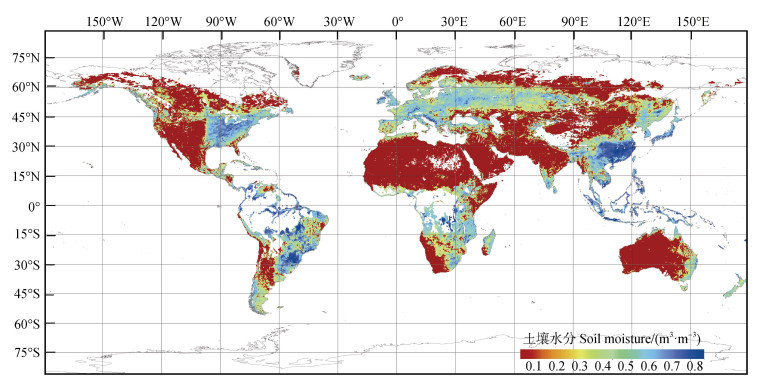
有毒有机物污染是土壤主要污染类型,多环芳烃、抗生素等高毒性有毒有机物可在生物体内积累或通过食物链进入人体,对人类健康和生态环境造成巨大威胁[1-2]。有毒有机物在土壤中赋存形态和生物有效性主要受其转化行为影响[3],土壤中活性矿物(铁锰矿物和富含金属离子的黏土矿物)可驱动有毒有机物化学转化,降低污染风险[4-5]。以往土壤矿物与有毒有机物界面行为研究主要集中于水溶液、矿物悬浊液、土壤悬浊液,悬浊液中矿物浓度可能在0.05~4.0 g·L–1,该体系并不能代表所有土壤真实状况[6-7]。除淹水水稻土外,受植物蒸腾和地表蒸发影响,绝大多数土壤特别是表层土壤常处于干燥、新鲜、湿润等水分非饱和状态。砂土、壤砂土、壤土、黏壤土最大田间持水量也分别仅有5%、8%、18%、30%[8]。国际土壤水分网络(International Soil Moisture Network)显示,许多国家或城市(如中国西藏、美国阿拉斯加州、澳大利亚堪培拉等)表层土壤质量含水量在旱季低于10%,部分甚至低于5%[3]。非洲许多地区表层土壤质量含水量常低于5%,沿海沙漠表层土壤质量含水量可小于2.3%[9]。有趣的是,部分有毒有机物污染也是造成土壤水分非饱和的因素之一,土壤被油类污染后由亲水性转变为疏水性,保水能力下降[10]。例如,中国莫莫格湿地油井附近受石油污染的25处沼泽土壤的平均质量含水量仅有7.8%[11]。卫星观测数据显示,中国湿润区、半湿润区、半干旱区和干旱区土壤平均质量含水量分别在21.7%、18.2%%、13.7%、8.2%,相对低值出现在春季和秋季[12]。图 1显示了2022年4月全球表层土壤体积含水量空间分布,世界范围内有大量低水分土壤分布。
|
图 1 2022年4月全球表层土壤(2~5 cm)体积含水量空间分布(数据来自欧洲太空局,采用ArcMap10.7绘制) Fig. 1 Spatial distribution of surface soil(2-5 cm) volumetric water content globally in April 2022(Data were obtained from the European Space Agency and plotted with ArcMap10.7) |
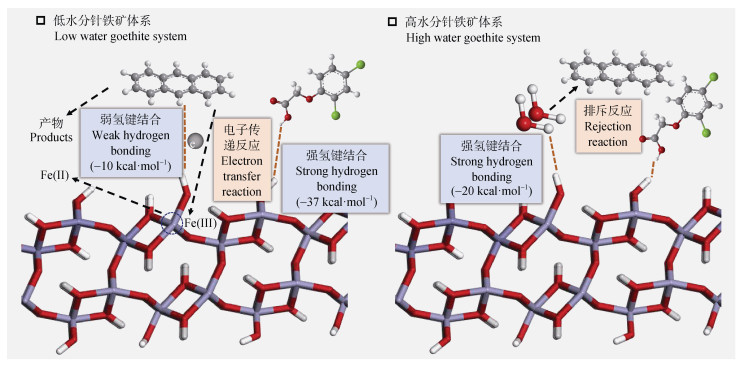
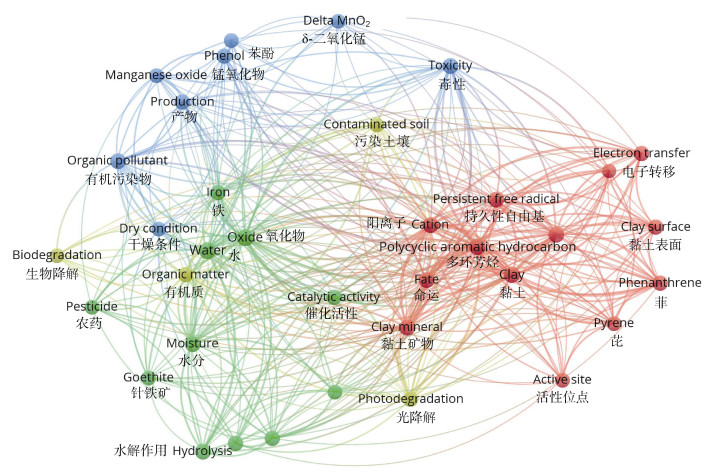
土壤矿物水分状况对其界面有毒有机物转化具有重要影响[13-14]。例如,水相体系中多环芳烃(Polycyclic Aromatic Hydrocarbon,PAHs)等疏水性有毒有机物受矿物表面水膜排斥很难被吸附,其转化行为也未被发现[3]。而低含水量铁锰氧化物能够与PAHs结合并通过电子转移反应驱动其氧化转化,随着含水量升高,PAHs转化也逐渐受到抑制[15-16]。如图 2所示,针铁矿与含有羧基的极性有毒有机物在低水分和高水分下均能结合,而非极性PAH蒽仅在低水分情况下才能被针铁矿吸附进而发生转化。此外,研究发现在低水分土壤矿物界面有毒有机物转化过程中可产生持久性自由基(Environmental Persistent Free Radicals,EPFRs)和卤代二噁英,而这些中间产物却很难在高水分体系中检测到[17-18]。图 3总结了Web of Science数据库中与水分非饱和、土壤矿物、有毒有机物相关研究的高频词汇,包括铁锰氧化物、黏土矿物、水分、电子转移、催化、水解、持久性自由基等。目前,水分非饱和条件下有毒有机物在铁锰氧化物、黏土矿物以及富含金属离子黏土矿物界面转化及机制已得到广泛关注。从土壤矿物催化性能、有毒有机物活性、水分状况影响规律以及电子转移、自由基催化、水解转化机制等方面展开了研究,逐步填补了该研究领域的空白[3,13]。然而,此类研究所涉及土壤矿物和有毒有机物种类繁多,所揭示转化机制十分复杂,相关成果尚缺乏系统梳理和总结。基于此,本文综述了水分非饱和条件下土壤矿物界面有毒有机物转化机制研究进展,提出未来重点研究方向和内容,试图为人们系统、全面地认识土壤中有毒有机物的迁移转化提供参考。
|
图 2 蒽在高/低水分针铁矿表面的界面行为[3,19-20] Fig. 2 Interfacial behavior of anthracene on high/low moisture goethite surface[3, 19-20] |
|
图 3 水分非饱和条件下土壤矿物界面有毒有机物转化机制研究热点图(数据统计于2022年9月17日Web of Science数据库) Fig. 3 Hot spot map of transformation mechanism of toxic organic pollutants on soil mineral interface under water-unsaturated conditions(Web of Science database, September 17, 2022) |
土壤矿物表面过量水分子会抑制有毒有机物转化[3]。Wu等[21]研究表明低含水量针铁矿、磁铁矿、赤铁矿和菱铁矿可通过氢键或质子酸作用促进氯霉素水解,而质量含水量大于10%的铁矿物表面氯霉素转化几乎完全受到抑制。Jia等[22]发现蒽在土壤表面的转化速率与铁氧化物含量呈正相关,蒽的转化速率随空气湿度从30%升高至60%而明显降低。本课题组的研究表明随着针铁矿质量含水量从4.8%升高至14.5%,蒽及其衍生物转化速率均逐渐降低[3]。锰矿物对PAHs的转化也呈现相同规律,将潮湿富含锰矿物的尾矿进行干燥处理,尾矿对蒽的转化能力明显增强[23]。Villalobos等[24]在正己烷溶剂里开展水钠锰矿对芴和蒽转化的实验,发现无水分子参与两者能够被快速转化。研究者认为,水分子和污染物竞争反应位点,随着含水量增加有毒有机物分子会被从矿物界面表面排除。此外,水分状况也会影响矿物结构和活性,低湿度环境会使土壤矿物发生脱水[3]。蒙脱石在脱水过程中,其结构Fe(Ⅲ)会向基底面硅氧空腔移动,与有毒有机物结合更强,更有利于有毒有机物转化[25]。富含金属离子的蒙脱石表面Brønsted酸度也会受水分状况影响,随着水分含量降低会显著升高,低水分条件下催化有毒有机物水解效率更高[26]。
