2. 中国科学院大学, 北京 100049;
3. 南京大学环境学院, 南京 210023
2. University of the Chinese Academy of Sciences, Beijing 100049, China;
3. School of the Environment, Nanjing University, Nanjing 210023, China
溶解性有机物(DOM)和亚硝酸盐/硝酸盐(NO2–/NO3–)是天然地表水和农业区水体中普遍存在的重要成分。DOM和NO2–/NO3–具有较强的光化学活性,光照时可产生一系列活性组分,包括三重激发态有机质(3CDOM*)、单线态氧(1O2)和羟基自由基(•OH)[1]。由于这些活性组分具有很高的化学反应活性,能够参与各种地球化学反应过程,对环境中的物质循环和污染物转化具有重要意义。例如,•OH具有极强的氧化能力,氧化电位高达2.8 V,可快速氧化大分子的有机质(反应速率达2.7×104 s–1·(mg·L–1)–1)产生CO2 [2],从而介导了碳在环境中的循环转化。此外,•OH和超氧阴离子(O2•–)等自由基能够介导有机污染物的降解和有毒变价金属(如三价砷)的转化,对水体中有机污染物和土壤中农药残留的消减均具有重要贡献[3]。
稻米是全球约1/2人口的主食,也是全球种植最广泛的作物之一,2020年稻田面积约1.62亿hm2[4]。由于水稻种植期间需要长时间淹水,稻田上覆水正在成为除草剂、杀虫剂和重金属等污染物的汇[5]。相比于河、湖、海洋等天然地表水体,在稻田生态系统中,存在着强烈的微生物活动、土壤有机质溶出、植物根系分泌作用以及施肥、耕作等人为干预活动,从而导致稻田上覆水中溶解性有机质、硝酸盐和亚硝酸盐等光敏物质(即能被光激发的成分)的浓度和结构与其他自然地表水存在着较大的差异。因此,稻田上覆水中的自由基过程要较普通地表水更剧烈,其对稻田水体中污染物的转化贡献更加显著。本文介绍了稻田上覆水中光活性组分的产生过程和机制,及其随着水稻生长过程的变化趋势;探讨了水质参数、太阳光照和农艺处理等因素对活性组分产生的影响机制;阐述了该过程对稻田中污染物非生物转化的作用,并进一步展望了上覆水光化学过程的研究方向。
1 光致活性组分的形成过程及其环境效应光化学反应是指物质受光照而引发的化学反应,其先决条件是吸收辐射,当基质吸收光辐射时,光子将其能量传递给吸收分子。从而使分子从基态转换为第一激发单重态,进而可能发生二次化学反应,或将激发能量传递至其他化合物,或以光(如磷光)、热(如热辐射)形式释放至环境中。在环境中,光化学反应主要发生在地表水体和大气中有机或无机基质的结构表面上。凡是在紫外-可见光区(200~800 nm)能够吸收辐射的物质,均可被太阳光照激发,从而发生一系列的光化学反应。
1.1 水体环境中光致活性组分的形成过程自然水体中的光敏物质主要包括溶解有机质(Dissolved Organic Matters,DOM)、硝酸盐(NO3–)、亚硝酸盐(NO2–)和三价铁离子(Fe3+)等[1]。其中,DOM在地表水中的含量约为1~20 mg·L–1(以C计),包含了从简单小分子有机酸(如柠檬酸、草酸等)到富里酸(Fulvic acid)、腐殖酸(Humic acid)等复杂大分子等一系列有机物,由于其来源的复杂性和多样性,主要分为水生生物的代谢活动、动植物残体的腐解、土壤有机质的释放、生活污水的排放和人类活动等造成的有机物质进入水体途径[6]。DOM中的生色团(Chromophoric Dissolved Organic Matters,CDOM)通常在200~500 nm具有较高的吸光度,极易被太阳辐射激发,产生激发单重态有机质(1CDOM*),1CDOM*主要以热辐射的形式释放其激发能量,也会以纳秒尺度的荧光形式释放[7]。此外,醌类和含羰基的芳香化合物被激发后形成的1CDOM*很容易通过系间窜跃(ISC)生成三重激发态有机质(3CDOM*),3CDOM*则可通过能量、电子和氢转移等途径降解水体中的有机污染物[8]。