2. 中国科学院土壤环境与污染修复重点实验室(南京土壤研究所), 南京 210008;
3. 省部共建国家重点实验室培育基地-江苏省食品质量安全重点实验室, 南京 210014;
4. 南京师范大学分析测试中心, 南京 210046
2. Key Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China;
3. State Key Laboratory Cultivation Base, Ministry of Science and Technology—Jiangsu Key Laboratory for Food Quality and Safety, Nanjing 210014, China;
4. Nanjing Normal University Center for Analysis and Testing, Nanjing 210046, China
生物修复是一种常用的污染土壤原位修复技术,能够利用微生物净化土壤污染物,其效率和经济性优于传统的化学和物理方法。相较于常规微生物修复技术,生物膜中的细胞在基质中受到保护,具有更好的适应恶劣环境和生存的机会。因此,生物膜主导的污染土壤生物修复技术凭借其高效且安全的特点,展现出更好的应用前景。生物膜技术指利用浮游态微生物在非生物载体表面附着、富集、繁殖并最终形成生物膜结构,使环境总生物量密度增加,促使疏水性有毒化合物的高效代谢。与浮游细胞相比,在生存竞争压力大、环境条件恶劣或者存在有害毒素时,生物膜基质能给予微生物更强的抵御力,例如营养物耗竭、pH变化或环境中存在氧自由基、消毒剂和抗生素等[1-2]。由于细胞接触更紧密,生物膜内微生物之间遗传物质的转移速度较快,转化频率是浮游细胞的10倍~600倍[3]。基因表达和调控的变化使生物膜细胞在表型和代谢方面不同于浮游细胞,例如基质中营养物质局部浓度的变化以及微生物间分工的不同,能够诱导生物膜中不同基因的差异性表达,有利于微生物通过多代谢途径去除难降解污染物。同时,微生物趋化及鞭毛运动能够促使微生物接近目标污染物,提高微生物对污染物的降解效率,该过程是影响微生物降解污染物的另一重要因素[4]。
生物膜的产生和扩散受到群体感应(Quorum sensing,QS)的调控,群体感应通过信号分子调控胞外聚合物(Extracellular polymeric substances,EPS)的产生,改变生物膜特性,从而增强对污染物的修复效果[5-6]。群体感应是微生物细胞间通讯的一种形式,它利用信号分子(自诱导剂)在细胞间扩散,当细胞密度和物种复杂性发生变化时,信号分子浓度达到一定阈值,从而启动菌体的基因表达[7]。因此,可以通过信号分子检测细胞的生理功能。到目前为止,已经确定了许多结构不同的QS信号。虽然其中许多是物种特异性的,但一些QS信号可以被许多物种识别,从而允许物种间的交流。细菌通过产生、释放和检测QS信号分子以调节多种生理活动,如生物膜形成[6]、生物表面活性剂的产生[4]、胞外多糖的合成[8]、分解代谢基因的表达[9]等。其中,革兰氏阴性菌的QS行为通常由LuxR/I型信息系统所调控,并以N-酰基高丝氨酸内酯(N-acyl-homoserine lactones,AHLs)作为信号分子[10]。革兰氏阳性细菌主要通过寡肽类信号分子进行交流。此外,QS信号可用于具有强化降解能力的工程生物膜的制备[11]。
本综述旨在总结生物膜和群体感应的功能和作用,明确基于群体感应调控的生物膜修复技术强化污染物去除的机制及其工程化应用,主要涉及QS调控生物膜的形成和对污染物的降解过程,并且通过操纵QS系统,能够实现生物膜对污染物的高效去除,为生物膜群体感应系统原位修复污染土壤提供一定的理论依据和参考。
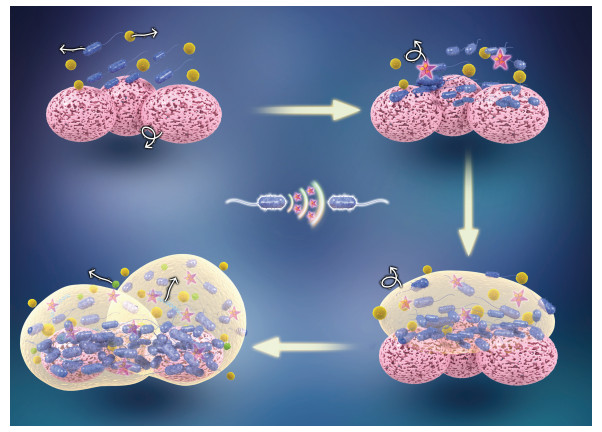
1 生物膜与群体感应生物膜是地球上微生物存在的一种基本形式,广泛存在于地球表面环境中[12],驱动了地球上所有的生物化学过程[13],并且对污染物具有高效的迁移转化和降解能力。生物膜是由细菌、真菌、原生动物或藻类组成的聚集体或群落,由于其具有较高的生物量和固定化合物的能力,生物膜系统尤其适用于处理难降解的污染物。同时,微生物间的趋化性能够增强生物膜内微生物间的基因转移以及提高污染物生物有效性,从而促进污染物的微生物修复。
1.1 群体感应对生物膜形成的影响细菌通过QS系统控制通道和支柱状结构的形成和EPS的产生,改变生物膜特性,从而调节生物膜的形成与变化(图 1)。当生物膜内细胞高密度下对营养的竞争加剧时,该结构有助于营养充分分散,从而确保将营养有效的输送到细胞[14]。群体感应信号分子调控EPS的合成及有机物的降解是强化污染物去除的重要环节。其中,EPS由特定的多糖、蛋白质、脂类、核酸、腐殖酸和水结合形成[4]。不同细菌的生物膜和EPS产物在结构组成上存在差别,主要由微生物所处的生长条件、生物膜表面结构以及环境胁迫共同决定[2]。此外,构成生物膜的微生物可与污染物共同固定于EPS中[9]。EPS表面含有很多携带负电荷的官能团,可与重金属包括铅、铜、锌、镉、铁和镍等及有机污染物形成复合物[15]。
|
图 1 群体感应调控生物膜的形成过程 Fig. 1 Quorum sensing regulates biofilm formation |