一些研究发现虽然低含水量有利于土壤矿物界面有毒有机物转化,但当含水量过低时则会抑制有毒有机物转化。Wu等[21]研究表明氯霉素水解反应随湿度(0~33.0%)降低而减弱,认为少量水分子可提高传质作用,进而促进氯霉素转移至矿物表面合适的活性位点。Jia等[17]发现富含Fe(Ⅲ)蒙脱石表面蒽转化速率随湿度从8%升高至11%而显著增加,转化反应需要少量水分子参与,但过量水分子又会覆盖反应位点,水分子对蒽转化表现出双面性。Wang等[27]指出水分子很难与铁黏土矿物中活性位点结构铁结合,却会与2-溴酚形成氢键,促进2-溴酚中H脱出,加速2-溴酚的转化。理论计算结果表明,将水分子加入2-溴酚转化途径中,会使反应能和活化能分别降低6.91和0.52 kcal·mol–1。Ding等[18]发现向干燥针铁矿、锰矿物中添加50%水分,三氯生转化率分别提高9.9%和29.9%。适量水分可促进三氯生吸附在矿物表面活性位点,并将Fe(Ⅱ)和Mn(Ⅱ)释放,加快电子传递反应。因此,水分非饱和条件有利于矿物界面有毒有机物转化,但转化反应仍需少量水分子参与,不同矿物界面有毒有机物转化对水分的最佳需求量仍需进一步探究。此外,土壤是一个复杂体系,土壤含水量与矿物并不一致,实际研究中需要特别注意。
矿物表面含水量不同,有毒有机物转化产物可能具有明显差异。Gu等[28]发现五氯苯酚在Fe(Ⅲ)-蒙脱土界面可通过电子转移、自由基耦合、脱氯和环闭合等反应生成二噁英,环境湿度从4%升高至18%会抑制二噁英形成。三氯生在干燥针铁矿和锰矿物表面可生成卤代二噁英,添加50%水分后未检测到卤代二噁英,推测水分子可促进自由基中醚键断裂,从而抑制有机自由基间耦合反应发生[18]。Cheng等[16]研究证实光照下金属氧化物/二氧化硅悬浮液较固体颗粒产生更多·OH和1O2,三氯生在悬浊液中会产生更多羟基化中间体。EPFRs是有毒有机物在矿物界面反应过程中重要的中间产物,而水分子是影响EPFRs生成和衰减的重要因素。一方面水分子通过抑制有毒有机物转化降低EPFRs生成,另一方面水分子可与EPFRs反应抑制它的积累[17]。土壤界面蒽转化生成EPFRs的量在30%湿度要高于60%湿度,EPFRs在30%和60%湿度环境下的半衰期分别为5.57~19.39和3.26~13.5 d[22]。本课题组研究发现三氯生在2%氧化铝/二氧化硅表面生成的EPFRs在悬浊液中半衰期30.6 h,而在干燥颗粒中半衰期为10.9 d[16]。
2 土壤矿物对有毒有机物的界面转化作用纯净黏土矿物性质相对稳定,而天然黏土矿物常与周围金属离子发生离子交换和同晶置换,其表面或结构中活性金属离子具有催化活性[29]。研究中常采用过量金属离子处理黏土矿物以获取金属离子饱和黏土矿物,从而探究金属离子对矿物活性和有毒有机物转化的影响[30]。水分非饱和条件下,金属离子饱和黏土矿物表面未水合化金属离子可将有毒有机物络合吸附,随后发生电子转移或水解反应[27,29]。Li等[31]发现金属阳离子含量与PAHs转化速率呈正相关,PAHs在Fe(Ⅲ)含量最高的Fe(Ⅲ)-蒙脱石表面转化最快,然后是Fe(Ⅲ)-伊利石、Fe(Ⅲ)-叶腊石和Fe(Ⅲ)-高岭土。2︰1型蒙脱石、绿泥石和伊利石较1︰1型高岭石具有更高的负电荷,而2︰1膨胀型蒙脱石表面负电荷量又较2︰1非膨胀型绿泥石、伊利石、叶腊石更高[25]。Jia等[29]发现金属离子饱和蒙脱石表面苯并(a)芘转化速率与金属离子的氧化还原电势呈正相关,表现为Fe(Ⅲ)(0.77 eV) > Cu(Ⅱ)(0.15 eV) > Ni(Ⅱ)(–0.25 eV) > Co(Ⅱ)(–0.28 eV) > Zn(Ⅱ)(–0.76 eV)。Jin等[26]采用指示剂法测定的干燥Fe(Ⅲ)、Ni(Ⅱ)和K(Ⅰ)饱和蒙脱石Brønsted酸性分别为C(H)+ < 3.16 moL L–1、0.10 moL L–1 < C(H)+ < 1.00 moL L–1、C(H)+ > 0.01 moL L–1,Brønsted酸性强的Fe(Ⅲ)饱和蒙脱石对氯霉素水解表现最高催化活性。综上可知,金属离子饱和黏土矿物催化活性由金属离子负载量、氧化还原电势以及Bronsted酸性决定,其中Fe(Ⅲ)饱和蒙脱石在众多金属离子饱和黏土矿物中活性最高,应用更加广泛[30]。黏土矿物也可携带结构型Fe(Ⅲ),绿脱石结构型Fe(Ⅲ)含量最高(15%~31%),其次伊利石(约5.0%),蒙脱石中结构铁含量相对较少,通常小于5%[25,32]。Wang等[25]研究表明2,4,6-三氯酚在含结构Fe(Ⅲ)的蒙脱石和绿脱石界面生成羟基化卤联苯醚速率与Fe(Ⅲ)总含量并无明显关系,认为八面体Fe(Ⅲ)较四面体Fe(Ⅲ)具有更高活性,是转化反应中主要活性位点。除活性金属离子外,黏土矿物中边缘Al-OH也可与有毒有机物形成氢键,加速有毒有机物水解转化[26]。因此,黏土矿物及其含有金属离子的材料可驱动有毒有机物转化,但仍未能清楚了解矿物结构和氧化还原位点,未来仍需通过同步辐射等检测技术和计算化学手段进一步探究。