当水体中存在溶解氧(O2)时,3CDOM*将能量传递给氧气产生单线态氧(1O2),在氧气充足的水体中1O2的稳态浓度通常高达10–14~10–12 mol·L–1[9]。此外,3CDOM*经过电子转移后可产生带有正或负电的有机自由基(CDOM•+/•–),该自由基可与O2通过单电子传递反应,产生O2•–,O2•–进一步得到电子通过歧化反应,产生过氧化氢(H2O2)[10]。H2O2作为水体中一种常见的氧化剂,也可在光照情况下产生氧化性更强的•OH,由于•OH与各种化合物均具有较高的反应速率,因此其在水体中的迁移受到传质效应的限制,其稳态浓度通常仅有10–18~10–16 mol·L–1,但对污染物的转化和物质循环却具有重要的意义[11]。
除DOM以外,自然水体中的硝酸根(NO3–)、亚硝酸根(NO2–)、三价铁离子(Fe3+)和其他具有光活性的过渡金属离子也是•OH等活性组分的重要来源[3]。如NO3–和NO2–在吸收光子后,均能直接产生亚硝基自由基(•NO2/•NO)和•OH,亚硝酸盐的浓度通常较硝酸盐低两个数量级,但亚硝酸盐产生•OH的能力更强,在太阳光谱范围内,NO2–的光量子产率也可达0.065,而NO3–的光量子产率仅为0.01[3]。因此,在自然水体中,亚硝酸盐和硝酸盐均为•OH的重要来源。此外,三价铁离子(Fe3+)在光照下可吸收电子,被还原为二价铁离子(Fe2+),Fe2+进一步与水体中的痕量H2O2发生芬顿(Fenton)反应产生•OH[12]。在水体中的含铁颗粒物,例如Fe-有机质复合体,也可发生类Fenton反应产生•OH[13]。研究表明,在农业区浅层水体、河流交汇处和入海口,颗粒有机质对•OH的生成具有重要贡献[14]。
综上,自然水体中的这些光化学过程,可产生3CDOM*、1O2、H2O2、•OH等具有较强反应活性的物质,其对环境中污染物的转化和碳氮等元素的循环具有重要作用。
1.2 光致活性组分形成对污染物转化与碳氮元素循环的贡献自然水体中产生的3CDOM*、1O2和•OH等活性组分对环境中污染物的转化和碳氮元素的循环具有重要作用。表 1总结了3CDOM*、1O2和•OH与部分农药、药物和个人护理品类污染物的二级反应速率常数。可以看出,3CDOM*与上述污染物的二级反应速率常数大多数在107~1010 mol·L–1·s–1,3CDOM*与酚类、苯脲类除草剂和磺胺类抗生素的反应速率相对较快,达到109~1010 mol·L–1·s–1,其在自然水体中对这些污染物光转化贡献率也高达50%~90%[15-18]。3CDOM*被溶解氧淬灭后可产生1O2,其反应活性主要取决于污染物的类型和结构[19]。如表 1所示,1O2难以与苯脲类农药和头孢类药物发生反应,但其对氯酚类污染物的转化反应速率常数在104~108 mol·L–1·s–1 [20-21],1O2还能与一些易被氧化的氨基酸快速反应[22],因此,其对天然水体中有机氮转化作用不可忽视,如1O2诱导了核苷酸、尿素等溶解性有机氮的光氨化反应[23]。
|
|
表 1 污染物与各活性物质的反应速率汇总 Table 1 A summary of the reaction rates of contaminants with reactive intermediates |
除了3CDOM*和1O2,地表水中•OH的作用也不可忽视,•OH是地表水中反应活性最高的活性组分之一,可无选择性地与大多数有机污染物和DOM发生快速反应[3]。表 1中列出了•OH与常见农药、药品和个人护理品类污染物的反应速率,其二级反应速率常数均高于109 mol·L–1·s–1 [24],因此•OH对于污染物在环境中的转化具有很重要的贡献。此外,每1 mol •OH氧化有机质会产生约0.3 mol CO2[2]。除了直接矿化,•OH氧化使得大分子有机质转化为易被生物利用的小分子有机酸,间接地促进了水体中有机碳的转化与循环[25]。
