土壤胞外呼吸是土壤微生物将胞内代谢产生的电子通过胞外电子传递链,传递至胞外并还原土壤中氧化态的铁、锰、腐殖质等电子受体同时产生能量的过程[1-4]。因此,胞外电子传递是实现胞外呼吸的必要条件。一般地,胞外电子传递可通过细胞表面一系列的氧化还原蛋白(如细胞色素c)与胞外电子受体直接接触而实现[5-7]。但是该方式仅适用于微生物与胞外电子受体可直接接触的情况,无法实现细胞与胞外受体之间长距离的电子传递。通过利用可溶性的具有氧化还原特性的电子中介体(如黄素类物质或腐殖质等),微生物可实现长距离的胞外电子传递[8-11]。然而并非所有的微生物均可分泌电子中介体,并且电子中介体的胞外电子传递效率较低,会受到浓度、自身扩散速率等因素的影响[12-13]。相比之下,微生物纳米导线被认为可弥补微生物通过直接接触或电子中介体传递电子的不足[5,14]。微生物纳米导线是一类生长于微生物表面、长达数十微米并具有导电性的纤维状结构,其可长距离传递电子至距离细胞较远的胞外电子受体[15-17]。研究发现,微生物纳米导线可直接还原土壤中铁、锰矿物与重金属元素,从而影响土壤矿物的迁移转化及重金属污染土壤的修复[18-23]。此外,微生物纳米导线广泛表达于产甲烷及甲烷氧化微生物群落,参与了微生物种间的电子传递,影响了全球温室气体排放[24-28]。基于上述重要的生态、环境功能,微生物纳米导线成为土壤胞外呼吸研究的前沿与热点。
Geobacter是表达微生物纳米导线的典型微生物,广泛存在于稻田、湿地、还原性土壤及地表沉积物等厌氧环境中并在厌氧微生物食物链中占据关键生态位[29]。微生物纳米导线最初发现于还原水铁矿的Geobacter sulfurreducens表面,被认为是G. sulfurreducens主要的胞外电子传递机制[2]。它们具有良好的导电性,可传递电子并通过直接接触还原胞外三价铁氧化物。因具有完整的基因组信息及成熟的基因操作手段,G. sulfurreducens已成为微生物纳米导线研究的模式微生物[30-34]。长期以来,基于分子生物学实验数据,G. sulfurreducens纳米导线被认为是由PilA-N蛋白单体组成的四型菌毛,并衍生出一套完整的“菌毛导电理论”[35-38]。然而,随着冷冻电镜技术的应用,PilA-N菌毛被鉴定为新型细胞色素纳米导线,“菌毛导电理论”遇到重大挑战。那么G. sulfurreducens能否表达PilA-N菌毛?其纳米导线的本质是什么?针对上述问题,本文以G. sulfurreducens纳米导线理论发展为主线,综述了纳米导线的研究进展(图 1),提出纳米导线(导电菌毛)研究存在的争议,为推进土壤胞外呼吸研究及微生物纳米导线的应用研究提供科学参考。
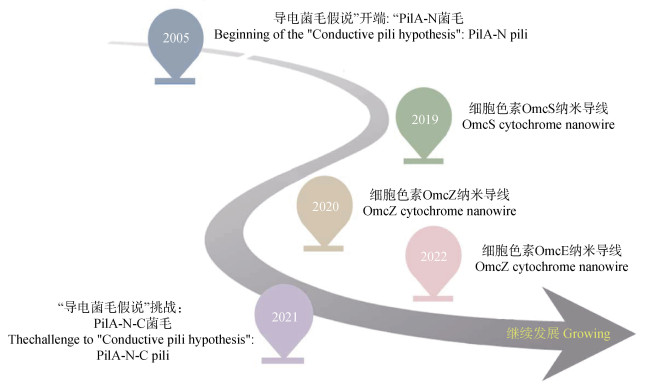
|
图 1 微生物纳米导线的重要研究历程 Fig. 1 Milestones of critical advances in microbial nanowire studies |
2005年,美国麻省理工学院Derek Lovley教授研究团队利用导电原子力显微镜率先在G. sulfurreducens上鉴定到直径约3 nm的导电菌毛,并发表在Nature上[2]。随后,基于基因、蛋白水平分析表明这些导电菌毛属于四型菌毛,由单一的菌毛蛋白单体PilA-N(编码基因为GSU1496)构成。相比于传统的四型菌毛蛋白,PilA-N菌毛蛋白具有明显的截短结构(仅含有61个氨基酸):N端主要由α螺旋构成,C端缺少传统四型菌毛蛋白具有的由多个β折叠形成的球形结构域[39-41]。此外,PilA-N菌毛蛋白富含芳香族氨基酸(占比9.8%)[42]。这些芳香族氨基酸被丙氨酸取代后会严重降低菌毛的导电性,而增加PilA-N中芳香族氨基酸含量可提高导电性[35,43]。因此,导电菌毛假说认为PilA-N截短的结构利于其紧密组装,并导致芳香族氨基酸芳香环间彼此紧密堆叠,从而形成电子传递的通道,即赋予菌毛导电能力[44-45]。然而,有研究表明PilA-N的结构不直接贡献菌毛的导电性,菌毛蛋白芳香族氨基酸的密度直接影响菌毛导电能力[46]。
pilA-N基因下游为GSU1497基因,它们共用启动子,实现共转录[39](图 2a)。基因进化分析发现,pilA-N与GSU1497可能源自原始长型四型菌毛蛋白基因分裂导致的进化事件,而胞外氧化铁还原压力被认为驱动了该进化过程[47]。因此,GSU1497基因在有些研究中被命名为pilA-C。分子动力学分析发现由于PilA-N菌毛蛋白存在疏水表面,其不能以单体形式单独存在于细胞中。此时,GSU1497蛋白被认为可作为分子伴侣蛋白,通过与PilA-N静电相互作用,稳定PilA-N蛋白单体并协助其组装[39]。因此,GSU1497蛋白也被简称为Spc(Short pili chaperon)蛋白。亦因此,异源表达PilA-N菌毛时常需要共表达Spc[48]。值得注意的是,虽然经过二十多年的发展“导电菌毛假说”逐渐成熟,但是PilA-N菌毛结构尚不清楚,仅有拟合或者计算模型(图 2b)。
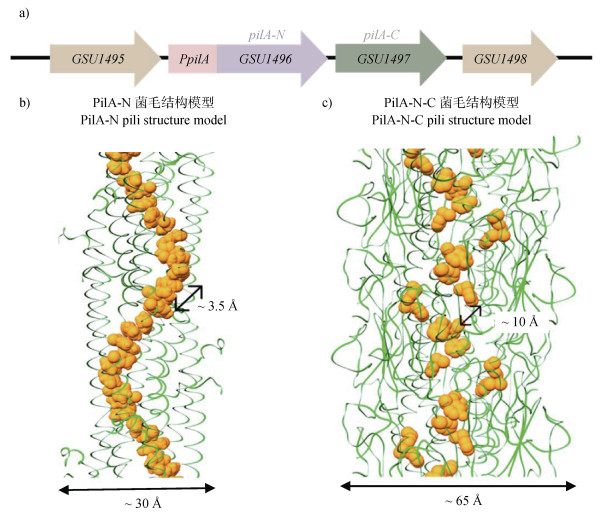
|