铁锰矿物具有高氧化性,是驱动有毒有机物化学转化的典型活性物质[33-34]。研究发现,在水分非饱和条件下PAHs等非极性有毒有机物可与铁锰矿物结合并发生转化,加速了它们在土壤环境中衰减[18,22]。铁锰矿物以不同物相和结构存在,其氧化性受表面积、氧化度、氧化还原电位和电导率等影响。早期研究认为,氧化度高的锰矿物对有毒有机物转化程度更高,Mn(Ⅳ)相比Mn(Ⅲ)转化为Mn(Ⅱ)要多转移1个电子,具有更高的氧化容量[35]。近期研究却得出相反结果,有毒有机物更容易被Mn(Ⅲ)氧化,Mn(Ⅲ)(1.49 eV)不仅较Mn(Ⅳ)(1.23 eV)具有更高的氧化还原电势,而且与Mn(Ⅳ)相比Mn(Ⅲ)可直接与有毒有机物发生电子转移不需要改变Mn自旋状态[36-37]。Wang等[36]研究发现蒽的转化速率与锰矿物中Mn(Ⅲ)含量呈正相关,与锰矿物氧化态呈负相关。而最近研究发现不同晶相锰矿中活性氧自由基(Reactive Oxide Species,ROS)产量可能是影响有毒有机物转化效率的主要原因,苯并(a)芘在水钠锰矿、隐钾锰矿和软锰矿界面的转化速率与其ROS产量呈正相关[15,36]。综上可知,锰矿物对有毒有机物的转化包括自由基和非自由基途径,但对Mn(Ⅲ)、Mn(Ⅳ)以及ROS对有毒有机物转化的贡献尚不清楚,未来可通过猝灭、遮蔽等实验手段进一步探究。同一矿物不同晶面上有毒有机物也会有明显差异,例如赤铁矿的{012}和{104}晶面能与邻苯二甲酸二甲酯形成双齿配位络合,相比于{001}晶面更能诱导污染物的水解反应[14]。铁锰矿物与黏土矿物相似,也会与周围环境中金属离子发生离子交换和同晶置换作用。Cheng等[3]发现水钠锰矿的Fe(Ⅲ)交换能力要强于隐钾锰矿,其次是水锰矿和软锰矿。Fe(Ⅲ)饱和锰矿物具有更高的表面积、氧化能力和自由基产量,对苯并(a)芘转化也具有更高的催化性能。同晶置换过程相较于离子交换对矿物的影响更为显著,除增加矿物表面积外,还可使矿物产生更多氧空穴[38]。Sun等[39]认为金属离子掺杂锰矿物中活性氧反应性和可用性与金属离子电负性保持一致,电负性高的金属离子产生较大的电子离域,导致M(金属)-O-Mn中Mn-O键更弱,活性氧迁移率和反应性也更高。金属离子掺杂隐钾锰矿界面二甲醚转化效率顺序为:Ni(Ⅱ) > Cu(Ⅱ) > Co(Ⅱ) > Fe(Ⅱ) > Cr(Ⅲ),金属离子电负性分别为1.91、1.90、1.88、1.83和1.66。本质上其仍为一种推测性意见,未来可通过计算化学分析矿物氧空穴和自由基形成能力。
3 土壤矿物对有毒有机物界面转化机制研究有毒有机物在土壤矿物界面转化机制,对于评估其在土壤中的环境行为具有指导意义。目前,已有研究表明有毒有机物转化机制主要包括电子转移反应、自由基催化反应以及水解反应。表 1总结了土壤中常见土壤矿物对常见有毒有机物的转化及其机制。
|
|
表 1 水分非饱和条件下有毒有机物在土壤矿物表面转化 Table 1 Transformation of toxic organic pollutants on soil mineral surface under water-unsaturated conditions |
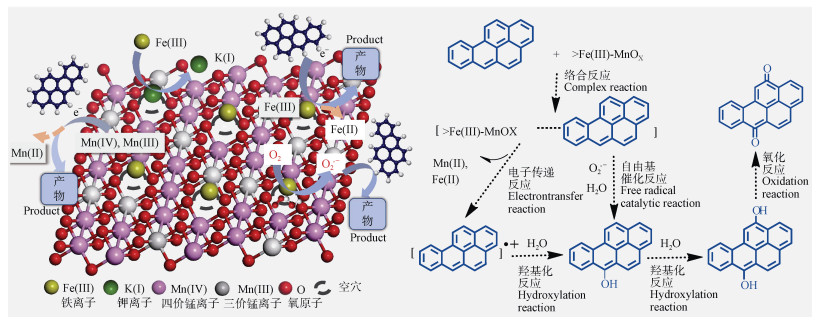
电子转移反应是土壤矿物驱动有毒有机物转化最主要的途径,其反应过程包括:(1)有毒有机物和土壤矿物形成络合前驱体;(2)有毒有机物与土壤矿物中活性金属元素(如Fe(Ⅲ)和Mn(Ⅳ))发生电子转移,并伴随有机自由基的释放和低价金属离子的释放;(3)由有机自由基引发的进一步反应。PAHs及其衍生物富含离域电子,可通过电子供体-受体相互作用与缺电子或带正电荷的土壤矿物发生强烈的相互作用[52]。土壤中含有过渡金属的氧化物(如铁锰氧化物)、碳酸盐矿物(如菱铁矿)、硅铝酸盐矿物(如含铁的蒙脱石、高岭石)均可通过以上途径与有毒有机物发生电子转移反应。图 2展示了低水分针铁矿中Fe(Ⅲ)与蒽通过弱氢键络合,然后发生电子转移反应将其转化,Fe(Ⅲ)被还原了Fe(Ⅱ)。图 4为水分非饱和条件下Fe(Ⅲ)饱和锰氧化物中Mn(Ⅳ)/Mn(Ⅲ)和负载Fe(Ⅲ)通过与苯并(a)芘间的电子转移反应将其转化。碳酸盐矿物菱铁矿中Fe(Ⅲ)也可通过电子转移反应直接介导氯酚等有毒有机物转化[53-54]。而含金属阳离子的黏土矿物介导PAHs转化的主要机制为:阳离子和PAHs形成强烈的阳离子-π键,然后PAHs和金属阳离子之间发生电子转移,随后发生氧化、羟基化以及聚合反应[29]。
|
图 4 水分非饱和条件下苯并(a)芘在Fe(Ⅲ)饱和锰氧化物表面转化机制[15] Fig. 4 The transformation mechanism of B(a)P on Fe(Ⅲ) saturation manganese oxides under water-unsaturated conditions[15] |
土壤矿物既可氧化苯酚、抗生素等有毒有机物,也可促进有机氯农药、硝基苯等污染物的还原转化。李芳柏等[55-56]报道了有机氯类污染物可被亚热带土壤胶体界面还原转化释放出氯离子,并发现土壤中针铁矿、赤铁矿等铁氧化物对它们具有较好的还原转化能力。铁氧化物中Fe(Ⅲ)在还原性较强的淹水土壤中极易被还原为Fe(Ⅱ),而根系分泌物以及还原菌的存在也会促进Fe(Ⅱ)形成,Fe(Ⅱ)作为电子供体可与有机氯、硝基苯发生电子转移反应使其还原降解。同理Mn(Ⅱ)可由有机物或者微生物将Mn(Ⅲ)和Mn(Ⅳ)还原而产生,进而可介导有毒有机物还原转化[57]。此外,当黏土矿物表面吸附有Fe(Ⅱ)离子时,其对有毒有机物的还原转化速率明显增强[58]。相关研究主要集中在矿物悬浊液和厌氧体系,用于模拟淹水厌氧条件下有毒有机物自然衰减过程,尚未发现非水相体系下的研究。氧气充足的干燥土壤表层中氧化反应更容易发生,但低价金属的存在仍可驱动有毒有机物还原转化,未来仍需开展相关研究。
有毒有机物与土壤矿物的结合是电子转移反应发生的前提,是有毒有机物转化的关键因素[59]。极性酚类化合物中羟基可与铁锰矿物表面羟基形成强烈氢键作用,被牢牢吸附在铁锰矿物表面[60]。铁锰矿物表面羟基则使非极性PAHs的π-体系极化,π-体系接受质子,两者形成弱氢键[19]。铁锰矿物悬浊液或含水量较高的铁锰矿中,水分子与铁锰矿物表面羟基形成强烈的氢键,将PAHs从铁锰矿物表面排除,PAHs几乎不能被吸附至铁锰矿物表面[3,61]。Villalobos等[24]发现线性强、分子尺寸小的蒽(5 Å)会被水钠锰矿吸附和氧化,而分子尺寸较大的荧蒽(10 Å)未能被吸附,所以荧蒽并未被氧化转化。土壤矿物与有毒有机物结合作用研究仍然较少,水分子对两者结合作用的影响仍处于推测阶段,未来仍需通过计算化学进一步阐明。