2 稻田上覆水中光致活性组分的形成 2.1 稻田上覆水中的光敏物质稻田中有机质等光敏物质的含量和结构与天然地表水存在着较大的差异,且在大多数时期光敏物质的含量要高于一般的河湖地表水[4]。此外,河湖地表水中DOM主要是水体微生物的自生源性以及人为排放废水的外源输入性有机质,成分主要是类蛋白和类腐殖质物质,而稻田上覆水中DOM主要是富含大量芳香结构和生色团的类富里酸和类腐殖酸,具有显著的陆源性特征[36]。在水稻生长过程中,这些光敏物质的含量和结构也会发生显著变化。Zeng等[4]研究表明,育苗期稻田上覆水中硝酸盐、亚硝酸盐和DOM的含量要显著高于其他时期。亚硝酸盐浓度高的原因主要是基肥的施用,大量含氮的化肥未被有效利用,经反硝化作用后转化为亚硝态氮[37]。DOM累积主要归因于土壤有机质的溶出,在上一轮种植后稻田经历了长期的落干,而水稻种植初期的淹水使得土壤有机质中的可溶解部分大量溶出,此外田间残留的水稻根茎腐解也带来了大量DOM[38]。随着水稻生长,上覆水中DOM的浓度逐渐下降,主要归因于微生物对DOM的分解和上覆水表层DOM的光降解漂白作用。此外,水稻的根系分泌物、稻田中常用的土壤改良剂也对上覆水DOM具有显著影响,根系分泌物可迅速地被根际微生物利用,提高土壤微生物活性,加速土壤有机质的分解与溶出[39]。Liu等[13]研究表明,秸秆还田会显著增加稻田上覆水DOM富里酸组分的浓度,且当化肥和秸秆同时施用时,上覆水中DOM的腐殖化程度明显提高,这是由于添加的化肥提高了微生物的活性,进而促进了微生物对秸秆的分解作用。关连珠等[40]研究发现,稻壳生物质炭能够溶出大量的DOM,其主要成分为类富里酸和类腐殖酸,生物质炭的施用显著增加了稻田上覆水DOM的浓度。关于稻田土壤改良的研究发现,酸性土壤中生石灰的施用会通过调节土壤酸碱度,促进稻田土壤中胡敏酸的溶出,且溶出的有机质主要为单宁酸和木质素等物质,具有较高的吸光度和光化学活性[41]。综上,稻田上覆水含有丰富的光敏物质,具备光致产生活性组分的条件,但光致活性组分的产生过程与地表自然水体存在显著差异。
2.2 稻田上覆水中光致活性组分的产生机制Zeng等[4]的研究发现,稻田上覆水中的光敏物质在太阳光照时可产生大量的活性组分,包括三重激发态有机质3CDOM*、1O2和•OH。其产生机制如下式(1)~式(11)所示,DOM中的生色团在光照下产生了激发单重态有机质,再经过系间窜跃(ISC)形成3CDOM*。3CDOM*可被溶解氧淬灭,通过能量传递生成1O2。上覆水中的•OH主要来源于有机质和(亚)硝酸盐的光化学反应过程。上覆水中的DOM在光照下可发生直接电子转移,同时激发三重态也可经历该过程,产生带有正、负电子的有机质自由基,其进一步与溶解氧、质子等发生自由基链式反应,最终产生•OH。此外,上覆水中的(亚)硝酸盐也是•OH的重要来源,其在光照下可直接产生•OH。而Fe3+、Mn2+等具有光化学活性的过渡金属在光照下对•OH的贡献通常可以忽略,这主要是因为其在上覆水中的浓度一般低于0.18 nmol·L–1 [4]。
| $ {\text{CDOM}} + hv\xrightarrow{{}}{}_{}^1{\text{CDO}}{{\text{M}}^*} $ | (1) |
| $ {}_{}^1{\text{CDO}}{{\text{M}}^*}\xrightarrow{{ISC}}{}_{}^3{\text{CDO}}{{\text{M}}^*} $ | (2) |
| $ {}_{}^3{\text{CDO}}{{\text{M}}^*} + {{O} _2}\xrightarrow{{}}{}_{}^1{{\text{O}}_2} $ | (3) |
| $ {}_{}^3{\text{CDO}}{{\text{M}}^*}\xrightarrow{{}}{\text{CDO}}{{\text{M}}^{{\text{ + }} \cdot {\text{/}} - \cdot }} $ | (4) |