对聚集于固体表面的金黄色葡萄球菌(Staphylococcus aureus),黏附素介导其与表面基质的连接,群体感应通过调控黏附素合成来增强其对表面的吸附作用,这些黏附素介导与宿主基质的接触并促进其附着到各种表面,从而促进生物膜的形成[16]。Vibrio cholerae生物膜QS系统通过弧菌多糖(Vibrio polysaccharides,vps)操纵子编码胞外多糖基因,控制胞外多糖的产生与分泌,进而调控细胞聚集和细胞对表面的黏附[17]。以AHLs为信号分子的生物膜QS能够诱导Serratia liquefaciens生物膜的形成与成熟,还能够控制Rhodobacter sphaeroides的聚集[18]。革兰氏阴性菌Novosphingobium pentaromativorans US6-1通过QS生成AHLs调控细胞表面疏水性和胞外多糖的产生,从而提高该降解菌对菲的去除效率[19]。Kang和Park[20]首次发现Acinetobacter sp. DR1分泌的信号分子(C12-HSL)可能是十六烷降解和生物膜形成过程中的关键因素。笔者研究发现,3-oxo-C12-HSL为Bordetella sp. F2生物膜形成过程中的主导型信号分子,其生成规律与生物膜的生长、1,2,4-三氯苯的降解紧密相关[21]。以上研究证实,细菌QS在生物膜的形成过程中发挥着至关重要的作用(表 1)。
|
|
表 1 群体感应系统在生物膜形成和污染物降解过程中的作用 Table 1 Involvement of quorum sensing system in the development of biofilm and biodegradation of pollutants |
研究发现,操纵信号分子可控制生物膜形成和扩散过程,生物膜的形成和扩散属于遗传过程,因此,可以像其他遗传系统一样通过合成生物学工具对其进行操控[14,22]。吲哚作为QS信号分子的一种,其生成可以抑制大肠杆菌生物膜的形成。例如,向Pseudomonas fluorescens体内克隆邻甲苯单氧酶可改变含有Escherichia coli和Pseudomonas群落的胞外吲哚浓度,Pseudomonas内单加氧酶的嵌合型表达可以将吲哚转换成不溶性异靛,导致吲哚胞外浓度减少22倍,大肠杆菌生物膜的生成量则增加12倍。该结果表明,改变特定的QS信号分子浓度可介导生物膜的形成过程[23]。
1.2 群体感应淬灭对生物膜形成的影响群体淬灭是指自诱导器介导的群体感应被中断的过程,群体感应淬灭(Quorum quenching,QQ)和群体感应往往共存于生物群体中[6],对群体感应的干扰可能会导致所需表型的抑制,如生物膜的形成[6]。研究发现,通过使用QQ介导的方法来控制生物膜形成方面具有较大潜力。针对研究较多的革兰氏阴性菌,淬灭过程通过三种途径:1)通过抑制AHLs合成基因的表达,使微生物QS系统不再生成AHLs;2)生成AHLs降解酶,通过化学降解、微生物代谢干扰细菌间正常交流;3)阻断AHLs与LuxI/LuxR型QS受体的结合,通过信号分子类似物与信号分子受体蛋白竞争性结合来阻断细菌QS系统[28]。以上所述的三种QS淬灭途径,能够有效干扰和破坏AHLs调控的QS系统,达到抑制目的基因的表达并调节细菌表型的目的。在群体淬灭中,发生反应的靶点不是细菌生存所必需的,因此微生物不会像使用传统抗生素那样受到不良影响。
1.3 群体感应调控生物膜形成的环境影响因素微生物间的信息交流受到环境中众多因素的影响,例如土壤理化性质、温度、pH、纳米金属离子等。土壤的理化性质影响着AHLs的迁移与扩散,能够一定程度上降低其生物有效性[29]。在中性或弱酸性环境中,AHLs容易发生水解,其半衰期较短。环境温度越高,AHLs越不稳定。Gómez-Gómez等[30]报道环境中金属纳米离子的性质和浓度能够影响微生物间QS系统,ZnONPs(ZnO纳米颗粒,10~100 mg·L–1)能够干扰微生物间信号感知和响应,TiO2NPs(TiO2纳米颗粒,500 mg·L–1)和AgNPs(纳米银,4 mg·L–1)能够抑制AHLs的合成。此外,局部环境的疏水性、传质条件等也会影响细胞间信息交流,传质条件越高越有利于微生物间的信息交流。
2 生物膜修复技术应用生物膜修复技术的成功应用取决于微生物、污染物和载体材料之间的相互作用,其中载体材料对微生物的降解功能至关重要。迄今为止,传统的有机、无机、复合载体材料已得到广泛应用,但这些材料存在一定缺陷,如无机载体材料与微生物结合力较弱,部分有机载体存在微生物毒性。为完善复杂环境中载体材料性能,科研人员不再局限于单一的有机、无机材料,通过改性、功能化等方式制备新型载体材料,挖掘更多特殊功能,旨在提高土壤中污染物的去除效率(表 2)。将微生物固定在经腐殖酸改性的生物质灰烬表面,其对土壤中石油烃的降解率是游离微生物的两倍,并且该固定降解菌能够显著增加土壤中微生物的多样性[31]。固定多环芳烃降解菌株W1、W2于菠萝皮制备的生物炭表面,其对污染土壤中芘的降解效率显著高于游离菌[32]。此外,工农业废弃物,如葵花籽壳,也可以作为微生物的固定载体,并对其修复过程有一定的促进作用[33]。目前利用工程化生物膜技术进行污染土壤修复的研究越来越多,其中工程化的生物膜修复技术包括原位修复技术和异位修复技术,可成功应用于土壤中有机污染物的降解。
|
|