有毒有机物得失电子能力是其能否被转化的关键因素,研究中已占有电子的能级最高的轨道(HOMO)、未占有电子的能级最低的轨道(LUMO)、电离能等运算符常用于评估其得失电子能力[3]。电离能7.55 eV被认为是PAHs生物和化学降解的阈值[62],Villalobos等[24]发现菲(7.90 eV)不能被水钠锰矿转化,而蒽(7.44 eV)可在水钠锰矿表面迅速转化。Jia等[29]则发现Fe(Ⅲ)-蒙脱石对蒽、芘、苯并(a)芘、苯并(a)蒽具有较高催化性,转化速率与PAHs电离能呈正相关。而芳香烃上引入取代官能团会改变其电子性质,进而影响其转化能力。-CH3和-NH2等给电子基可增加芳香环电子密度,增加HOMO能量值。相反,-NO2和-Cl等吸电子基会降低HOMO能量[63]。本课题组研究[3]表明,蒽(ANT)及其衍生物在针铁矿界面转化效率与其HOMO能量呈正相关,转化速率顺序为:9-NH2-ANT > 9-CH3-ANT > ANT > 9-Cl-ANT > 9-NO2-ANT,HOMO能量值分别为–5.03、–5.43、–5.55、–5.43、和–5.47 eV。
金属元素价态变化是矿物与有毒有机物发生电子转移反应的证据之一,通常采用分光光度计、电感耦合等离子体发射光谱仪(ICP-OES)以及X射线光电子能谱等技术测定[3,18,47]。但现有技术仍不够灵敏,需要较高的低价金属生成量,未来仍需发展同步辐射、穆斯堡尔谱等更灵敏精确的测试方法[3]。有机自由基是电子转移发生的另一证据,部分有机自由基可在低含水量矿物表面稳定存在,现有研究称其为持久性自由基[29]。有机自由基可通过电子顺磁共振光谱(EPR)和原位漫反射光谱检测[30],EPR主要测定有机自由基中未配对电子,而红外光谱则通过有毒有机物官能团的变化证明有机自由基的产生。
3.2 自由基催化反应早期研究认为土壤矿物与有毒有机物的自由基催化反应需在光照下进行。前期研究发现光照下Fe(Ⅲ)-蒙脱石可产生羟基(·OH)和超氧(O2-)自由基,且随空气湿度10%升高至80%两者浓度分别增加1.07倍和62.79倍[30]。最近研究发现,部分土壤矿物在黑暗常温环境下也会产生活性氧自由基(ROS)。如锰氧化物氧空穴上方可产生O2-,其产生过程为电子从氧空穴转移至表面吸附氧(图 4)[64]。不同结构锰氧化物表面超氧自由基产量与其表面吸附氧(Oads)和晶格氧(Oaltt)比值呈正相关,锰氧化物表面吸附氧对O2-形成起主导作用[15,36]。锰氧化物表面O2-对有毒有机物的转化有显著贡献,如O2-对水钠锰矿表面7种氯酚类污染物转化的贡献在4%~28%[64],以及不同结构锰氧化物表面O2-的浓度与蒽的转化速率呈正相关[15,36]。近期研究发现,隐钾锰矿经过Fe(Ⅲ)和Cu(Ⅱ)离子交换处理后表面O2-产量均有增加,因为离子交换过程不仅导致隐钾锰矿结晶减弱、比表面积增加,而且含有Fe(Ⅲ)和Cu(Ⅱ)的隐钾锰矿具有更高的氧空位形成能[65]。此外,金属离子引入会降低锰氧化物水接触角并增加干燥矿物吸附水含量,水分子不仅促进活性氧在催化剂表面扩散,其解离形成的羟基更有利于O2吸附[15,66]。虽然锰矿物表面自由基驱动的有毒有机物转化已逐渐受到关注,但相关研究仍基于锰矿物自身性质,土壤环境因子对转化影响规律及机制仍需进一步研究。
普遍认为铁矿物在暗环境中不会有ROS产生,仅在受到强光形成导带电子和空穴时,才能与表面O2和H2O反应形成ROS[16]。但也有学者发现铁矿物表面具有ROS,其所用矿物可能具备较多缺陷结构,未来仍需进一步研究[67]。而金属离子饱和黏土矿物表面ROS的形成机理研究仍处于初级阶段。Zhao等[52]在苯并(a)芘与Cu(Ⅱ)-蒙脱石体系中检测到O2-和·OH,认为ROS的生成与EPFRs有关,EPFRs可作为电子供体诱导ROS形成,反应过程中ROS和EPFRs浓度均呈现先增加后减小的趋势。此外,也有研究认为黏土矿物中结构Fe2+可传递电子给表面吸附O2生成O2-,而O2-可进一步转化H2O2和·OH等活性物种[68]。
3.3 水解反应水解反应是土壤中脂类和酰胺类有毒有机物的主要转化行为,涕灭威、丁硫克百威、卡巴呋喃等农药均可通过水解反应生成高溶解性和低毒性的酚类或胺类小分子化合物[69]。水分非饱和条件下,矿物易与农药和抗生素类有毒有机物结合,降低有毒有机物水解活化能,加速水解反应发生[70]。不同矿物催化有毒有机物水解的机制取决于矿物晶体结构和表面性质。高岭石和氧化铝表面Al-OH形成的氢键作用是两种矿物表面有毒有机污染物水解的主要驱动力[26]。Jin等[42]发现抗生素氯霉素能够在高岭石表面快速水解,红外等光谱结果显示Al-OH与氯霉素中C=O形成氢键,理论结果表明氢键作用显著降低了水解活化能。而金属离子饱和蒙脱石的活性主要来源于Fe(Ⅲ)、Cu(Ⅱ)等层间阳离子,存在着Brønsted酸和Lewis酸两种催化机制。Brønsted酸机制为阳离子与蒙脱石层间水分子产生极化作用释放出H+,使有毒有机物生成质子化形态,质子化有毒有机物较母体具有更低的水解活化能。Fe(Ⅲ)、Al(Ⅲ)、Ni(Ⅱ)饱和蒙脱石(pH < 1.0)相对于K(Ⅰ)、Ca(Ⅱ)、Mg(Ⅱ)等离子饱和蒙脱石(pH > 2.0)具有更强的Brønsted酸性,对有毒有机物水解具有更高催化活性[26]。Lewis酸机制为阳离子与有毒有机物形成络合物,降低了有毒有机物水解活化能[26]。Reddy等[70]发现蒙脱石Al(Ⅲ)离子改性处理后,其表面Brønsted酸和Lewis酸位点显著增加。Xu等[43]发现聚二甲基硅氧烷在蒙脱石上的水解反应受交换性阳离子影响,转化速率顺序为:Al(Ⅲ) > Ca(Ⅱ) > K(Ⅰ)。
此外,不同金属氧化物和碳酸盐矿物界面有毒有机物水解机制也具有明显差异。针铁矿表面具有丰富的Fe-OH,针铁矿驱动的水解反应以Fe-OH与有毒有机物形成氢键作用为主导[71]。赤铁矿表面Fe-OH基团较少,对有毒有机物转化则以Lewis酸催化为主,氢键作用为辅。与金属氧化物相比,碳酸盐矿物菱铁矿中CO32-在水分子作用下可发生质子化形成HCO32-,HCO32-基团中-OH和C=O均可与有毒有机物形成氢键作用。锰氧化物是典型的氧化剂,但阿拉特津在其表面脱烷基过程为水解反应,阿特拉津氮和氧原子与Mn(Ⅳ)形成双键,进而脱去质子[49]。土壤环境中锰矿物相比于铁矿物更加丰富,有毒有机物在锰矿物界面水解反应的实验和理论研究仍然非常匮乏,未来可重点关注。