图 2 PilA-N菌毛与PilA-N-C菌毛的结构模型(a. 菌毛相关的基因图谱;b. PilA-N菌毛结构模型;c. PilA-N-C菌毛结构模型)(基于Gu等[49]) Fig. 2 Structure models of PilA-N and PilA-N-C nanowires(a. pili-related genetic map; b. PilA-N pili structure model; c. PilA-N-C pili structure model)(modified from Gu et al.[49]) |
2021年,Gu等[49]企图通过高分辨冷冻电镜技术解析PilA-N菌毛结构。他们未检测到直径3 nm的PilA-N菌毛,却发现直径约为6 nm的PilA-N-C菌毛,并在近原子分辨率水平上解析其结构:正如字面意思所示,PilA-N-C菌毛由PilA-N和PilA-C组成。具体而言,PilA-N与PilA-C首先通过静电作用与疏水作用形成稳定的异二聚体PilA-N-C,然后完成组装。然而,冷冻电镜结构分析发现,在PilA-N-C菌毛中芳香族氨基酸芳香环间无π−π堆叠,并且芳香环之间的间隙大于10 Å,暗示不能依靠其形成电子传递通道(图 2c)。不足为奇,导电性测量发现PilA-N-C菌毛的电导率几乎可忽略不计,即PilA-N-C菌毛不导电。此外,结果显示,相比于传统意义上附着于细胞表面的PilA-N菌毛,PilA-N-C菌毛主要位于周质空间,功能上类似二型分泌假菌毛,主要作用于细胞色素c的分泌。需要注意的是,该论文似乎重新定义了PilA-C的功能,但是笔者认为需谨慎对待该研究结论,因为部分关键数据自相矛盾。例如,在分析纯化的PilA-N-C菌毛组成时,蛋白质印迹Western Blot显示PilA-C大小为13 kDa而十二烷基硫酸钠-聚丙烯酰胺凝胶电泳SDS-PAGE显示其大小接近25 kDa(原文扩充数据图 1)。紧接着,2022年Wang等[50]利用冷冻电镜也在G. sulfurreducens表面观察到PilA-N-C菌毛结构。
1.3 菌毛之争:PilA-N菌毛是否存在?冷冻电镜仅观察到PilA-N-C菌毛,这是否意味着PilA-N菌毛不存在呢?Derek Lovley教授认为该冷冻电镜实验未原位观察细胞表面纤维状物质的结构,仅分析了分离纯化后的菌毛,而菌毛纯化过程可能引入误差。为此,Derek Lovley教授利用原子力显微镜原位分析了G. sulfurreducens表面纤维状物质的结构[51],结果显示:G. sulfurreducens表面90%的纳米导线直径呈3 nm,这与推测的PilA-N菌毛直径一致;胞外细胞色素c突变株仍然表达直径3 nm的纳米导线;G. sulfurreducens菌毛蛋白PilA-N羧基端的5个芳香族氨基酸残基替换为丙氨酸后表达的直径3 nm的“纳米导线”导电性显著降低。因此,这些间接证据强烈地暗示着PilA-N菌毛的存在。更为直接的证据可能来自对PilA-N菌毛的原位标记。Ueki等[52]使用六聚组氨酸标签标记了G. sulfurreducens中的PilA-N蛋白,发现胞外仍然表达直径3 nm的纳米导线;胞外纤维结构含有被组氨酸标记的PilA-N蛋白。需要注意的是,这两个结论来自完全不同的独立实验,无直接证据表明这些被组氨酸标记的纤维结构直径为3 nm。
与此同时,研究者们尝试在PilA-C缺席的情况下在异源物种中表达PilA-N菌毛,并似乎取得了成功。例如,Liu等[53]克隆了pilA-N基因至Pseudomonas aeruginosa菌中,实现了在P. aeruginosa表面表达直径大约3 nm的PilA-N菌毛;Ueki等[54]同样克隆了pilA-N基因,并利用E. coli本身的四型菌毛组装机制,在E. coli中成功表达了直径3 nm的PilA-N菌毛;进一步基于该E. coli底盘菌株,Lekbach等[55]在E. coli中表达了功能化修饰的PilA-N菌毛;同时,Szmuc等[56]在Shewanella oneidensis中不但表达了PilA-N菌毛,而且通过改变氨基酸残基种类实现了PilA-N菌毛与金纳米粒子的复合。上述证据似乎支撑了导电PilA-N菌毛的存在。然而,最近的综述中Wang等[57]指出Derek Lovley教授等在E. coli上异源表达的直径约3 nm的“PilA-N纳米导线”并未得到证实,其很可能是B型DNA。该综述也对PilA-N导电菌毛假说进行了否定。因此,未来要坐实PilA-N菌毛存在似乎仅能通过解析其组装结构。
值得注意的是,无论PilA-N菌毛是否存在,基于芳香环的生物分子导电机制却是合理的:Cosert等[58]表达、纯化了N端截短的PilA-N蛋白并诱导其组装形成纳米导线,其中芳香环侧链被认为贡献了导电性;Malvankar研究团队不但发现淀粉样蛋白可依靠酪氨酸残基的芳香环间的堆叠获得导电性[59],而且证明通过增加E. coli菌毛蛋白中色氨酸含量可显著提升菌毛的导电性[60]。