表 2 不同载体材料的生物膜应用 Table 2 The applied biofilm with different carrier materials |
原位生物膜修复包括生物刺激和生物强化。生物刺激指通过添加外源营养物质(如氮、磷、空气等)或通过施加添加剂提高氧浓度,从而加快生物膜生长,最终促进污染物的降解[42]。在实际污染场地土壤修复中,可通过提供营养物质、生长基质、以及电子供体和受体进行生物刺激,提高微生物的代谢活动,从而有效去除污染物[4]。当污染物浓度较低时,通常采用自然衰减法,该技术已在石油烃污染场地修复中得到广泛应用[43]。生物强化指向污染场地接种特异性功能微生物或菌群来促进污染物的降解,同时可利用绿色荧光蛋白和荧光素酶的生物标记物追踪接种微生物的代谢活性,实时监测生物强化过程[44]。生物强化可以通过整合传统的基因工程方法、提高营养浓度或保持微生物持久性的策略以及生物刺激来增强污染物的降解效果[45]。因此,当污染场地本身含有可降解污染物的微生物时,可通过加入辅助基质和营养物质进行生物刺激。如果污染场地缺乏可降解污染物的土著微生物,或土著微生物降解能力较差,则优选生物强化。
2.2 异位生物膜修复技术在缺少污染物的土著降解菌群或缺乏微生物降解有利条件的情况下,异位修复反应器可实现污染场地土壤或地下水目标污染物的快速降解。例如,将生物膜应用于惰性载体生物反应器,可以吸附并降解污染物,尤其是重金属、烃类化合物等[46-47]。该反应器可以有效解决悬浮液中微生物生长缓慢或生物反应器进料的稀释效应所导致的生物量不足问题,从而保证生物量维持较长时间。典型的生物膜反应器包括气升式反应器、升流式厌氧污泥床、生物膜气升式间歇反应器等。与传统处理工艺相比,生物膜反应器具有以下优点:生物量密度大、好氧/厌氧代谢活动共存且代谢活性高、处理流速快、有毒污染物耐受性强等。
2.2.1 生物膜修复有机污染物生物膜的形成和降解特性是修复持久性有机污染物的关键因素[48-49]。从持久性有机污染物污染土壤、水体等环境介质中筛选出多种具备生物膜生成能力的微生物,经进一步优化降解性能可应用于生物膜修复技术中[50]。随着工业生产中石油使用量的增加,其在水环境中的积累和对海洋生物的毒性随之增加,同时石油泄漏也会影响土壤中烃类降解菌的生长,其中Alcanivorax、Arthrobacter、Pseudomonas、Bacillus、Cycloclasticus和Rhodococcus已被证实可用于降解石油烃[51]。对微生物固定载体如活性炭、沸石等进行修饰,能够提高氧获得率以及土壤持水性,从而有效提高微生物修复石油污染土壤的效率[52-53]。Shimada等[54]报道在利用Pseudomonas stutzeri T102修复石油污染土壤过程中,尽管T102生物膜对萘的降解速率最初低于浮游细胞,然而随着时间的推移,生物膜较浮游细菌表现出更高的降解效果,细菌具有更高的生存能力和存活率。另有研究发现,固定于黏土矿物上的Bordetella对1,2,4-三氯苯的矿化程度大于游离降解菌,其中1,2,4-三氯苯的矿化速率与QS信号AHLs浓度呈线性相关[55]。此外,接种功能微生物至生物膜反应器,能够有效降解多环芳烃,其中接种剂量和生物刺激起到至关重要的作用[56]。
2.2.2 生物膜修复重金属生物膜在污染环境中有毒重金属的生物修复中具有广阔的应用前景。栖息在重金属污染区域的微生物分布和多样性,以及负责编码重金属-微生物相互作用的基因的分布和多样性是重金属微生物修复的关键要素。生物膜具备独特的耐高浓度重金属能力,能够通过固定化、吸附和生物转化等方式,经过沉淀或外排泵过程,将高毒性重金属离子转变为低浓度形态,从而降低其生物有效性,最大限度地减少预期危害[4]。研究发现,在不同的天然和合成基质上开发的纤维素微生物膜能够去除90%的铬酸盐(500 mg·L–1)[57]。硫酸盐还原细菌生物膜能够通过沉淀作用有效去除工业废水中的Cu(98%)、Ni(82%)、Fe(78%)等重金属[58]。微藻生物膜能够通过生物吸附方式去除水体中Cd(70%)、Pb(71%)和Ni(74%)等重金属,此外,微藻生物膜在处理过程中产生了高浓度的活性氧,能够高效降解环境中的有机污染物[59]。以上研究表明生物膜EPS基质在重金属累积和重金属离子螯合过程中起着至关重要的作用[60]。由于EPS存在带负电荷的官能团,如羧基和羟基等,这些官能团的比例根据EPS的组成变化,因此EPS表现出较强的金属螯合能力[61]。其中,生物膜EPS的金属螯合或生物吸附能力受其化学性质、离子强度、重金属离子浓度、结合位点和表面性能的影响[62]。
3 群体感应在生物膜修复过程中的作用生物膜修复过程中,群体感应系统能够调控微生物对污染物的降解。研究人员希望能够针对细菌AHLs介导的QS系统进行干扰和促进,即通过群体感应促进或淬灭的方式,从而达到强化生物膜降解污染物的目的[14]。
