pH是主控土壤微生物群落组成的重要因子之一[10,35-36]。王晓洁等[35]研究发现,pH是影响不同类型水稻土微生物群落组成的主要环境因子;陈美淇等[10]研究发现pH主导了外源有机物料添加后水稻土细菌群落组成差异,这可能是由于微生物生活习性受pH调控,如Actinobacteria嗜好在中性偏碱环境中生存,而Acidobacteria活性随pH升高而降低[37],与本研究结果相同(图 2a)。SOM含量同样会显著影响土壤微生物群落组成。Chen等[38]研究发现,短期秸秆还田后,微生物群落结构差异主要由SOM和MBC主导,这可能是由于SOM含量增加,增加了养分供给,有利于Proteobacteria和Actinobacteria等富营养型微生物的生长,这与本研究结果一致(图 2a);而土壤MBC含量往往用来表征微生物生物量的大小,其常与SOM含量显著正相关。DOC是土壤中易被微生物快速吸收利用的碳组分,微生物的代谢产物也是DOC的重要来源,施用有机物料会增加DOC含量进而影响微生物群落组成[26]。
3.3 细菌群落组成与水稻产量之间关系基于网络分析和路径分析发现,作物产量主要与决定模块1相对丰度变化的优势物种,及其中不同处理间(即CK、RJ、RJM1、RJM2+RJM3)的差异物种显著相关(图 3,图 5,表 3),其中盖勒氏菌、类诺卡氏菌、土壤球菌、从毛单胞菌、WD2101 unidentified、鞘脂杆菌、SC-I-84 unidentified丰度与产量呈显著正相关,黄色土源菌和红螺菌丰度与产量呈显著负相关(图 5a,表 3)。RJ处理显著增加了黄色土源菌和SC-I-84 unidentified丰度,而显著降低了红螺菌丰度(图 6,图 7),表示该处理产量增加主要与SC-I-84 unidentified丰度增加和红螺菌丰度降低对产量的正效应超过黄色土源菌丰度增加对产量的负效应有关;RJM1处理盖勒氏菌、类诺卡氏菌、土壤球菌、从毛单胞菌、WD2101 unidentified丰度显著增加,而厌氧蝇菌丰度显著降低(图 6,图 7),表示该处理产量增加与前5个物种丰度显著增加有关;RJM2+RJM3导致盖勒氏菌、类诺卡氏菌、土壤球菌、从毛单胞菌、WD2101 unidentified、鞘脂杆菌丰度显著增加(图 6,图 7),表示该组产量显著增加与上述6个物种丰度显著增加有关。上述结果进一步表明,RJM2+RJM3较RJM1处理刺激更多的与作物产量正相关的优势物种。
盖勒氏菌是一种植物有益菌,可以通过分泌多种活性物质抑制土壤病原菌的生长,促进作物生长[39]。类诺卡氏菌和土壤球菌属于放线菌,可以通过分解秸秆中纤维素为作物提供养分[28,32-33]。从毛单胞菌属于变形菌,作为一类有益菌,能分泌多种物质抑制病原菌的生长,促进作物增产[29,40]。WD2101 unidentified作为一类富营养型细菌,在泥炭中多有分布,主要参与泥炭中稳定性碳向活性碳的转变,增加土壤中碳的可利用性,促进作物生长[41-42]。有研究表明鞘脂杆菌具有代谢多种碳源的能力,其在有机质转化方面具有重要作用[43]。水稻虽然是喜铵作物,但可以通过特定的植物根系转运体吸收NO3–-N,为水稻生长提供15%~40%的氮素养分[44];SC-I-84 unidentified可以通过分泌代谢产物参与土壤NH4+-N向NO3–-N的生化反应,促进作物生长[45]。黄色土源菌在土壤C/N过高的情况下,可能会与作物竞争氮素,不利于作物的生长[46]。红螺菌在土壤中会与其他微生物竞争活性有机质用于自身生长和繁殖,可能会降低有益菌的丰度,进而抑制作物生长[47]。
4 结论本文研究结果表明,不同比例下的木本泥炭和秸秆配施均能显著提高水稻产量,其中木本泥炭和秸秆施用量比例为3︰1(RJM2)和4︰1(RJM3)的SOM和DOC含量显著高于2︰1(RJM1),表示RJM2和RJM3提升有机质含量更为明显。RJM2和RJM3的细菌群落组成相似,并主要受制于pH、SOM、DOC的变化,但与RJM1的群落组成显著不同。相关性分析发现,RJM2和RJM3处理的产量增加主要与其刺激的6个优势物种有关,即盖勒氏菌、类诺卡氏菌、土壤球菌、从毛单胞菌、WD2101 unidentified、鞘脂杆菌,其丰度均在RJM2和RJM3中最高,而RJM1的产量增加主要与刺激上述除鞘脂杆菌外的5个物种有关,表示RJM2和RJM3较RJM1能刺激更多与作物产量正相关的优势物种。因此,从经济效益考虑,木本泥炭与秸秆施用量比例为3︰1时能通过改善土壤理化性质、刺激更多与作物增产有关的优势物种来达到土壤有机质和作物产量同时快速提升的效应,故为适宜施用比例。
| [1] |
Zech W, Senesi N, Guggenberger G, et al. Factors controlling humification and mineralization of soil organic matter in the tropics[J]. Geoderma, 1997, 79(1/4): 117-161.