2 细胞色素型纳米导线 2.1 OmcS纳米导线既然冷冻电镜显示细胞表面PilA-N-C菌毛不导电,那么G. sulfurreducens纳米导线是什么呢?2019年,Wang等[61]利用高分辨率的冷冻电镜解析G. sulfurreducens纳米导线的三维结构,发现c型细胞色素OmcS可首尾相接组装成OmcS纳米导线,并得到分辨率高达3.7 Å的纳米导线结构,其特征如下(图 3):直径约4 nm,呈正弦形态,每个OmcS亚基含有6个血红素,血红素分子连续排列,彼此间距约3.5~6 Å,组成了电子传递通道。巧合的是,同一时期Filman等[62]也发现了同样结构的OmcS纳米导线。
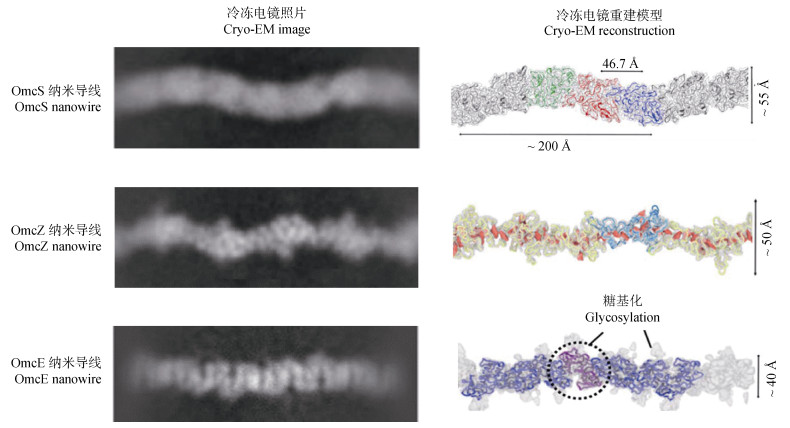
|
图 3 三种细胞色素型纳米导线的结构(基于Wang等[50,61,74]) Fig. 3 Structures of three cytochrome nanowires(modified from Wang et al.[50, 61, 74]) |
OmcS是G. sulfurreducens表达的一种胞外细胞色素c,被认为直接参与了胞外Mn(Ⅳ)/Fe(Ⅲ)氧化物等的还原、微生物与电极间电子传递、地杆菌种间直接电子互营等胞外电子传递过程[63-71]。2010年Leang等[72]尝试用免疫金标记分析了胞外OmcS的分布,发现OmcS广泛分布于胞外,并且部分OmcS与PilA-N菌毛结合。现在认为这些被纳米金识别的PilA-N菌毛可能实际上是OmcS纳米导线。但是,PilA-N蛋白确实可以通过与OmcS相互作用协助其分泌甚至组装[49]。考虑到OmcS在胞外电子传递中的重要作用,虽然OmcS可组装形成纳米导线,但是不能排除胞外仍然存在以单体形式存在的OmcS。
2.2 OmcZ纳米导线2020年,Yalcin等[73]利用扫描探针成像和光谱学分析等技术发现,在电场刺激条件下G. sulfurreducens表面会生长大量由c型细胞色素OmcZ聚合而成的丝状结构,即OmcZ纳米导线。随后,Wang等[74]利用高分辨率的冷冻电镜对OmcZ纳米导线的原子结构进行了分析,获得了近4.2 Å分辨率的OmcZ纳米导线结构(图 3),其具有以下特征:1)每个亚基约为58 Å;2)未表现出强烈的正弦形态;3)轴向最宽处约50 Å;4)每个亚基含有8个血红素;5)大部分血红素按T型或反向平行排列。此外,分析发现OmcZ纳米导线的导电性较OmcS纳米导线高出1 000倍并且硬度较OmcS纳米导线高出3倍。这种高的导电性被认为源自OmcZ亚基中有四分之三的血红素可暴露于溶剂中,从而增加了与外界环境传递电子的可能性[75]。
OmcZ同样是G. sulfurreducens表达的一种胞外细胞色素c[76]。一般认为其不参与G. sulfurreducens胞外铁锰矿物的还原,却是G. sulfurreducens电活性生物膜产生高电流密度的必要条件[77-79]。免疫金标记实验显示,OmcZ大量分布于贴近电极表面的一侧,作为“电化学门”介导了微生物与阳极间电子传递[80]。最近研究发现,OmcZ(纳米导线)在电活性生物膜结构稳定及跨膜电子传递中同样发生着重要的作用[81]。此外,Yalcin等[73]发现改变OmcZ纳米导线的构象后,OmcZ纳米导线可将机械与化学刺激转化为电信号,实现传感与能量生产等功能。
2.3 OmcE纳米导线2022年,Wang等[50]再次利用冷冻电镜在OmcS表达缺陷的G. sulfurreducens表面发现另一种细胞色素型纳米导线—OmcE纳米导线(图 3)。该结构具有以下几个特征:1)每个亚基约为34 Å;2)每个亚基旋转约59°;3)未表现出清晰的正弦形态;4)轴向最宽处约40 Å;5)每个亚基含有4个血红素;6)血红素之间的最小边界距离介于3.5~4.0 Å;7)每个亚基均有一个界面血红素与邻近亚基的组氨酸配体配位;8)呈高度糖基化。
OmcE与OmcS、OmcZ细胞色素相同,为G. sulfurreducens胞外最丰富的细胞色素c之一,在G. sulfurreducens的胞外呼吸过程中发挥重要作用[63,78],并且其功能与OmcS细胞色素相似。例如,研究证明缺失OmcE,G. sulfurreducens突变株也会在还原Fe(Ⅲ)氧化物、Mn(Ⅳ)氧化物、电极、U(Ⅵ)和腐殖酸等电子受体时出现不同程度的缺陷[64,82-84],而并不影响可溶性三价铁的还原[84]。不同的是,OmcE与OmcS细胞色素对上述胞外电子受体的影响程度可能不同。例如,敲除OmcE细胞色素对腐殖质还原的影响最大,而缺失OmcS对蒽醌-2,6-二磺酸(AQDS)还原的影响最显著[84]。
2.4 其他细胞色素纳米导线OmcS纳米导线缺失后,G. sulfurreducens表达出OmcE纳米导线。这暗示细胞可能通过表达其他的细胞色素纳米导线来补偿该纳米导线部分或完全丧失的功能。据推测,G. sulfurreducens可编码100多种细胞色素c[85-86],那么G. sulfurreducens是否可表达更多种类的细胞色素纳米导线?同时,其他种类的地杆菌也依赖胞外细胞色素c实现胞外电子传递,而细胞色素OmcS、OmcZ等在地杆菌中并不保守。其他地杆菌中是否会表达更加丰富的细胞色素纳米导线?进一步,细胞色素广泛分布于各种微生物,细胞色素纳米导线是否也广泛分布?以此类推,Derek Lovley教授报道的其他菌种中的“导电菌毛”[27]是否可能也是细胞色素纳米导线?