3.1 群体感应调控微生物降解能力Kumari等[9]研究发现,群体感应不仅在Pseudomonas aeruginosa N6P6生物膜形成过程中起着关键作用,并且控制萘降解基因ndo表达,从而影响萘的降解。另有研究发现,不动杆菌株DR1通过QS信号分子调控生物膜的形成和对十六烷烃降解[20]。通过比较不动杆菌DR1和DR1R发现,DR1和DR1R菌株分别可以生成3种和1种AHLs,其中aqsI(LuxI类似物)突变体不能生成AHLs。蛋白质组学分析表明,DR1R和aqsI突变体内AHLs降解酶大幅增加。在aqsI突变体中,生物膜的形成和对十六烷烃降解能力下降明显。但外源添加野生型细胞培养上清液和AHLs均可以恢复aqsI突变体对十六烷烃的降解能力。此外,添加外源信号分子也能够改变微生物群落参数结构,从而影响污染修复效果。例如,在处理工业有机废水时,往底泥样品中外源添加AHLs,污泥的群落功能和组成均发生了变化,在此过程中苯酚的降解更加高效稳定[63],该结果表明AHLs可以在调节微生物群落参数方面发挥重要作用,并对土壤生态系统功能和工业废水处理具有重要意义。
3.2 群体感应调控微生物分泌物产生在土壤污染修复过程中,QS信号分子对微生物分泌物(乳化剂、表面活性剂等)也起到一定的调控作用。例如,生物膜中同时存在乳化剂生成菌和烃类降解菌时,乳化剂生成菌有助于烃类降解菌的代谢[14]。当细胞处于增长期和生长稳定期时,生物膜QS系统能够调控乳化剂的生成,Serratia和Pseudomonas均可利用AHLs调节不同表面活性剂的产生[18]。Pseudomonas释放的信号分子能够控制鼠李糖脂的产生,从而影响细菌运动、细胞与细胞之间的相互作用、细胞分化以及Pseudomonas生物特有水通道的形成。在序批式生物膜处理系统中,鼠李糖脂的生成能够使自体诱导物活性提高十倍左右[64]。据报道,鼠李糖脂表面活性剂溶解莠去津等农药的能力优于传统的化学表面活性剂[65],其在在污染土壤(工业废物、原油、多环芳烃、炼油产品、农药和重金属等)修复中发挥着重要作用[66]。
3.3 群体感应淬灭调控生物膜修复群体感应淬灭是调控生物膜修复的一个重要手段。在膜生物反应器中,单宁酸的添加能够抑制QS基因的表达,多环芳烃的降解效率随之降低,其中QS信号分子的表达与PAHs的降解呈显著正相关(P < 0.05)[67]。在污水处理过程中,膜生物反应器生物膜表面的微生物积累粘连导致生物污损,群体感应淬灭可有效控制废水处理过程中生物膜的形成[68]。群体感应系统常被选择作为膜反应器污染物去除的靶点,通过调控主导型QS信号分子在膜反应器的浓度防止生物絮凝,进而优化膜反应器的运行效率[69]。富集活性污泥中的QS淬灭菌可分泌γ-己内酯降解AHLs,通过抑制EPS的产生有效控制膜反应器中的生物污损[70]。群体感应淬灭不仅打破了膜改性、膜清洗等传统防污方法资源利用率低和运行成本高的局限性[71],还可防止抗菌化合物或抗生素使用带来的多重耐药性和二次污染[68]。另有研究表明,外源加入游离酰化酶I能够破坏AHLs酰胺键,有效抑制跨膜压力升高,在磁性颗粒上固定酰化酶I形成磁酶载体,成功实现了生活污水处理中对生物膜的抑制[6]。然而,由于群体淬灭可能会抑制所有QS调节的路径,必须优先确定膜生物反应器中功能微生物群体的相互作用不会受到特定群体淬灭策略的影响。
4 结论与展望群体感应在控制各种微生物生理功能方面起着重要作用,如生物膜的形成和污染环境的生物膜修复等。生物膜为细胞间相互作用、遗传物质的细胞间交换、通讯信号和代谢物扩散提供了最佳环境。群体感应介导的生物膜技术在修复污染土壤方面具有较好的应用前景,对生物膜QS系统而言,厘清信号分子的生成规律和在微生物间的信号传导路径及机制至关重要,此过程有助于功能菌群的工程化设计及应用。
目前,合成生物学家常采取操控QS的策略,通过细胞的基因工程改造,控制QS信号分子的生成规律,从而调控工程菌对污染物降解等目的性状的表达。现有大多数较成熟的研究主要针对革兰氏阴性菌,其他微生物物种(包括革兰氏阳性菌和真菌)的生物膜QS系统也需引起关注。另一个重要的研究领域包括生物膜QS系统干扰和信号特异性,其在调控生物膜的形成和土壤中污染物的去除方面起着关键作用。此外,群体感应信号分子在特定环境下的调控可以帮助维持土壤中微生物群落稳定性,因此,合成生物学家可利用生物膜QS系统来调控微生物群落行为,充分发挥功能微生物的修复能力,混合菌群的微生物群落工程也将成为该研究方向的重要目标。
| [1] |
Mukherjee S, Sarkar B, Aralappanavar V K, et al. Biochar-microorganism interactions for organic pollutant remediation: Challenges and perspectives[J]. Environmental Pollution, 2022, 308: 119609. DOI:10.1016/j.envpol.2022.119609
(  0) 0) |
| [2] |
Sonawane J M, Rai A K, Sharma M, et al. Microbial biofilms: Recent advances and progress in environmental bioremediation[J]. Science of the Total Environment, 2022, 824: 153843. DOI:10.1016/j.scitotenv.2022.153843
(  0) 0) |
| [3] |
Nesse L L, Simm R. Biofilm: A hotspot for emerging bacterial genotypes[J]. Advances in Applied Microbiology, 2018, 103: 223-246.