(  0) 0) |
| [2] |
Sarker J R, Singh B P, Dougherty W J, et al. Impact of agricultural management practices on the nutrient supply potential of soil organic matter under long-term farming systems[J]. Soil and Tillage Research, 2018, 175: 71-81. DOI:10.1016/j.still.2017.08.005
(  0) 0) |
| [3] |
Brady N C, Ray R W. The nature and properties of soils . New Jersey: Prentice Hall, 2008.
(  0) 0) |
| [4] |
Xu J S, Zhao B Z, Chu W Y, et al. Chemical nature of humic substances in two typical Chinese soils(upland vs paddy soil): A comparative advanced solid state NMR study[J]. Science of the Total Environment, 2017, 576: 444-452. DOI:10.1016/j.scitotenv.2016.10.118
(  0) 0) |
| [5] |
Yuan Y R, Li L J, Li N, et al. Effect of long-term fertilization on soil organic carbon pools in Mollisols of Northeast China (In Chinese)[J]. Chinese Journal of Ecology, 2016, 35(6): 1435-1439. [苑亚茹, 李禄军, 李娜, 等. 长期施肥对东北黑土不同活性有机碳库的影响[J]. 生态学杂志, 2016, 35(6): 1435-1439.]
(  0) 0) |
| [6] |
Zhang H, Tang J, Liang S, et al. Early thawing after snow removal and no straw mulching accelerates organic carbon cycling in a paddy soil in Northeast China[J]. Journal of Environmental Management, 2018, 209: 336-345.
(  0) 0) |
| [7] |
Xu J, Han H F, Ning T Y, et al. Long-term effects of tillage and straw management on soil organic carbon, crop yield, and yield stability in a wheat-maize system[J]. Field Crops Research, 2019, 233: 33-40. DOI:10.1016/j.fcr.2018.12.016
(  0) 0) |
| [8] |
Blanco-Canqui H, Lal R. Crop residue removal impacts on soil productivity and environmental quality[J]. Critical Reviews in Plant Sciences, 2009, 28(3): 139-163. DOI:10.1080/07352680902776507
(  0) 0) |
| [9] |
Liu B B, Wu Q R, Wang F, et al. Is straw return-to-field always beneficial? Evidence from an integrated cost-benefit analysis[J]. Energy, 2019, 171: 393-402. DOI:10.1016/j.energy.2019.01.031
(  0) 0) |
| [10] |
Chen M Q, Ma L, Zhao B Z, et al. Effects of woody peat on quick improvement of soil organic matter and bacterial community composition in newly reclaimed red-yellow paddy soils (In Chinese)[J]. Soils, 2020, 52(2): 279-286. [陈美淇, 马垒, 赵炳梓, 等. 木本泥炭对红黄壤性水田土壤有机质提升和细菌群落组成的影响[J]. 土壤, 2020, 52(2): 279-286.]
(  0) 0) |
| [11] |
Zheng Y Y, Zhang J B, Tan J, et al. Chemical composition and structure of humus relative to sources (In Chinese)[J]. Acta Pedologica Sinica, 2019, 56(2): 386-397. DOI:10.11766/trxb201805070241 [郑延云, 张佳宝, 谭钧, 等. 不同来源腐殖质的化学组成与结构特征研究[J]. 土壤学报, 2019, 56(2): 386-397.]
(  0) 0) |
| [12] |
Wang Z Q, Zhang X Y, Meng X M, et al. The enlightenment of nature peat formation to peat substitute research (In Chinese)[J]. Journal of Natural Resources, 2012, 27(7): 1252-1258. [王忠强, 张心昱, 孟宪民, 等. 泥炭形成过程对泥炭基质替代物研究的启示[J]. 自然资源学报, 2012, 27(7): 1252-1258.]
(  0) 0) |
| [13] |
Zhao W H, Ma L, Xu J S, et al. Effect of application of straw and wood peat for a short period on soil organic matter and microbial community in composition and function in fluvo-aquic soil (In Chinese)[J]. Acta Pedologica Sinica, 2020, 57(1): 153-164. DOI:10.11766/trxb201811300537 [赵文慧, 马垒, 徐基胜, 等. 秸秆与木本泥炭短期施用对潮土有机质及微生物群落组成和功能的影响[J]. 土壤学报, 2020, 57(1): 153-164.]