序列比对分析发现,尽管OmcS与OmcE纳米导线无总体序列或结构相似性,但它们中血红素堆叠方式几乎相同[50]。其中OmcE亚基中的四个血红素与OmcS亚基中的前四个血红素排列高度相似。这种看似巧合暗示两种细胞色素纳米导线可能具有一个共同的进化起源,而血红素可能驱动了细胞色素纳米导线的进化。相比之下,OmcZ纳米导线在序列、蛋白质折叠或血红素堆叠等方面均无相似性。例如,OmcZ亚基中的8个血红素均为与来自同一亚基的组氨酸进行轴向协调,而在OmcS和OmcE中,相邻亚基中的血红素均为与相邻亚基中的组氨酸相互配合[74]。这种不一致性,暗示多血红素细胞色素的聚合物(即细胞色素纳米导线)至少独立进化过两次。因此,理论上存在更多种类的细胞色素纳米导线。值得注意的是,细胞色素纳米导线的导电机制尚不清楚[87]。相比于发现更多种类的细胞色素纳米导线,揭示其电子传递机制显得尤为重要与紧迫。
3 展望无论是无氧化还原活性的菌毛蛋白可导电,还是细胞色素c可组装形成数十微米甚至更长的纳米导线,微生纳米导线的发现、理论的发展促进并拓展了对蛋白导电及生命体电子传递的认识。回望微生物纳米导线发展历程,笔者发现争议可以源自科学技术进步后对事物的深入认识,也可能因为使用了不同的实验材料而造成认知偏差。微生物纳米导线的表达受培养条件的影响。例如,代谢阳极时omcZ、omcS纳米导线基因均会上调,而pilA-N基因受到抑制;以富马酸为电子受体时,pilA-N基因表达同样受到抑制[88-89];PilA-N-C菌毛似乎仅产生于细菌受环境胁迫的时候[49]。因此,未来在利用冷冻电镜研究PilA-N菌毛时应选取利于PilA-N菌毛表达环境(比如以水铁矿为电子受体)中分离的纳米导线。同时,纳米导线导电性测量仍缺乏有效方法,获得生理环境下微生物纳米导线真实导电率将是未来的研究方向。
微生物纳米导线的发现丰富了对土壤胞外呼吸的认识。作为天然导电结构,微生物纳米导线不但在个体水平上实现了微生物与环境直接电子传递,而且赋予微生物群落导电能力,实现通过传递“电子”影响整个群落的功能。此外,可以预想微生物纳米导线也显著贡献了土壤的导电能力,将沟通不同深度土壤及土壤与空气等不同氧化还原梯度环境间更大范围的电子传递,进而驱动各种生态过程。不同细胞色素型纳米导线蛋白结构存在巨大差别,而其血红素亚基排列形式却高度统一。铁是血红素的活性,也是地球上最古老而丰富的元素之一。基于三价铁的还原过程被认为是地球上最古老的呼吸方式。这不禁让人思考,血红素与呼吸的相互进化有何关系?血红素的自组装是否促进了胞外呼吸链的进化?
尽管对微生物纳米导线的认识还处于初级阶段,微生物纳米导线应用已展现出巨大前景[90]。例如,Wang等[91]通过过表达纳米导线蛋白显著提高了微生物的电活性并促进了微生物成膜,为提高微生物燃料电池性能提供了新途径;利用微生物纳米导线特殊的纳米尺寸效应、导电属性与表面化学性质,Liu等[92]及Lovley和Yao[93]开发了一种低成本无污染的新型空气湿度电池;基于微生物纳米导线的优良电子传递能力与机械硬度[94],Sun等[95]将纳米导线与聚乙烯醇制成复合材料,成功研发出较传统聚合物导线材料具有更高的热稳定性、更宽的导电范围并具有可调导电性的复合材料;基于微生物纳米导线可调控的导电性能,Smith等[96]制作了高效的氨气电子传感器,可以检测到低浓度的氨气(0.01 mg·L–1);Liu等[97]制备了厚度约2 μm的纳米导线薄膜,用于实时监测人体呼吸和皮肤水分等生理状况。此外,微生物纳米导线在极端环境下具有独特优势。例如,在低pH条件下,微生物纳米导线依旧具有很强的电子传递能力[98]。发展能够在极端环境下耐受并发挥作用的微生物纳米导线型电子材料,将为未来生物传感器和pH传感器等的发展奠定基础[97]。
| [1] |
Richardson D J. Bacterial respiration: A flexible process for a changing environment[J]. Microbiology, 2000, 146(3): 551-571. DOI:10.1099/00221287-146-3-551
(  0) 0) |
| [2] |
Reguera G, McCarthy K D, Mehta T, et al. Extracellular electron transfer via microbial nanowires[J]. Nature, 2005, 435(7045): 1098-1101. DOI:10.1038/nature03661
(  0) 0) |
| [3] |
Shi L, Dong H L, Reguera G, et al. Extracellular electron transfer mechanisms between microorganisms and minerals[J]. Nature Reviews Microbiology, 2016, 14(10): 651-662. DOI:10.1038/nrmicro.2016.93
(  0) 0) |
| [4] |
Logan B E, Rossi R, Ragab A, et al. Electroactive microorganisms in bioelectrochemical systems[J]. Nature Reviews: Microbiology, 2019, 17(5): 307-319. DOI:10.1038/s41579-019-0173-x
(  0) 0) |
| [5] |
Lovley D R, Holmes D E. Electromicrobiology: The ecophysiology of phylogenetically diverse electroactive microorganisms[J]. Nature Reviews Microbiology, 2022, 20(1): 5-19. DOI:10.1038/s41579-021-00597-6
(  0) 0) |
| [6] |
Lovley D R. Electromicrobiology[J]. Annual Review of Microbiology, 2012, 66: 391-409. DOI:10.1146/annurev-micro-092611-150104
(  0) 0) |
| [7] |
Reguera G, Pollina R B, Nicoll J S, et al. Possible nonconductive role of Geobacter sulfurreducens pilus nanowires in biofilm formation[J]. Journal of Bacteriology, 2007, 189(5): 2125-2127. DOI:10.1128/JB.01284-06
(  0) 0) |
| [8] |
Light S H, Su L, Rivera-Lugo R, et al. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria[J]. Nature, 2018, 562(7725): 140-144. DOI:10.1038/s41586-018-0498-z
(  0) 0) |
| [9] |
Newman D K, Kolter R. A role for excreted quinones in extracellular electron transfer[J]. Nature, 2000, 405(6782): 94-97. DOI:10.1038/35011098
(  0) 0) |
| [10] |
Okamoto A, Saito K, Inoue K, et al. Uptake of self-secreted flavins as bound cofactors for extracellular electron transfer in Geobacter species[J]. Energy & Environmental Science, 2014, 7(4): 1357-1361.