4 有机质和微生物对土壤矿物界面有毒有机物转化的影响土壤是一个复杂体系,矿物、有机质和微生物是土壤主要组成。有机质和微生物对有毒有机物在自然土壤中溶解、迁移及转化起着重要作用,对矿物界面有毒有机物转化具有重要影响。
4.1 有机质土壤中有机质与矿物颗粒紧密结合,会改变矿物表面性质和反应性,影响矿物界面有毒有机物迁移和转化。矿物表面有机质会减弱有毒有机物与矿物结合,从而抑制有毒有机物转化[72]。但有机质中酚羟基、羧基和酰胺基等会促进铁锰矿物还原溶解,生成的还原态铁、锰离子则有利于矿物界面有毒有机物还原转化[56,73]。同时,有机质可能含有持久性自由基,可直接或间接介导活性自由基产生,例如诱导H2O2和抗坏血酸产生活性自由基,促进矿物界面有毒有机物转化[74]。此外,土壤有机质可作为光敏剂诱导自由基生成,进而引发有毒有机物光降解。Jia等[75]发现富里酸和胡敏酸可促进黏土矿物界面多环芳烃光降解,Li等[76]也揭示了草酸可促进双酚A在铁矿物表面光降解。而不同有机质的官能团、分子量和氧化还原位点不同,对矿物界面有毒有机物光降解影响也不同。可溶性有机质中酚类分子具有较高电子供体能力,会与有毒有机物竞争活性自由基,抑制矿物界面有毒有机物转化[75]。因此,有机质既能促进也能抑制矿物界面有毒有机物的转化,有机质对矿物界面有毒机物转化的影响规律及机制有待进一步研究。
4.2 微生物土壤中矿物与微生物之间的电子传递过程对有毒有机物转化具有重要意义,土壤矿物可作为电子受体促进微生物胞外呼吸,提高有毒有机物转化效率。铁氧化物具有半导体结构,可传递胞外呼吸电子,形成微生物外膜细胞色素c-铁氧化物-有毒有机物的电子传递途径,加速有毒有机物转化[77]。在铁还原菌脱色希瓦氏菌-针铁矿-滴滴涕体系中,针铁矿虽然不能转化滴滴涕,但针铁矿被还原菌还原产生的Fe(Ⅱ)能够将滴滴涕还原脱氯[55]。此外,土壤矿物可为微生物繁殖提供场所和营养物质,进而促进有毒有机物微生物降解。萘降解菌还原溶解菱铁矿和磁铁矿生成的Fe(Ⅱ)也可为降解菌繁殖提供营养物质,进而促进降解菌对萘转化[78]。Ruan等[79]发现向鞘氨醇单胞菌GY2B和菲体系中加入蒙脱石,细菌可吸附在蒙脱石表面,促进了细菌生长和菲转化。
5 研究展望本文综述了非水分饱和条件下土壤矿物界面有毒有机物转化及机制。水分非饱和条件有利于土壤矿物驱动多环芳烃、抗生素、农药等疏水性有毒有机物化学转化。水分非饱和条件下,水分子与有毒有机物竞争矿物界面活性位点作用较弱,矿物更容易与有毒有机物结合,且矿物脱水情况下具有高活性结构。同时,水分非饱和条件有利于土壤矿物与有毒有机物产生持久性自由基和卤代二噁英等中间产物。
根据目前的知识和该领域的最新进展,建议后期从以下几个方面进行深入研究:
(1)现有研究仍基于纯净矿物与单一污染物之间的界面行为,但实际土壤中矿物结晶程度不高,且复合污染更普遍。此外,土壤是由多组分组成的复杂体系,有机物、微生物等会对矿物界面有毒有机物转化产生影响。未来应尝试采用来源于实际土壤的非纯净矿物为研究对象,在水分非饱和实际土壤体系中开展有毒有机物转化研究,这样更具有现实意义。
(2)实际环境中有毒有机物还原转化过程也是其在土壤矿物界面的重要环境行为,但相关研究仍十分匮乏。未来可尝试在水分非饱和情况下探究硝基和卤代芳香烃在低价铁锰矿物界面还原转化过程,以及微生物或有机质-矿物-有毒有机物体系中矿物还原溶解和有毒有机物还原转化。
(3)尽管现有技术已实现持久性自由基等中间产物检测,但尚难达到原位、高精度、瞬时检测的实际需求。矿物界面反应极易受周围环境影响,为保障高效原位检测反应,未来可建立原位反应-电子顺磁共振波谱仪等在线研究界面反应中间产物有机自由基的联用装置。此外,可尝试使用瞬态吸收光谱、低温电子顺磁共振波谱等技术检测铁锰矿物界面产生的不稳定有机自由基。
(4)现有研究仍局限于土壤矿物整体活性及其对有毒有机物的转化效能。而土壤矿物不同位点、不同晶面与水分子以及有毒有机物的结合方式不同,对有毒有机物的转化机制也具有差异。未来仍需通过同步辐射红外光谱、傅里叶变换离子回旋共振质谱、微束X射线荧光光谱等技术在微纳米尺度和分子水平解析有毒有机物在矿物中的结合方式和转化机制。
| [1] |
Ge F, Zhang Z X, Fu H, et al. Distribution of organic contaminated sites in China: Statu quo and prospect (In Chinese)[J]. Soils, 2021, 53(6): 1132-1141. [葛锋, 张转霞, 扶恒, 等. 我国有机污染场地现状分析及展望[J]. 土壤, 2021, 53(6): 1132-1141.]
(  0) 0) |
| [2] |
Gao Y Z, Li H. Agro-environmental contamination, food safety and human health: An introduction to the special issue[J]. Environment International, 2021, 157: 106812. DOI:10.1016/j.envint.2021.106812
(  0) 0) |
| [3] |
Cheng P F, Zhang W, Zhao X Q, et al. Nano-goethite-mediated transformation of anthracene derivatives under low moisture conditions[J]. Environmental Science: Nano, 2022, 9(1): 289-301. DOI:10.1039/D1EN00570G
(  0) 0) |
| [4] |
Remucal C K, Ginder-Vogel M. A critical review of the reactivity of manganese oxides with organic contaminants[J]. Environmental Science: Processes & Impacts, 2014, 16(6): 1247-1266.