(  0) 0) |
| [14] |
Duran-Pinedo A E, Paster B, Teles R, et al. Correlation network analysis applied to complex biofilm communities[J]. PLoS ONE, 2011, 6(12): e28438. DOI:10.1371/journal.pone.0028438
(  0) 0) |
| [15] |
Fan K K, Delgado-Baquerizo M, Guo X S, et al. Microbial resistance promotes plant production in a four-decade nutrient fertilization experiment[J]. Soil Biology & Biochemistry, 2020, 141: 107679.
(  0) 0) |
| [16] |
Shi Y, Delgado-Baquerizo M, Li Y T, et al. Abundance of kinless hubs within soil microbial networks are associated with high functional potential in agricultural ecosystems[J]. Environment International, 2020, 142: 105869. DOI:10.1016/j.envint.2020.105869
(  0) 0) |
| [17] |
Bao Y Y, Dolfing J, Guo Z B, et al. Important ecophysiological roles of non-dominant Actinobacteria in plant residue decomposition, especially in less fertile soils[J]. Microbiome, 2021, 9(1): 1-17. DOI:10.1186/s40168-020-00939-1
(  0) 0) |
| [18] |
Fan K K, Delgado-Baquerizo M, Guo X S, et al. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment[J]. The ISME Journal, 2021, 15(2): 550-561. DOI:10.1038/s41396-020-00796-8
(  0) 0) |
| [19] |
Lu R K. Analytical methods for soil and agro-chemistry[M]. China Agricultural Science and technology Press, 2000.[鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000.]
(  0) 0) |
| [20] |
Jones D L, Willett V B. Experimental evaluation of methods to quantify dissolved organic nitrogen(DON)and dissolved organic carbon(DOC)in soil[J]. Soil Biology and Biochemistry, 2006, 38(5): 991-999. DOI:10.1016/j.soilbio.2005.08.012
(  0) 0) |
| [21] |
Lefroy R D B, Blair G J, Strong W M. Changes in soil organic matter with cropping as measured by organic carbon fractions and ^13C natural isotope abundance[J]. Plant and Soil, 1993, 155/156(1): 399-402. DOI:10.1007/BF00025067
(  0) 0) |
| [22] |
Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks[J]. International Conference on Web and Social Media 8, 2009, 361-362.
(  0) 0) |
| [23] |
Fu W, Yong C X, Ma D H, et al. Rapid fertilization effect in soils after gully control and land reclamation in loess hilly and gully region of China (In Chinese)[J]. Transactions of the Chinese Society of Agricultural Engineering, 2019, 35(21): 252-261. [付威, 雍晨旭, 马东豪, 等. 黄土丘陵沟壑区治沟造地土壤快速培肥效应[J]. 农业工程学报, 2019, 35(21): 252-261.]
(  0) 0) |
| [24] |
Zhao Y C, Wang M Y, Hu S J, et al. Economics- and policy-driven organic carbon input enhancement dominates soil organic carbon accumulation in Chinese croplands[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(16): 4045-4050.
(  0) 0) |
| [25] |
Fu W, Fan J, Wang S, et al. Woody peat addition increases soil organic matter but its mineralization is affected by soil clay in the four degenerated erodible soils[J]. Agriculture, Ecosystems & Environment, 2021, 318: 107495.
(  0) 0) |
| [26] |
Ge Y H, Zou D S. Study on interaction between soil organic carbon and microbial communities (In Chinese)[J]. Journal of Anhui Agricultural Sciences, 2012, 40(11): 6468-6470. [葛云辉, 邹冬生. 土壤有机碳与微生物群落互作关系的研究[J]. 安徽农业科学, 2012, 40(11): 6468-6470.]
(  0) 0) |
| [27] |
Xi G H, Shi G R, Li Q, et al. Adsorption performance of woody peat for Cr(Ⅵ) (In Chinese)[J]. Environmental Chemistry, 2019, 38(1): 202-208. [席改红, 石国荣, 李强, 等. 木本泥炭对Cr(Ⅵ)的吸附性能[J]. 环境化学, 2019, 38(1): 202-208.]
(  0) 0) |
| [28] |
Lei Y P, Xiao Y L, Li L F, et al. Impact of tillage practices on soil bacterial diversity and composition under the tobacco-rice rotation in China[J]. Journal of Microbiology: Seoul, Korea, 2017, 55(5): 349-356.
(  0) 0) |
| [29] |
Wen T, Yuan J, He X M, et al. Enrichment of beneficial cucumber rhizosphere microbes mediated by organic acid secretion[J]. Horticulture Research, 2020, 7(10.1038): s41438-20.