(  0) 0) |
| [4] |
Mishra S, Huang Y H, Li J Y, et al. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants[J]. Chemosphere, 2022, 294: 133609. DOI:10.1016/j.chemosphere.2022.133609
(  0) 0) |
| [5] |
Mangwani N, Kumari S, Das S. Effect of synthetic N-acyl-homoserine lactones on cell-cell interactions in marine Pseudomonas and biofilm mediated degradation of polycyclic aromatic hydrocarbons[J]. Chemical Engineering Journal, 2016, 302: 172-186. DOI:10.1016/j.cej.2016.05.042
(  0) 0) |
| [6] |
Sun Z Q, Xi J Y, Yang C P, et al. Quorum sensing regulation methods and their effects on biofilm in biological waste treatment systems: A review[J]. Frontiers of Environmental Science & Engineering, 2022, 16(7): 87.
(  0) 0) |
| [7] |
Sheng H J, Song Y, Bian Y R, et al. Advance in study on methods for analysis of N-acyl-homoserine lactones (In Chinese)[J]. Acta Pedologica Sinica, 2016, 53(4): 832-844. [生弘杰, 宋洋, 卞永荣, 等. 信号分子N-酰基高丝氨酸内酯分析方法研究进展[J]. 土壤学报, 2016, 53(4): 832-844.]
(  0) 0) |
| [8] |
Chattopadhyay I, J R B, Usman T M M, et al. Exploring the role of microbial biofilm for industrial effluents treatment[J]. Bioengineered, 2022, 13(3): 6420-6440. DOI:10.1080/21655979.2022.2044250
(  0) 0) |
| [9] |
Kumari S, Mangwani N, Das S. Naphthalene catabolism by biofilm forming marine bacterium Pseudomonas aeruginosa N6P6 and the role of quorum sensing in regulation of dioxygenase gene[J]. Journal of Applied Microbiology, 2021, 130(4): 1217-1231. DOI:10.1111/jam.14867
(  0) 0) |
| [10] |
Xiao Y P, Zou H C, Li J J, et al. Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: Current understanding and future perspectives[J]. Gut Microbes, 2022, 14(1): 2039048. DOI:10.1080/19490976.2022.2039048
(  0) 0) |
| [11] |
Xiao Y, Lu Q Q, Yi X, et al. Synergistic degradation of pyrethroids by the quorum sensing-regulated carboxylesterase of Bacillus subtilis BSF01[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 889. DOI:10.3389/fbioe.2020.00889
(  0) 0) |
| [12] |
Flemming H C, Wingender J, Szewzyk U, et al. Biofilms: an emergent form of bacterial life[J]. Nature Reviews Microbiology, 2016, 14(9): 563-575. DOI:10.1038/nrmicro.2016.94
(  0) 0) |
| [13] |
Flemming H C, Wuertz S. Bacteria and archaea on Earth and their abundance in biofilms[J]. Nature Reviews Microbiology, 2019, 17(4): 247-260. DOI:10.1038/s41579-019-0158-9
(  0) 0) |
| [14] |
Mangwani N, Kumari S, Das S. Bacterial biofilms and quorum sensing: Fidelity in bioremediation technology[J]. Biotechnology and Genetic Engineering Reviews, 2016, 32(1/2): 43-73.
(  0) 0) |
| [15] |
Li W W, Yu H Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption[J]. Bioresource Technology, 2014, 160: 15-23. DOI:10.1016/j.biortech.2013.11.074
(  0) 0) |
| [16] |
Ioana Cotar A, Ionescu B, Pelinescu D, et al. Current solutions for the interception of quorum sensing in Staphylococcus aureus[J]. Current Organic Chemistry, 2013, 17(2): 97-104. DOI:10.2174/1385272811317020004
(  0) 0) |
| [17] |
Parsek M R, Greenberg E P. Sociomicrobiology: The connections between quorum sensing and biofilms[J]. Trends in Microbiology, 2005, 13(1): 27-33. DOI:10.1016/j.tim.2004.11.007
(  0) 0) |
| [18] |
Pan X W, Tang M, You J J, et al. PsrA is a novel regulator contributes to antibiotic synthesis, bacterial virulence, cell motility and extracellular polysaccharides production in Serratia marcescens[J]. Nucleic Acids Research, 2022, 50(1): 127-148. DOI:10.1093/nar/gkab1186
(  0) 0) |
| [19] |
Chen A N, Huang Y L. Acyl-homoserine lactone based quorum sensing affects phenanthrene removal by Novosphingobium pentaromativorans US6-1 through altering cell surface properties[J]. International Biodeterioration & Biodegradation, 2020, 147: 104841.