(  0) 0) |
| [5] |
Cheng P F, Wang Y, Li F B, et al. Progresses in research on surface acid-base properties of variable charge soils and their models (In Chinese)[J]. Acta Pedologica Sinica, 2019, 56(3): 516-527. [程鹏飞, 王莹, 李芳柏, 等. 可变电荷土壤表面酸碱性质与模型研究进展[J]. 土壤学报, 2019, 56(3): 516-527.]
(  0) 0) |
| [6] |
Chen G, Zhao L, Hua D. Oxidative degradation kinetics and products of chlortetracycline by manganese dioxide[J]. Journal of Hazardous Materials, 2011, 193: 128-138. DOI:10.1016/j.jhazmat.2011.07.039
(  0) 0) |
| [7] |
Cheng P F, Wang Y, Cheng K, et al. The acid-base buffer capacity of red soil variable charge minerals and its surface complexation model[J]. Acta Chimica Sinica, 2017, 75: 637-644. DOI:10.6023/A17020056
(  0) 0) |
| [8] |
Goldberg D, Gornat B, Rimon D. Drip irrigation: Principles, design and agricultural practices[M]. Kfar Shmariahu: Drip Irrigation Scientific Publications, 1976.
(  0) 0) |
| [9] |
Agam N, Berliner P R. Diurnal water content changes in the bare soil of a coastal desert[J]. Journal of Hydrometeorology, 2004, 5: 922-933. DOI:10.1175/1525-7541(2004)005<0922:DWCCIT>2.0.CO;2
(  0) 0) |
| [10] |
Abousnina R, Manalo A, Lokuge W, et al. Effects of light crude oil contamination on the physical and mechanical properties of geopolymer cement mortar[J]. Cement & Concrete Composites, 2018, 90: 136-149.
(  0) 0) |
| [11] |
Wang Y, Feng J, Lin Q, et al. Effects of crude oil contamination on soil physical and chemical properties in momoge wetland of China[J]. Chinese Geographical Science, 2013, 23: 708-715. DOI:10.1007/s11769-013-0641-6
(  0) 0) |
| [12] |
Liu R H, Zhang K, Chao L J, et al. Analysis of spatiotemporal characteristics of surface soil moisture across China based on multi-satellite observations (In Chinese)[J]. Advances in Water Science, 2017, 28(4): 479-487. [刘荣华, 张珂, 晁丽君, 等. 基于多源卫星观测的中国土壤湿度时空特征分析[J]. 水科学进展, 2017, 28(4): 479-487.]
(  0) 0) |
| [13] |
Wang Y, Jin X, Peng A, et al. Transformation and toxicity of environmental contaminants as influenced by Fe containing clay minerals: A review[J]. Bulletin of Environmental Contamination and Toxicology, 2020, 104: 8-14. DOI:10.1007/s00128-019-02747-2
(  0) 0) |
| [14] |
Jin X, Wu D, Liu C, et al. Facet effect of hematite on the hydrolysis of phthalate esters under ambient humidity conditions[J]. Nature Communications, 2022, 13: 6125. DOI:10.1038/s41467-022-33950-1
(  0) 0) |
| [15] |
Cheng P F, Lin Z P, Zhao X Q, et al. Enhanced transformation capability towards benzo(a) pyrene by Fe(Ⅲ)-modified manganese oxides[J]. Journal of Hazardous Materials, 2022, 431: 128637. DOI:10.1016/j.jhazmat.2022.128637
(  0) 0) |
| [16] |
Cheng P F, Zhao X Q, El-Ramady H, et al. Formation of environmentally persistent free radicals from photodegradation of triclosan by metal oxides/silica suspensions and particles[J]. Chemosphere, 2022, 290: 133322. DOI:10.1016/j.chemosphere.2021.133322
(  0) 0) |
| [17] |
Jia H Z, Nulaji G, Gao H W, et al. Formation and stabilization of environmentally persistent free radicals induced by the interaction of anthracene with Fe(Ⅲ)-modified clays[J]. Environmental Science & Technology, 2016, 50(12): 6310-6319.
(  0) 0) |
| [18] |
Ding J, Su M, Wu C, et al. Transformation of triclosan to 2, 8-dichlorodibenzo-p-dioxin by iron and manganese oxides under near dry conditions[J]. Chemosphere, 2015, 133: 41-46. DOI:10.1016/j.chemosphere.2015.03.055
(  0) 0) |
| [19] |
Tunega D, Gerzabek M H, Haberhauer G, et al. Model study on sorption of polycyclic aromatic hydrocarbons to goethite[J]. Journal of Colloid and Interface Science, 2009, 330(1): 244-249. DOI:10.1016/j.jcis.2008.10.056
(  0) 0) |
| [20] |
Aquino A, Tunega D, Haberhauer G, et al. Quantum chemical adsorption studies on the(110) surface of the mineral goethite[J]. Journal of Physical Chemistry C, 2006, 111: 877-885.
(  0) 0) |
| [21] |
Wu D, Huang S, Zhang X, et al. Iron minerals mediated interfacial hydrolysis of chloramphenicol antibiotic under limited moisture conditions[J]. Environmental Science & Technology, 2021, 55: 9569-9578.
(  0) 0) |
| [22] |
Jia H, Zhao S, Shi Y, et al. Formation of environmentally persistent free radicals during the transformation of anthracene in different soils: Roles of soil characteristics and ambient conditions[J]. Journal of Hazardous Materials, 2018, 362: 214-224.
(  0) 0) |
| [23] |
Clarke C, Tourney J, Johnson K. Oxidation of anthracene using waste Mn oxide minerals: The importance of wetting and drying sequences[J]. Journal of Hazardous Materials, 2012, 205: 126-130.
(  0) 0) |
| [24] |
Villalobos M, Carrillo-Cárdenas M, Gibson R, et al. The influence of particle size and structure on the sorption and oxidation behaviour of birnessite: Ⅱ. Adsorption and oxidation of four polycyclic aromatic hydrocarbons[J]. Environmental Chemistry, 2014, 11: 279-288. DOI:10.1071/EN13161
(  0) 0) |
| [25] |
Wang Y, Liu C, Peng A, et al. Formation of hydroxylated polychlorinated diphenyl ethers mediated by Structural Fe(Ⅲ) in smectites[J]. Chemosphere, 2019, 226: 94-102. DOI:10.1016/j.chemosphere.2019.03.082
(  0) 0) |
| [26] |
Jin X, Wu D, Ling J, et al. Hydrolysis of chloramphenicol catalyzed by clay minerals under non-aqueous conditions[J]. Environmental Science & Technology, 2019, 53: 10645-10653.
(  0) 0) |
| [27] |
Wang Y, Peng A, Chen Z, et al. Transformation of gaseous 2-bromophenol on clay mineral dust and the potential health effect[J]. Environmental Pollution, 2019, 250: 686-694. DOI:10.1016/j.envpol.2019.04.072
(  0) 0) |
| [28] |
Gu C, Li H, Teppen B J, et al. Octachlorodibenzodioxin formation on Fe(Ⅲ)-montmorillonite clay[J]. Environmental Science & Technology, 2008, 42: 4758-4763.
(  0) 0) |
| [29] |
Jia H Z, Zhao S, Shi Y F, et al. Transformation of polycyclic aromatic hydrocarbons and formation of environmentally persistent free radicals on modified montmorillonite: The role of surface metal ions and polycyclic aromatic hydrocarbon molecular properties[J]. Environmental Science & Technology, 2018, 52(10): 5725-5733.