(  0) 0) |
| [30] |
Ding Y, Xiong J S, Zhou B W, et al. Odor removal by and microbial community in the enhanced landfill cover materials containing biochar-added sludge compost under different operating parameters[J]. Waste Management, 2019, 87: 679-690.
(  0) 0) |
| [31] |
Lei X M, Wang F, Zhou B Q, et al. Effects of long-term fertilization on soil soluble organic nitrogen and free amino acid profile variations in paddy fields (In Chinese)[J]. Journal of Agro-Environment Science, 2019, 38(7): 1550-1559. [雷秀美, 王飞, 周碧青, 等. 长期施肥对稻田土壤可溶性有机氮和游离氨基酸剖面分异的影响[J]. 农业环境科学学报, 2019, 38(7): 1550-1559.]
(  0) 0) |
| [32] |
Anderson C R, Condron L M, Clough T J, et al. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus[J]. Pedobiologia, 2011, 54(5/6): 309-320.
(  0) 0) |
| [33] |
Kruglova A, Gonzalez-Martinez A, Kråkström M, et al. Bacterial diversity and population shifts driven by spotlight wastewater micropollutants in low-temperature highly nitrifying activated sludge[J]. Science of the Total Environment, 2017, 605/606: 291-299.
(  0) 0) |
| [34] |
Delgado-Baquerizo M, Eldridge D J, Ochoa V, et al. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe[J]. Ecology Letters, 2017, 20(10): 1295-1305.
(  0) 0) |
| [35] |
[35]Wang X J, Bei Q C, Liu G, et al. Microbial abundance and community composition in different types of paddy soils in China and their affecting factors (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(3): 767-776. [王晓洁, 卑其成, 刘钢, 等. 不同类型水稻土微生物群落结构特征及其影响因素[J]. 土壤学报, 2021, 58(3): 767-776.]
(  0) 0) |
| [36] |
Tripathi B M, Stegen J C, Kim M, et al. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria[J]. The ISME Journal, 2018, 12(4): 1072-1083.
(  0) 0) |
| [37] |
Wang J C, Song Y, Ma T F, et al. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil[J]. Applied Soil Ecology, 2017, 112: 42-50.
(  0) 0) |
| [38] |
Chen Z M, Wang H Y, Liu X W, et al. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice-wheat cropping system[J]. Soil and Tillage Research, 2017, 165: 121-127.
(  0) 0) |
| [39] |
Trivedi P, Delgado-Baquerizo M, Trivedi C, et al. Keystone microbial taxa regulate the invasion of a fungal pathogen in agro-ecosystems[J]. Soil Biology and Biochemistry, 2017, 111: 10-14.
(  0) 0) |
| [40] |
Li X G, Zhang Y N, Ding C F, et al. Declined soil suppressiveness to Fusarium oxysporum by rhizosphere microflora of cotton in soil sickness[J]. Biology and Fertility of Soils, 2015, 51(8): 935-946.
(  0) 0) |
| [41] |
Ivanova A A, Wegner C E, Kim Y, et al. Metatranscriptomics reveals the hydrolytic potential of peat-inhabiting Planctomycetes[J]. Antonie Van Leeuwenhoek, 2018, 111(6): 801-809.
(  0) 0) |
| [42] |
Dedysh S N, Ivanova A A. Planctomycetes in boreal and subarctic wetlands: Diversity patterns and potential ecological functions[J]. FEMS Microbiology Ecology, 2018, 95(2): fiy227.
(  0) 0) |
| [43] |
Bach E M, Williams R J, Hargreaves S K, et al. Greatest soil microbial diversity found in micro-habitats[J]. Soil Biology and Biochemistry, 2018, 118: 217-226.
(  0) 0) |
| [44] |
Gao Z, Wang Y, Chen G, et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency[J]. Nature Communications, 2019, 10: 5207.
(  0) 0) |
| [45] |
Yang Z C, Sun H M, Zhou Q, et al. Nitrogen removal performance in pilot-scale solid-phase denitrification systems using novel biodegradable blends for treatment of waste water treatment plants effluent[J]. Bioresource Technology, 2020, 305: 122994.
(  0) 0) |
| [46] |
Liu C J, Lin H, Li B, et al. Responses of microbial communities and metabolic activities in the rhizosphere during phytoremediation of Cd-contaminated soil[J]. Ecotoxicology and Environmental Safety, 2020, 202: 110958.
(  0) 0) |
| [47] |
Madigan M, Cox S S, Stegeman R A. Nitrogen fixation and nitrogenase activities in members of the family Rhodospirillaceae[J]. Journal of Bacteriology, 1984, 157(1): 73-78.
(  0) 0) |
 2023, Vol. 60
2023, Vol. 60