(  0) 0) |
| [20] |
Kang Y S, Park W. Contribution of quorum-sensing system to hexadecane degradation and biofilm formation in Acinetobacter sp. strain DR1[J]. Journal of Applied Microbiology, 2010, 109(5): 1650-1659.
(  0) 0) |
| [21] |
Sheng H J, Harir M, Boughner L A, et al. N-acyl-homoserine lactone dynamics during biofilm formation of a 1, 2, 4-trichlorobenzene mineralizing community on clay[J]. Science of the Total Environment, 2017, 605/606: 1031-1038. DOI:10.1016/j.scitotenv.2017.06.233
(  0) 0) |
| [22] |
Sahreen S, Mukhtar H, Imre K, et al. Exploring the function of quorum sensing regulated biofilms in biological wastewater treatment: A review[J]. International Journal of Molecular Sciences, 2022, 23(17): 9751. DOI:10.3390/ijms23179751
(  0) 0) |
| [23] |
Trebino M A, Shingare R D, MacMillan J B, et al. Strategies and approaches for discovery of small molecule disruptors of biofilm physiology[J]. Molecules, 2021, 26(15): 4582. DOI:10.3390/molecules26154582
(  0) 0) |
| [24] |
Yong Y C, Zhong J J. Regulation of aromatics biodegradation by rhl quorum sensing system through induction of catechol meta-cleavage pathway[J]. Bioresource Technology, 2013, 136: 761-765. DOI:10.1016/j.biortech.2013.03.134
(  0) 0) |
| [25] |
Wang J H, He H Z, Wang M Z, et al. Bioaugmentation of activated sludge with Acinetobacter sp. TW enhances nicotine degradation in a synthetic tobacco wastewater treatment system[J]. Bioresource Technology, 2013, 142: 445-453. DOI:10.1016/j.biortech.2013.05.067
(  0) 0) |
| [26] |
Wang M Z, Zheng X, Zhang K, et al. A new method for rapid construction of a Pseudomonas sp. HF-1 bioaugmented system: Accelerating acylated homoserine lactones secretion by pH regulation[J]. Bioresource Technology, 2014, 169: 229-235. DOI:10.1016/j.biortech.2014.06.098
(  0) 0) |
| [27] |
Xiong F Z, Zhao X X, Wen D H, et al. Effects of N-acyl-homoserine lactones-based quorum sensing on biofilm formation, sludge characteristics, and bacterial community during the start-up of bioaugmented reactors[J]. Science of the Total Environment, 2020, 735: 139449. DOI:10.1016/j.scitotenv.2020.139449
(  0) 0) |
| [28] |
Grandclément C, Tannières M, Moréra S, et al. Quorum quenching: Role in nature and applied developments[J]. FEMS Microbiology Reviews, 2016, 40(1): 86-116. DOI:10.1093/femsre/fuv038
(  0) 0) |
| [29] |
Sheng H J, Wang F, Gu C G, et al. Sorption characteristics of N-acyl homserine lactones as signal molecules in natural soils based on the analysis of kinetics and isotherms[J]. RSC Advances, 2018, 8(17): 9364-9374. DOI:10.1039/C7RA10421A
(  0) 0) |
| [30] |
Gómez-Gómez B, Arregui L, Serrano S, et al. Unravelling mechanisms of bacterial quorum sensing disruption by metal-based nanoparticles[J]. Science of the Total Environment, 2019, 696: 133869. DOI:10.1016/j.scitotenv.2019.133869
(  0) 0) |
| [31] |
Wang X L, Zheng J, Han Z T, et al. Bioremediation of crude oil-contaminated soil by hydrocarbon-degrading microorganisms immobilized on humic acid-modified biofuel ash[J]. Journal of Chemical Technology & Biotechnology, 2019, 94(6): 1904-1912.
(  0) 0) |
| [32] |
Wang C H, Gu L F, Ge S M, et al. Remediation potential of immobilized bacterial consortium with biochar as carrier in pyrene-Cr(Ⅵ)co-contaminated soil[J]. Environmental Technology, 2019, 40(18): 2345-2353. DOI:10.1080/09593330.2018.1441328
(  0) 0) |
| [33] |
Cubitto M A, Gentili A R. Bioremediation of crude oil-contaminated soil by immobilized bacteria on an agroindustrial waste—sunflower seed husks[J]. Bioremediation Journal, 2015, 19(4): 277-286. DOI:10.1080/10889868.2014.995376
(  0) 0) |
| [34] |
Zhang S N, Wang J H. Removal of chlortetracycline from water by immobilized Bacillus subtilis on honeysuckle residue–derived biochar[J]. Water, Air, & Soil Pollution, 2021, 232(6): 236.
(  0) 0) |
| [35] |
Li X, Wang Y X, Luo T, et al. Remediation potential of immobilized bacterial strain with biochar as carrier in petroleum hydrocarbon and Ni co-contaminated soil[J]. Environmental Technology, 2022, 43(7): 1068-1081. DOI:10.1080/09593330.2020.1815858
(  0) 0) |
| [36] |
You Z Y, Xu H Y, Shah K J, et al. Enhance Pyrene degradation of sodium alginate embedding immobilization by adding PAC[J]. Chemical Engineering Transactions, 2020, 81: 19-24.
(  0) 0) |
| [37] |
Liu X P, Xue P, Jia F, et al. Tailoring polymeric composite gel beads-encapsulated microorganism for efficient degradation of phenolic compounds[J]. Chinese Journal of Chemical Engineering, 2021, 32: 301-306. DOI:10.1016/j.cjche.2020.08.002
(  0) 0) |
| [38] |
Tariq A, Latif Z. Bioremediation of mercury compounds by using immobilized nitrogen-fixing bacteria[J]. International Journal of Agriculture and Biology, 2014, 16(6): 1129-1134.