(  0) 0) |
| [30] |
Zhao X Q, Cheng P F, Borch T, et al. Humidity induces the formation of radicals and enhances photodegradation of chlorinated-PAHs on Fe(Ⅲ)-montmorillonite[J]. Journal of Hazardous Materials, 2022, 423: 127210. DOI:10.1016/j.jhazmat.2021.127210
(  0) 0) |
| [31] |
Li L, Jia H, Li X, et al. Transformation of anthracene on various cation-modified clay minerals[J]. Environmental Science and Pollution Research, 2015, 22: 1261-1269. DOI:10.1007/s11356-014-3424-4
(  0) 0) |
| [32] |
Wang Y, Ling J Y, Gu C, et al. Dissolution of Fe from Fe-bearing minerals during the brown-carbonization processes in atmosphere[J]. Science of the Total Environment, 2021, 791: 148133. DOI:10.1016/j.scitotenv.2021.148133
(  0) 0) |
| [33] |
Li Q Q, Huang X C, Su G J, et al. The regular/persistent free radicals and associated reaction mechanism for the degradation of 1, 2, 4-trichlorobenzene over different MnO2 polymorphs[J]. Environmental Science & Technology, 2018, 52(22): 13351-13360.
(  0) 0) |
| [34] |
Hu S W, Liu T X, Li F B, et al. The abiotic and biotic transformation processes of soil iron-bearing minerals and its interfacial reaction mechanisms of heavy metals: A review (In Chinese)[J]. Acta Pedologica Sinica, 2022, 59(1): 54-65. [胡世文, 刘同旭, 李芳柏, 等. 土壤铁矿物的生物-非生物转化过程及其界面重金属反应机制的研究进展[J]. 土壤学报, 2022, 59(1): 54-65.]
(  0) 0) |
| [35] |
Liu C, Zhang L, Li F, et al. Dependence of sulfadiazine oxidative degradation on physicochemical properties of manganese dioxides[J]. Industrial & Engineering Chemistry Research, 2009, 48: 10408-10413.
(  0) 0) |
| [36] |
Wang Z Q, Jia H Z, Zheng T, et al. Promoted catalytic transformation of polycyclic aromatic hydrocarbons by MnO2 polymorphs: Synergistic effects of Mn3+ and oxygen vacancies[J]. Applied Catalysis B: Environmental, 2020, 272: 119030. DOI:10.1016/j.apcatb.2020.119030
(  0) 0) |
| [37] |
Huang J Z, Zhong S F, Dai Y F, et al. Effect of MnO2 phase structure on the oxidative reactivity toward bisphenol a degradation[J]. Environmental Science & Technology, 2018, 52(19): 11309-11318.
(  0) 0) |
| [38] |
Liu J, Ke L, Liu J, et al. Enhanced catalytic ozonation towards oxalic acid degradation over novel copper doped manganese oxide octahedral molecular sieves nanorods[J]. Journal of Hazardous Materials, 2019, 371: 42-52. DOI:10.1016/j.jhazmat.2019.02.094
(  0) 0) |
| [39] |
Sun M, Yu L, Ye F, et al. Transition metal doped cryptomelane-type manganese oxide for low-temperature catalytic combustion of dimethyl ether[J]. Chemical Engineering Journal, 2013, 220: 320-327. DOI:10.1016/j.cej.2013.01.061
(  0) 0) |
| [40] |
Liyanapatirana C, Gwaltney S R, Xia K. Transformation of triclosan by Fe(Ⅲ)-saturated montmorillonite[J]. Environmental Science & Technology, 2010, 44: 668-674.
(  0) 0) |
| [41] |
Qin C, Troya D, Shang C, et al. Surface catalyzed oxidative oligomerization of 17β-estradiol by Fe3+-saturated montmorillonite[J]. Environmental Science & Technology, 2015, 49(2): 956-964.
(  0) 0) |
| [42] |
Jin X, Wu D, Chen Z, et al. Surface catalyzed hydrolysis of chloramphenicol by montmorillonite under limited surface moisture conditions[J]. Science of the Total Environment, 2021, 770: 144843. DOI:10.1016/j.scitotenv.2020.144843
(  0) 0) |
| [43] |
Xu S H, Lehmann R G, Miller J R, et al. Degradation of polydimethylsiloxanes(silicones) as influenced by clay minerals[J]. Environmental Science & Technology, 1998, 32(9): 1199-1206.
(  0) 0) |
| [44] |
Mingelgrin U, Gerstl Z, Yaron B. Pirimiphos ethyl-clay surface interactions[J]. Soil Science Society of America Journal, 1975, 39(5): 834-837. DOI:10.2136/sssaj1975.03615995003900050016x
(  0) 0) |
| [45] |
Yi P, Chen Q, Li H, et al. A comparative study on the formation of environmentally persistent free radicals(EPFRs) on hematite and goethite: Contribution of various catechol degradation byproducts[J]. Environmental Science & Technology, 2019, 53: 13713-13719.
(  0) 0) |
| [46] |
Li H, Guo H, Pan B, et al. Catechol degradation on hematite/silica-gas interface as affected by gas composition and the formation of environmentally persistent free radicals[J]. Scientific Reports, 2016, 6: 24494. DOI:10.1038/srep24494
(  0) 0) |
| [47] |
Smith B A, Siems W F, Teel A L, et al. Pyrolusite(beta-MnO2)-mediated, near dry-phase oxidation of 2, 4, 6-trichlorophenol[J]. Environmental Toxicology and Chemistry, 2010, 25: 1474-1479.
(  0) 0) |
| [48] |
Feitosa-Felizzola J, Hanna K, Chiron S. Adsorption and transformation of selected human-used macrolide antibacterial agents with iron(Ⅲ) and manganese(Ⅳ) oxides[J]. Environmental Pollution, 2009, 157: 1317-1322. DOI:10.1016/j.envpol.2008.11.048
(  0) 0) |
| [49] |
Shin J Y, Cheney M A. Abiotic dealkylation and hydrolysis of atrazine by birnessite[J]. Environmental Toxicology and Chemistry, 2005, 24(6): 1353-1360. DOI:10.1897/04-248R.1
(  0) 0) |
| [50] |
Li Y Z, Fan Z Y, Shi J W, et al. Modified manganese oxide octahedral molecular sieves M'-OMS-2(M'=Co, Ce, Cu) as catalysts in post plasma-catalysis for acetaldehyde degradation[J]. Catalysis Today, 2015, 256: 178-185. DOI:10.1016/j.cattod.2015.02.003
(  0) 0) |
| [51] |
Miyake T, Koike K, Aoki I, et al. Synthesis of Al-containing octahedral molecular sieves and oxidation of cyclohexane with them[J]. Applied Catalysis A-General, 2005, 288(1): 216-219.
(  0) 0) |
| [52] |
Zhao S, Miao D, Zhu K, et al. Interaction of benzo[a]pyrene with Cu(Ⅱ)-montmorillonite: Generation and toxicity of environmentally persistent free radicals and reactive oxygen species[J]. Environment International, 2019, 129: 154-163. DOI:10.1016/j.envint.2019.05.037
(  0) 0) |
| [53] |
Paikaray S. Environmental stability of Schwertmannite: A review[J]. Mine Water and the Environment, 2021, 40: 570-586. DOI:10.1007/s10230-020-00734-2
(  0) 0) |
| [54] |
Zhong C, Jiang Y, Liu Q, et al. Natural siderite derivatives activated peroxydisulfate toward oxidation of organic contaminant: A green soil remediation strategy[J]. Journal of Environmental Sciences, 2023, 127: 615-627. DOI:10.1016/j.jes.2022.06.030
(  0) 0) |
| [55] |
Li F B, Li X M, Zhou S G, et al. Enhanced reductive dechlorination of DDT in an anaerobic system of dissimilatory iron-reducing bacteria and iron oxide[J]. Environmental Pollution, 2010, 158: 1733-1740. DOI:10.1016/j.envpol.2009.11.020
(  0) 0) |
| [56] |
Li F B, Wang X G, Zhou S G, et al. Reviews on abiotic transformation of organchlorines on the interface of iron oxides and water in red soil colloids (In Chinese)[J]. Ecology and Environment, 2006, 15(6): 1343-1351. DOI:10.3969/j.issn.1674-5906.2006.06.043 [李芳柏, 王旭刚, 周顺桂, 等. 红壤胶体铁氧化物界面有机氯的非生物转化研究进展[J]. 生态环境, 2006, 15(6): 1343-1351.]