(  0) 0) |
| [11] |
Marsili E, Baron D B, Shikhare I D, et al. Shewanella secretes flavins that mediate extracellular electron transfer[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(10): 3968-3973.
(  0) 0) |
| [12] |
von Canstein H, Ogawa J, Shimizu S, et al. Secretion of flavins by Shewanella species and their role in extracellular electron transfer[J]. Applied and Environmental Microbiology, 2008, 74(3): 615-623. DOI:10.1128/AEM.01387-07
(  0) 0) |
| [13] |
Lovley D R. Extracellular electron transfer: Wires, capacitors, iron lungs, and more[J]. Geobiology, 2008, 6(3): 225-231. DOI:10.1111/j.1472-4669.2008.00148.x
(  0) 0) |
| [14] |
Malvankar N S, Lovley D R. Microbial nanowires: A new paradigm for biological electron transfer and bioelectronics[J]. ChemSusChem, 2012, 5(6): 1039-1046. DOI:10.1002/cssc.201100733
(  0) 0) |
| [15] |
Clarke T A, Edwards M J. Uncovering nature's electronics[J]. Nature Chemical Biology, 2020, 16(10): 1041-1042. DOI:10.1038/s41589-020-00655-9
(  0) 0) |
| [16] |
Deane C. The new face of nanowires[J]. Nature Chemical Biology, 2019, 15(6): 551.
(  0) 0) |
| [17] |
Eshel Y, Peskin U, Amdursky N. Coherence-assisted electron diffusion across the multi-heme protein-based bacterial nanowire[J]. Nanotechnology, 2020, 31(31): 314002. DOI:10.1088/1361-6528/ab8767
(  0) 0) |
| [18] |
Cologgi D L, Lampa-Pastirk S, Speers A M, et al. Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(37): 15248-15252.
(  0) 0) |
| [19] |
Ueki T, Walker D J F, Nevin K P, et al. Generation of high current densities in Geobacter sulfurreducens lacking the putative gene for the PilB pilus asssembly motor[J]. Microbiology Spectrum, 2021, 9(2): e0087721. DOI:10.1128/Spectrum.00877-21
(  0) 0) |
| [20] |
Childers S E, Ciufo S, Lovley D R. Geobacter metallireducens accesses insoluble Fe(iii)oxide by chemotaxis[J]. Nature, 2002, 416(6882): 767-769. DOI:10.1038/416767a
(  0) 0) |
| [21] |
Anderson R T, Vrionis H A, Ortiz-Bernad I, et al. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer[J]. Applied and Environmental Microbiology, 2003, 69(10): 5884-5891. DOI:10.1128/AEM.69.10.5884-5891.2003
(  0) 0) |
| [22] |
Liang B, Cheng H, van Nostrand J D, et al. Microbial community structure and function of nitrobenzene reduction biocathode in response to carbon source switchover[J]. Water Research, 2014, 54: 137-148. DOI:10.1016/j.watres.2014.01.052
(  0) 0) |
| [23] |
Huang L P, Chai X L, Chen G H, et al. Effect of set potential on hexavalent chromium reduction and electricity generation from biocathode microbial fuel cells[J]. Environmental Science & Technology, 2011, 45(11): 5025-5031.
(  0) 0) |
| [24] |
Morita M, Malvankar N S, Franks A E, et al. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates[J]. mBio, 2011, 2(4): e00159-e00111.
(  0) 0) |
| [25] |
Liu X, Tremblay P L, Malvankar N S, et al. A Geobacter sulfurreducens strain expressing Pseudomonas aeruginosa type Ⅳ pili localizes OmcS on pili but is deficient in Fe(Ⅲ)oxide reduction and current production[J]. Applied and Environmental Microbiology, 2014, 80(3): 1219-1224. DOI:10.1128/AEM.02938-13
(  0) 0) |
| [26] |
Ueki T, Nevin K P, Rotaru A E, et al. Geobacter strains expressing poorly conductive pili reveal constraints on direct interspecies electron transfer mechanisms[J]. mBio, 2018, 9(4): e01273-e01218.