(  0) 0) |
| [39] |
Ahmad M, Liu S T, Mahmood N, et al. Synergic adsorption-biodegradation by an advanced carrier for enhanced removal of high-strength nitrogen and refractory organics[J]. ACS Applied Materials & Interfaces, 2017, 9(15): 13188-13200.
(  0) 0) |
| [40] |
Chen D K, Cai T M, Chen L W, et al. Biodegradation of acifluorfen using joint immobilized cells of sodium alginate and Fe3O4 nanoparticles (In Chinese)[J]. Chinese Journal of Environmental Engineering, 2017, 11(6): 3907-3913. [陈道康, 蔡天明, 陈立伟, 等. 海藻酸钠与纳米Fe3O4联合固定化菌对三氟羧草醚的降解[J]. 环境工程学报, 2017, 11(6): 3907-3913.]
(  0) 0) |
| [41] |
Meng C, Yang H, Wang S L, et al. Embedding immobilization of sulfate-reducing bacteria and the microbial community analysis (In Chinese)[J]. Chinese Journal of Environmental Engineering, 2019, 13(8): 1995-2003. [孟琛, 杨宏, 王少伦, 等. 硫酸盐还原菌包埋固定化及微生物群落分析[J]. 环境工程学报, 2019, 13(8): 1995-2003.]
(  0) 0) |
| [42] |
Vogt C, Richnow H H. Bioremediation via in situ microbial degradation of organic pollutants[J]. Advances in Biochemical Engineering/Biotechnology, 2014, 142: 123-146.
(  0) 0) |
| [43] |
Liu J B, Zhao S, Zhang R, et al. How important is abiotic dissipation in natural attenuation of polycyclic aromatic hydrocarbons in soil?[J]. Science of the Total Environment, 2021, 758: 143687. DOI:10.1016/j.scitotenv.2020.143687
(  0) 0) |
| [44] |
Zhao Q, Yue S J, Bilal M, et al. Comparative genomic analysis of 26 Sphingomonas and Sphingobium strains: Dissemination of bioremediation capabilities, biodegradation potential and horizontal gene transfer[J]. Science of the Total Environment, 2017, 609: 1238-1247. DOI:10.1016/j.scitotenv.2017.07.249
(  0) 0) |
| [45] |
Liu S, Luo X S, Xing Y H, et al. Natural bioaugmentation enhances the application potential of biochar for Cd remediation[J]. Separation and Purification Technology, 2022, 282: 119948. DOI:10.1016/j.seppur.2021.119948
(  0) 0) |
| [46] |
Maqsood Q, Hussain N, Mumtaz M, et al. Novel strategies and advancement in reducing heavy metals from the contaminated environment[J]. Archives of Microbiology, 2022, 204(8): 478. DOI:10.1007/s00203-022-03087-2
(  0) 0) |
| [47] |
Potnis A A, Raghavan P S, Rajaram H. Overview on cyanobacterial exopolysaccharides and biofilms: Role in bioremediation[J]. Reviews in Environmental Science and Bio/Technology, 2021, 20(3): 781-794. DOI:10.1007/s11157-021-09586-w
(  0) 0) |
| [48] |
Bhadra S, Chettri D, Kumar Verma A. Biosurfactants: Secondary metabolites involved in the process of bioremediation and biofilm removal[J]. Applied Biochemistry and Biotechnology, 2022. DOI:10.1007/s12010-022-03951-3
(  0) 0) |
| [49] |
Yesankar P J, Pal M, Patil A, et al. Microbial exopolymeric substances and biosurfactants as 'bioavailability enhancers' for polycyclic aromatic hydrocarbons biodegradation[J]. International Journal of Environmental Science and Technology, 2022. DOI:10.1007/s13762-022-04068-0
(  0) 0) |
| [50] |
Chen M, Xu P, Zeng G M, et al. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs[J]. Biotechnology Advances, 2015, 33(6): 745-755. DOI:10.1016/j.biotechadv.2015.05.003
(  0) 0) |
| [51] |
Zhu S F, Iyobosa E, Ning H J, et al. Screening and identification of highly efficient crude oil-degrading bacteria and construction of bacterial consortium (In Chinese)[J]. Biotechnology Bulletin, 2021, 37(4): 107-115. [朱淑芳, Eheneden Iyobosa, 宁海军, 等. 高效原油污染降解菌的筛选、鉴定及菌群的构建[J]. 生物技术通报, 2021, 37(4): 107-115.]