(  0) 0) |
| [57] |
Nasser A, Mingelgrin U. Birnessite-induced mechanochemical degradation of 2, 4-dichlorophenol[J]. Chemosphere, 2014, 107: 175-179. DOI:10.1016/j.chemosphere.2013.11.058
(  0) 0) |
| [58] |
Sun K W, Tao L, Zhong J H, et al. Interactively interfacial reaction between reductive transformation of 2-nitrophenol and Fe(Ⅱ) adsorbed on kaoline (In Chinese)[J]. Ecology and Environment, 2008, 17(6): 2177-2182. DOI:10.3969/j.issn.1674-5906.2008.06.013 [孙克文, 陶亮, 钟继洪, 等. 高岭土界面Fe(Ⅱ)吸附与邻硝基苯酚还原转化的交互反应研究[J]. 生态环境, 2008, 17(6): 2177-2182.]
(  0) 0) |
| [59] |
Ersan G, Apul O G, Perreault F, et al. Adsorption of organic contaminants by graphene nanosheets: A review[J]. Water Research, 2017, 126: 385-398. DOI:10.1016/j.watres.2017.08.010
(  0) 0) |
| [60] |
Lin K, Ding J, Wang H, et al. Goethite-mediated transformation of bisphenol A[J]. Chemosphere, 2012, 89: 789-795. DOI:10.1016/j.chemosphere.2012.04.053
(  0) 0) |
| [61] |
Guimaraes W G, de Lima G F, Duarte H A. Comparative DFT study of the oxy(hydr) oxides of iron and aluminum - structural, electronic and surface properties[J]. Surface Science, 2021, 708: 121821. DOI:10.1016/j.susc.2021.121821
(  0) 0) |
| [62] |
Jia H, Zhao J, Li L, et al. Transformation of polycyclic aromatic hydrocarbons(PAHs) on Fe(Ⅲ)-modified clay minerals: Role of molecular chemistry and clay surface properties[J]. Applied Catalysis B: Environmental, 2014, 154: 238-245.
(  0) 0) |
| [63] |
Shin D, Jung Y. Molecular electrostatic potential as a general and versatile indicator for electronic substituent effects: statistical analysis and applications[J]. Physical Chemistry Chemical Physics, 2022, 24: 25740-25752. DOI:10.1039/D2CP03244A
(  0) 0) |
| [64] |
Zhang S H, Lv J T, Han R X, et al. Sustained production of superoxide radicals by manganese oxides under ambient dark conditions[J]. Water Research, 2021, 196: 117034. DOI:10.1016/j.watres.2021.117034
(  0) 0) |
| [65] |
Cheng P, Zhao X, Chen X, et al. Improving the non-radical/radical transformation efficiency of halogenated polycyclic aromatic hydrocarbons by using metal ion-exchanged cryptomelane-type OMS-2 under moisture-unsaturated conditions[J]. Journal of Cleaner Production, 2023, 15: 136992.
(  0) 0) |
| [66] |
Deng J, Song W, Chen L, et al. The effect of oxygen vacancies and water on HCHO catalytic oxidation over Co3O4 catalyst: A combination of Density Functional Theory and microkinetic study[J]. Chemical Engineering Journal, 2018, 355: 540-550.
(  0) 0) |
| [67] |
Ni Z, Zhang C, Wang Z, et al. Performance and potential mechanism of transformation of polycyclic aromatic hydrocarbons(PAHs) on various iron oxides[J]. Journal of Hazardous Materials, 2021, 403: 123993. DOI:10.1016/j.jhazmat.2020.123993
(  0) 0) |
| [68] |
Sheng Y Z, Hu J L, Kukkadapu R, et al. Inhibition of extracellular enzyme activity by reactive oxygen species upon oxygenation of reduced iron-bearing minerals[J]. Environmental Science & Technology, 2023, 57(8): 3425-3433.
(  0) 0) |
| [69] |
Fang Y F, Cao X Q, Feng W Y, et al. High catalytic hydrolysis of microcystins on pyrite surface[J]. Environmental Chemistry Letters, 2020, 18: 483-487. DOI:10.1007/s10311-019-00948-z
(  0) 0) |
| [70] |
Reddy C R; Bhat Y S; Nagendrappa G; et al. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy[J]. Catalysis Today, 2009, 141(1): 157-160.
(  0) 0) |
| [71] |
Feng S, Ling J, Wang C, et al. Rapid hydrolysis of penicillin antibiotics mediated by adsorbed zinc on goethite surfaces[J]. Environmental Science & Technology, 2019, 53: 10705-10713.
(  0) 0) |
| [72] |
Pea A. A comprehensive review of recent research concerning the role of low molecular weight organic acids on the fate of organic pollutants in soil[J]. Journal of Hazardous Materials, 2022, 434: 128875. DOI:10.1016/j.jhazmat.2022.128875
(  0) 0) |
| [73] |
Hao T, Huang Y, Li F, et al. Facet-dependent Fe(Ⅱ) redox chemistry on iron oxide for organic pollutant transformation and mechanisms[J]. Water Research, 2022, 219: 118587. DOI:10.1016/j.watres.2022.118587
(  0) 0) |
| [74] |
Liu J, Zhang C, Jia H, et al. Abiotic transformation of polycyclic aromatic hydrocarbons via interaction with soil components: A systematic review[J]. Critical Reviews in Environmental Science and Technology, 2023, 53: 676-699. DOI:10.1080/10643389.2022.2083897
(  0) 0) |
| [75] |
Jia H, Li L, Fan X, et al. Visible light photodegradation of phenanthrene catalyzed by Fe(Ⅲ)-smectite: Role of soil organic matter[J]. Journal of Hazardous Materials, 2013, 256: 16-23.
(  0) 0) |
| [76] |
Li F B, Li X Z, Liu C S, et al. Effect of oxalate on photodegradation of bisphenol a at the interface of different iron oxides[J]. Industrial & Engineering Chemistry Research, 2007, 46(3): 781-787.
(  0) 0) |
| [77] |
Wu Y D, Li F B, Liu T X. Mechanism of extracellular electron transfer among microbe-humus-mineral in soil: A review (In Chinese)[J]. Acta Pedologica Sinica, 2016, 53(2): 277-291. [吴云当, 李芳柏, 刘同旭. 土壤微生物—腐殖质—矿物间的胞外电子传递机制研究进展[J]. 土壤学报, 2016, 53(2): 277-291.]
(  0) 0) |
| [78] |
Shen X, Dong W, Wan Y, et al. Influencing mechanisms of siderite and magnetite, on naphthalene biodegradation: Insights from degradability and mineral surface structure[J]. Journal of Environmental Management, 2021, 299: 113648. DOI:10.1016/j.jenvman.2021.113648
(  0) 0) |
| [79] |
Ruan B, Wu P, Lai X, et al. Effects of Sphingomonas sp GY2B on the structure and physicochemical properties of stearic acid-modified montmorillonite in the biodegradation of phenanthrene[J]. Applied Clay Science, 2018, 156: 36-44. DOI:10.1016/j.clay.2018.01.009
(  0) 0) |
 2024, Vol. 61
2024, Vol. 61