(  0) 0) |
| [27] |
Walker D J, Nevin K P, Holmes D E, et al. Syntrophus conductive pili demonstrate that common hydrogen-donating syntrophs can have a direct electron transfer option[J]. The ISME Journal, 2020, 14(3): 837-846. DOI:10.1038/s41396-019-0575-9
(  0) 0) |
| [28] |
Nagarajan H, Embree M, Rotaru A E, et al. Characterization and modelling of interspecies electron transfer mechanisms and microbial community dynamics of a syntrophic association[J]. Nature Communications, 2013, 4(1): 2809. DOI:10.1038/ncomms3809
(  0) 0) |
| [29] |
Esteve-Nunez A, Rothermich M, Sharma M, et al. Growth of Geobacter sulfurreducens under nutrient-limiting conditions in continuous culture[J]. Environmental Microbiology, 2005, 7(5): 641-648. DOI:10.1111/j.1462-2920.2005.00731.x
(  0) 0) |
| [30] |
Coppi M V, Leang C, Sandler S J, et al. Development of a genetic system for Geobacter sulfurreducens[J]. Applied and Environmental Microbiology, 2001, 67(7): 3180-3187. DOI:10.1128/AEM.67.7.3180-3187.2001
(  0) 0) |
| [31] |
Tabares M, Dulay H, Reguera G. Geobacter sulfurreducens[J]. Trends in Microbiology, 2020, 28(4): 327-328. DOI:10.1016/j.tim.2019.11.004
(  0) 0) |
| [32] |
Muhamadali H, Xu Y, Ellis D I, et al. Metabolic profiling of Geobacter sulfurreducens during industrial bioprocess scale-up[J]. Applied and Environmental Microbiology, 2015, 81(10): 3288-3298. DOI:10.1128/AEM.00294-15
(  0) 0) |
| [33] |
Mahadevan R, Bond D R, Butler J E, et al. Characterization of metabolism in the Fe(Ⅲ)-reducing organism Geobacter sulfurreducens by constraint-based modeling[J]. Applied and Environmental Microbiology, 2006, 72(2): 1558-1568. DOI:10.1128/AEM.72.2.1558-1568.2006
(  0) 0) |
| [34] |
Reguera G, Nevin K P, Nicoll J S, et al. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells[J]. Applied and Environmental Microbiology, 2006, 72(11): 7345-7348. DOI:10.1128/AEM.01444-06
(  0) 0) |
| [35] |
Vargas M, Malvankar N S, Tremblay P L, et al. Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens[J]. mBio, 2013, 4(2): e00105-00113.
(  0) 0) |
| [36] |
Malvankar N S, Yalcin S E, Tuominen M T, et al. Visualization of charge propagation along individual pili proteins using ambient electrostatic force microscopy[J]. Nature Nanotechnology, 2014, 9(12): 1012-1017. DOI:10.1038/nnano.2014.236
(  0) 0) |
| [37] |
Walker D J, Adhikari R Y, Holmes D E, et al. Electrically conductive pili from pilin genes of phylogenetically diverse microorganisms[J]. The ISME Journal, 2018, 12(1): 48-58. DOI:10.1038/ismej.2017.141
(  0) 0) |
| [38] |
Lovley D R. e-biologics: Fabrication of sustainable electronics with "green" biological materials[J]. mBio, 2017, 8(3): e00695-17.
(  0) 0) |
| [39] |
Liu X, Zhan J, Jing X Y, et al. A pilin chaperone required for the expression of electrically conductive Geobacter sulfurreducens pili[J]. Environmental Microbiology, 2019, 21(7): 2511-2522. DOI:10.1111/1462-2920.14638
(  0) 0) |
| [40] |
Malvankar N S, Vargas M, Nevin K, et al. Structural basis for metallic-like conductivity in microbial nanowires[J]. mBio, 2015, 6(2): e00084.
(  0) 0) |
| [41] |
McCallum M, Tammam S, Khan A, et al. The molecular mechanism of the type Ⅳa pilus motors[J]. Nature Communications, 2017, 8(1): 15091. DOI:10.1038/ncomms15091
(  0) 0) |
| [42] |
Tan Y, Adhikari R Y, Malvankar N S, et al. Synthetic biological protein nanowires with high conductivity[J]. Small, 2016, 12(33): 4481-4485. DOI:10.1002/smll.201601112
(  0) 0) |
| [43] |
Lovley D R, Holmes D E. Protein nanowires: The electrification of the microbial world and maybe our own[J]. Journal of Bacteriology, 2020, 202(20): e00331-20.
(  0) 0) |
| [44] |
Bond D R, Strycharz-Glaven S M, Tender L M, et al. On electron transport through Geobacter biofilms[J]. ChemSusChem, 2012, 5(6): 1099-1105. DOI:10.1002/cssc.201100748
(  0) 0) |
| [45] |
Lampa-Pastirk S, Veazey J P, Walsh K A, et al. Thermally activated charge transport in microbial protein nanowires[J]. Scientific Reports, 2016, 6(1): 23517. DOI:10.1038/srep23517
(  0) 0) |
| [46] |
Lovley D R. Electrically conductive pili: Biological function and potential applications in electronics[J]. Current Opinion in Electrochemistry, 2017, 4(1): 190-198. DOI:10.1016/j.coelec.2017.08.015
(  0) 0) |
| [47] |
Liu X, Ye Y, Xiao K, et al. Molecular evidence for the adaptive evolution of Geobacter sulfurreducens to perform dissimilatory iron reduction in natural environments[J]. Molecular Microbiology, 2020, 113(4): 783-793. DOI:10.1111/mmi.14443
(  0) 0) |
| [48] |
Tan Y, Adhikari R Y, Malvankar N S, et al. Expressing the Geobacter metallireducens PilA in Geobacter sulfurreducens yields pili with exceptional conductivity[J]. mBio, 2017, 8(1): e02203-16.