(  0) 0) |
| [52] |
Khanpour-Alikelayeh E, Partovinia A, Talebi A, et al. Enhanced biodegradation of light crude oil by immobilized Bacillus licheniformis in fabricated alginate beads through electrospray technique[J]. Environmental Monitoring and Assessment, 2021, 193(6): 328. DOI:10.1007/s10661-021-09104-z
(  0) 0) |
| [53] |
Laothamteep N, Naloka K, Pinyakong O. Bioaugmentation with zeolite-immobilized bacterial consortium OPK results in a bacterial community shift and enhances the bioremediation of crude oil-polluted marine sandy soil microcosms[J]. Environmental Pollution, 2022, 292: 118309. DOI:10.1016/j.envpol.2021.118309
(  0) 0) |
| [54] |
Shimada K, Itoh Y, Washio K, et al. Efficacy of forming biofilms by naphthalene degrading Pseudomonas stutzeri T102 toward bioremediation technology and its molecular mechanisms[J]. Chemosphere, 2012, 87(3): 226-233. DOI:10.1016/j.chemosphere.2011.12.078
(  0) 0) |
| [55] |
Wang F, Fekete A, Harir M, et al. Soil remediation with a microbial community established on a carrier: Strong hints for microbial communication during 1, 2, 4-Trichlorobenzene degradation[J]. Chemosphere, 2013, 92(11): 1403-1409. DOI:10.1016/j.chemosphere.2013.03.043
(  0) 0) |
| [56] |
Demeter M A, Lemire J A, Mercer S M, et al. Screening selectively harnessed environmental microbial communities for biodegradation of polycyclic aromatic hydrocarbons in moving bed biofilm reactors[J]. Bioresource Technology, 2017, 228: 116-124. DOI:10.1016/j.biortech.2016.12.086
(  0) 0) |
| [57] |
Naeem A, Batool R, Jamil N. Cr(Ⅵ) reduction by Cellulosimicrobium sp. isolated from tannery effluent[J]. Turkish Journal of Biology, 2013, 37(3): 315-322.
(  0) 0) |
| [58] |
Jong T, Parry D L. Removal of sulfate and heavy metals by sulfate reducing bacteria in short-term bench scale upflow anaerobic packed bed reactor runs[J]. Water Research, 2003, 37(14): 3379-3389. DOI:10.1016/S0043-1354(03)00165-9
(  0) 0) |
| [59] |
Ugya Y A, Hasan D B, Tahir S M, et al. Microalgae biofilm cultured in nutrient-rich water as a tool for the phycoremediation of petroleum-contaminated water[J]. International Journal of Phytoremediation, 2021, 23(11): 1175-1183. DOI:10.1080/15226514.2021.1882934
(  0) 0) |
| [60] |
Gupta P, Diwan B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies[J]. Biotechnology Reports, 2017, 13: 58-71. DOI:10.1016/j.btre.2016.12.006
(  0) 0) |
| [61] |
Ding P F, Song W F, Yang Z H, et al. Influence of Zn(Ⅱ) stress-induction on component variation and sorption performance of extracellular polymeric substances(EPS) from Bacillus vallismortis[J]. Bioprocess and Biosystems Engineering, 2018, 41(6): 781-791. DOI:10.1007/s00449-018-1911-6
(  0) 0) |
| [62] |
Costa O Y A, Raaijmakers J M, Kuramae E E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation[J]. Frontiers in Microbiology, 2018, 9: 1636. DOI:10.3389/fmicb.2018.01636
(  0) 0) |
| [63] |
Valle A, Bailey M J, Whiteley A S, et al. N-acyl-L-homoserine lactones(AHLs) affect microbial community composition and function in activated sludge[J]. Environmental Microbiology, 2004, 6(4): 424-433. DOI:10.1111/j.1462-2920.2004.00581.x
(  0) 0) |
| [64] |
Eslami P, Hajfarajollah H, Bazsefidpar S. Recent advancements in the production of rhamnolipid biosurfactants by Pseudomonas aeruginosa[J]. RSC Advances, 2020, 10(56): 34014-34032. DOI:10.1039/D0RA04953K
(  0) 0) |
| [65] |
Sarubbo L A, da Gloria C Silva M, Durval I J B, et al. Biosurfactants: Production, properties, applications, trends, and general perspectives[J]. Biochemical Engineering Journal, 2022, 181: 108377. DOI:10.1016/j.bej.2022.108377
(  0) 0) |
| [66] |
Cazals F, Colombano S, Huguenot D, et al. Polycyclic aromatic hydrocarbons remobilization from contaminated porous media by(bio) surfactants washing[J]. Journal of Contaminant Hydrology, 2022, 251: 104065. DOI:10.1016/j.jconhyd.2022.104065
(  0) 0) |
| [67] |
Mangwani N, Kumari S, Das S. Involvement of quorum sensing genes in biofilm development and degradation of polycyclic aromatic hydrocarbons by a marine bacterium Pseudomonas aeruginosa N6P6[J]. Applied Microbiology and Biotechnology, 2015, 99(23): 10283-10297. DOI:10.1007/s00253-015-6868-7
(  0) 0) |
| [68] |
Lade H, Paul D, Kweon J H. Quorum quenching mediated approaches for control of membrane biofouling[J]. International Journal of Biological Sciences, 2014, 10(5): 550-565. DOI:10.7150/ijbs.9028
(  0) 0) |
| [69] |
Wang J F, Liu Q J, Li X H, et al. In-situ monitoring AHL-mediated quorum-sensing regulation of the initial phase of wastewater biofilm formation[J]. Environment International, 2020, 135: 105326. DOI:10.1016/j.envint.2019.105326
(  0) 0) |
| [70] |
Yu H R, Liang H, Qu F S, et al. Biofouling control by biostimulation of quorum-quenching bacteria in a membrane bioreactor for wastewater treatment[J]. Biotechnology and Bioengineering, 2016, 113(12): 2624-2632. DOI:10.1002/bit.26039
(  0) 0) |
| [71] |
Huang J H, Liu L X, Zeng G M, et al. Influence of feed concentration and transmembrane pressure on membrane fouling and effect of hydraulic flushing on the performance of ultrafiltration[J]. Desalination, 2014, 335(1): 1-8. DOI:10.1016/j.desal.2013.11.038
(  0) 0) |
 2024, Vol. 61
2024, Vol. 61