(  0) 0) |
| [49] |
Gu Y Q, Srikanth V, Salazar-Morales A I, et al. Structure of Geobacter pili reveals secretory rather than nanowire behaviour[J]. Nature, 2021, 597(7876): 430-434. DOI:10.1038/s41586-021-03857-w
(  0) 0) |
| [50] |
Wang F B, Mustafa K, Suciu V, et al. Cryo-EM structure of an extracellular Geobacter OmcE cytochrome filament reveals tetrahaem packing[J]. Nature Microbiology, 2022, 7(8): 1291-1300. DOI:10.1038/s41564-022-01159-z
(  0) 0) |
| [51] |
Liu X Y, Walker D J F, Nonnenmann S S, et al. Direct observation of electrically conductive pili emanating from Geobacter sulfurreducens[J]. mBio, 2021, 12(4): e0220921. DOI:10.1128/mBio.02209-21
(  0) 0) |
| [52] |
Ueki T, Walker D J F, Tremblay P L, et al. Decorating the outer surface of microbially produced protein nanowires with peptides[J]. ACS Synthetic Biology, 2019, 8(8): 1809-1817. DOI:10.1021/acssynbio.9b00131
(  0) 0) |
| [53] |
Liu X, Wang S W, Xu A M, et al. Biological synthesis of high-conductive pili in aerobic bacterium Pseudomonas aeruginosa[J]. Applied Microbiology and Biotechnology, 2019, 103(3): 1535-1544. DOI:10.1007/s00253-018-9484-54
(  0) 0) |
| [54] |
Ueki T, Walker D J F, Woodard T L, et al. An Escherichia coli chassis for production of electrically conductive protein nanowires[J]. ACS Synthetic Biology, 2020, 9(3): 647-654. DOI:10.1021/acssynbio.9b00506
(  0) 0) |
| [55] |
Lekbach Y, Ueki T, Liu X M, et al. Microbial nanowires with genetically modified peptide ligands to sustainably fabricate electronic sensing devices[J]. Biosensors and Bioelectronics, 2023, 226: 115147. DOI:10.1016/j.bios.2023.115147
(  0) 0) |
| [56] |
Szmuc E, Walker D J F, Kireev D, et al. Engineering Geobacter pili to produce metal: Organic filaments[J]. Biosensors and Bioelectronics, 2023, 222: 114993. DOI:10.1016/j.bios.2022.114993
(  0) 0) |
| [57] |
Wang F B, Craig L, Liu X, et al. Microbial nanowires: Type Ⅳ pili or cytochrome filaments?[J]. Trends in Microbiology, 2023, 31(4): 384-392. DOI:10.1016/j.tim.2022.11.004
(  0) 0) |
| [58] |
Cosert K M, Castro-Forero A, Steidl R J, et al. Bottom-up fabrication of protein nanowires via controlled self-assembly of recombinant Geobacter pilins[J]. mBio, 2019, 10(6): e02721-19.
(  0) 0) |
| [59] |
Shipps C, Kelly H R, Dahl P J, et al. Intrinsic electronic conductivity of individual atomically resolved amyloid crystals reveals micrometer-long hole hopping via tyrosines[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(2): e2014139118.
(  0) 0) |
| [60] |
Shapiro D M, Mandava G, Yalcin S E, et al. Protein nanowires with tunable functionality and programmable self-assembly using sequence-controlled synthesis[J]. Nature Communication, 2022, 13(1): 829. DOI:10.1038/s41467-022-28206-x
(  0) 0) |
| [61] |
Wang F B, Gu Y Q, O'Brien J P, et al. Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers[J]. Cell, 2019, 177(2): 361-369.e310. DOI:10.1016/j.cell.2019.03.029
(  0) 0) |
| [62] |
Filman D J, Marino S F, Ward J E, et al. Cryo-EM reveals the structural basis of long-range electron transport in a cytochrome-based bacterial nanowire[J]. Communications Biology, 2019, 2(1): 219. DOI:10.1038/s42003-019-0448-9
(  0) 0) |
| [63] |
Mehta T, Coppi M V, Childers S E, et al. Outer membrane c-type cytochromes required for Fe(Ⅲ)and Mn(Ⅳ)oxide reduction in Geobacter sulfurreducens[J]. Applied and Environmental Microbiology, 2005, 71(12): 8634-8641. DOI:10.1128/AEM.71.12.8634-8641.2005
(  0) 0) |
| [64] |
Holmes D E, Chaudhuri S K, Nevin K P, et al. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens[J]. Environmental Microbiology, 2006, 8(10): 1805-1815. DOI:10.1111/j.1462-2920.2006.01065.x
(  0) 0) |
| [65] |
Leang C, Malvankar N S, Franks A E, et al. Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production[J]. Energy & Environmental Science, 2013, 6(6): 1901-1908.
(  0) 0) |
| [66] |
Summers Z M, Fogarty H E, Leang C, et al. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria[J]. Science, 2010, 330(6009): 1413-1415. DOI:10.1126/science.1196526
(  0) 0) |
| [67] |
Qian X, Mester T, Morgado L, et al. Biochemical characterization of purified OmcS, a c-type cytochrome required for insoluble Fe(Ⅲ)reduction in Geobacter sulfurreducens[J]. Biochimica et Biophysica Acta(BBA)-Bioenergetics, 2011, 1807(4): 404-412. DOI:10.1016/j.bbabio.2011.01.003
(  0) 0) |
| [68] |
Malvankar N S, Tuominen M T, Lovley D R. Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobacter sulfurreducens[J]. Energy & Environmental Science, 2012, 5(9): 8651-8659.
(  0) 0) |
| [69] |
Neu J, Shipps C C, Guberman-Pfeffer M J, et al. Microbial biofilms as living photoconductors due to ultrafast electron transfer in cytochrome OmcS nanowires[J]. Nature Communications, 2022, 13(1): 5150. DOI:10.1038/s41467-022-32659-5
(  0) 0) |
| [70] |
Salgueiro C A, Morgado L, Silva M A, et al. From iron to bacterial electroconductive filaments: Exploring cytochrome diversity using Geobacter bacteria[J]. Coordination Chemistry Reviews, 2022, 452.
(  0) 0) |
| [71] |
Shrestha P M, Rotaru A E, Aklujkar M, et al. Syntrophic growth with direct interspecies electron transfer as the primary mechanism for energy exchange[J]. Environmental Microbiology Reports, 2013, 5(6): 904-910. DOI:10.1111/1758-2229.12093
(  0) 0) |
| [72] |
Leang C, Qian X L, Mester T, et al. Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens[J]. Applied and Environmental Microbiology, 2010, 76(12): 4080-4084. DOI:10.1128/AEM.00023-10
(  0) 0) |
| [73] |
Yalcin S E, O'Brien J P, Gu Y Q, et al. Electric field stimulates production of highly conductive microbial OmcZ nanowires[J]. Nature Chemical Biology, 2020, 16(10): 1136-1142. DOI:10.1038/s41589-020-0623-9
(  0) 0) |
| [74] |
Wang F B, Chan C H, Suciu V, et al. Structure of Geobacter OmcZ filaments suggests extracellular cytochrome polymers evolved independently multiple times[J]. Elife, 2022, 11: e81551. DOI:10.7554/eLife.81551
(  0) 0) |
| [75] |
Gu Y Q, Guberman-Pfeffer M J, Srikanth V, et al. Structure of Geobacter cytochrome OmcZ identifies mechanism of nanowire assembly and conductivity[J]. Nature Microbiology, 2023, 8(2): 284-298. DOI:10.1038/s41564-022-01315-5
(  0) 0) |
| [76] |
Yalcin S E, Malvankar N S. The blind men and the filament: Understanding structures and functions of microbial nanowires[J]. Current Opinion in Chemical Biology, 2020, 59: 193-201. DOI:10.1016/j.cbpa.2020.08.004
(  0) 0) |
| [77] |
Inoue K, Qian X L, Morgado L, et al. Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens[J]. Applied and Environmental Microbiology, 2010, 76(12): 3999-4007. DOI:10.1128/AEM.00027-10
(  0) 0) |
| [78] |
Nevin K P, Kim B C, Glaven R H, et al. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells[J]. PLoS One, 2009, 4(5): e5628. DOI:10.1371/journal.pone.0005628
(  0) 0) |
| [79] |
Bonanni P S, Massazza D, Busalmen J P. Stepping stones in the electron transport from cells to electrodes in Geobacter sulfurreducens biofilms[J]. Physical Chemistry Chemical Physics, 2013, 15(25): 10300-10306. DOI:10.1039/c3cp50411e
(  0) 0) |
| [80] |
Inoue K, Leang C, Franks A E, et al. Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens[J]. Environmental Microbiology Reports, 2011, 3(2): 211-217. DOI:10.1111/j.1758-2229.2010.00210.x
(  0) 0) |
| [81] |
Ye Y, Liu X, Nealson Kenneth H, et al. Dissecting the structural and conductive functions of nanowires in Geobacter sulfurreducens electroactive biofilms[J]. mBio, 2022, 13(1): e03822-03821.
(  0) 0) |
| [82] |
Richter H, Nevin K P, Jia H F, et al. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type Ⅳ pili, and protons in extracellular electron transfer[J]. Energy & Environmental Science, 2009, 2(5): 506-516.
(  0) 0) |
| [83] |
Shelobolina E S, Coppi M V, Korenevsky A A, et al. Importance of c-type cytochromes for U(Ⅵ)reduction by Geobacter sulfurreducens[J]. BMC Microbiology, 2007, 7: 16. DOI:10.1186/1471-2180-7-16
(  0) 0) |
| [84] |
Voordeckers J W, Kim B C, Izallalen M, et al. Role of Geobacter sulfurreducens outer surface c-type cytochromes in reduction of soil humic acid and anthraquinone-2, 6-disulfonate[J]. Applied and Environmental Microbiology, 2010, 76(7): 2371-2375. DOI:10.1128/AEM.02250-09
(  0) 0) |
| [85] |
Kai A, Tokuishi T, Fujikawa T, et al. Proteolytic maturation of the outer membrane c-type cytochrome OmcZ by a subtilisin-like serine protease ss essential for optimal current production by Geobacter sulfurreducens[J]. Applied and Environmental Microbiology, 2021, 87(12): e02617-20.
(  0) 0) |
| [86] |
Steidl R J, Lampa-Pastirk S, Reguera G. Mechanistic stratification in electroactive biofilms of Geobacter sulfurreducens mediated by pilus nanowires[J]. Nature Communications, 2016, 7(1): 12217. DOI:10.1038/ncomms12217
(  0) 0) |
| [87] |
Dahl P J, Yi S M, Gu Y, et al. A 300-fold conductivity increase in microbial cytochrome nanowires due to temperature-induced restructuring of hydrogen bonding networks[J]. Science Advances, 2022, 8(19): eabm7193. DOI:10.1126/sciadv.abm7193
(  0) 0) |
| [88] |
Adhikari R Y, Malvankar N S, Tuominen M T, et al. Conductivity of individual Geobacter pili[J]. RSC Advances, 2016, 6(10): 8354-8357. DOI:10.1039/C5RA28092C
(  0) 0) |
| [89] |
Lovley D R, Walker D J F. Geobacter protein nanowires[J]. Frontiers in Microbiology, 2019, 10: 2078. DOI:10.3389/fmicb.2019.02078
(  0) 0) |
| [90] |
Boesen T, Nielsen L P, Schramm A. Pili for nanowires[J]. Nature Microbiology, 2021, 6(11): 1347-1348. DOI:10.1038/s41564-021-00990-0
(  0) 0) |
| [91] |
Wang Z G, Hu Y D, Dong Y R, et al. Enhancing electrical outputs of the fuel cells with Geobacter sulferreducens by overexpressing nanowire proteins[J]. Microbial Biotechnology, 2023, 16(3): 534-545. DOI:10.1111/1751-7915.14128
(  0) 0) |
| [92] |
Liu X, Gao H, Ward J E, et al. Power generation from ambient humidity using protein nanowires[J]. Nature, 2020, 578(7796): 550-554. DOI:10.1038/s41586-020-2010-9
(  0) 0) |
| [93] |
Lovley D R, Yao J. Intrinsically conductive microbial nanowires for 'green' electronics with novel functions[J]. Trends in Biotechnology, 2021, 39(9): 940-952. DOI:10.1016/j.tibtech.2020.12.005
(  0) 0) |
| [94] |
Myers B, Catrambone F, Allen S, et al. Engineering nanowires in bacteria to elucidate electron transport structural-functional relationships[J]. Scientific Reports, 2023, 13(1): 8843. DOI:10.1038/s41598-023-35553-2
(  0) 0) |
| [95] |
Sun Y L, Tang H Y, Ribbe A, et al. Conductive composite materials fabricated from microbially produced protein nanowires[J]. Small, 2018, 14(44): e1802624. DOI:10.1002/smll.201802624
(  0) 0) |
| [96] |
Smith A F, Liu X, Woodard T L, et al. Bioelectronic protein nanowire sensors for ammonia detection[J]. Nano Research, 2020, 13(5): 1479-1484. DOI:10.1007/s12274-020-2825-6
(  0) 0) |
| [97] |
Liu X M, Fu T D, Ward J, et al. Multifunctional protein nanowire humidity sensors for green wearable electronics[J]. Advanced Electronic Materials, 2020, 6(9): 200721.
(  0) 0) |
| [98] |
MacDiarmid A G. "Synthetic metals": A novel role for organic polymers[J]. Current Applied Physics, 2001, 1(4): 269-279.
(  0) 0) |
 2024, Vol. 61
2024, Vol. 61