| $ {\text{CDOM + }}hv\xrightarrow{{}}{\text{CDO}}{{\text{M}}^{{\text{ + }} \cdot {\text{/}} - \cdot }} $ | (5) |
| $ {\text{CDO}}{{\text{M}}^{{\text{ + }} \cdot {\text{/}} - \cdot }}{\text{ + }}{O_2}\xrightarrow{{}}{\text{CDO}}{{\text{M}}^{{\text{ + }} \cdot {\text{/}} - \cdot }} + {\text{O}}_2^{ \cdot - } $ | (6) |
| $ {\text{2O}}_2^{ \cdot - }{\text{ + 2}}{H^ + }\xrightarrow{{}}{{\text{H}}_2}{{\text{O}}_2} + {{\text{O}}_{\text{2}}} $ | (7) |
| $ {{\text{H}}_2}{{\text{O}}_2} + hv\xrightarrow{{}}{\text{2}}{}_{}^ \cdot {\text{OH}} $ | (8) |
| $ {\text{NO}}_{\text{3}}^ - {\text{ + }}hv\xrightarrow{{}}{}_{}^ \cdot {\text{N}}{{\text{O}}_{\text{2}}} + {{\text{O}}^{ - \cdot }} $ | (9) |
| $ {\text{NO}}_{\text{2}}^ - {\text{ + }}hv\xrightarrow{{}}{}_{}^ \cdot {\text{NO}} + {{\text{O}}^{ - \cdot }} $ | (10) |
| $ {{\text{O}}^{ - \cdot }}{\text{ + }}{{\text{H}}^ + }\overset {} \longleftrightarrow {}_{}^ \cdot {\text{OH}} $ | (11) |
如图 1所示,稻田上覆水中3CDOM*和1O2的浓度分别为1.56×10–13~11.08×10–13和2.88×10–13~14.25×10–13 mol·L–1,较河湖等天然地表水体中高0.5~1个数量级。而•OH的稳态浓度为1.95×10–18~49.11×10–18 mol·L–1,与河湖等天然水产生的•OH浓度相当[41]。上述差异主要与稻田水中光敏物质的浓度和结构差异密切相关。3CDOM*和1O2主要来源于DOM,如2.1中所述,稻田上覆水中DOM的含量显著高于河湖等天然地表水,因此稻田上覆水中3CDOM*和1O2的产率也更高。Zeng等[4]关于有机质结构与活性物质光量子产率的进一步研究表明,DOM中富含碳基、羧基基团的小分子物质是产生光活性物质的主要前驱体,而稠环芳烃和多酚类等组分则抑制了活性组分的产生。在水稻生长过程中,育苗和分蘖期的活性组分产率较高,这与水稻生长初期上覆水中光敏物质的含量更高相一致。随着水稻生长,至孕穗期后上覆水活性组分产率大大降低,这与上覆水中光敏物质有机质减少和漂白过程密切相关。稻田上覆水中较高的自由基等活性组分水平,可能导致上覆水中污染物转化和强烈的物质循环过程。
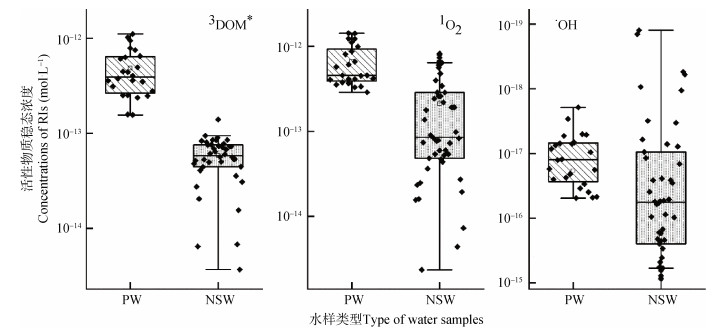
|
注:3CDOM*、1O2、•OH分别表示激发三重态溶解性有机质、单线态氧和羟基自由基。 Note: 3CDOM*, 1O2 and •OH represent triple excited organic matter, singlet oxygen and hydroxyl radicals, respectively. 图 1 稻田上覆水(PW)与河湖等天然地表水(NSW)中的光致活性组分稳态浓度对比 Fig. 1 Comparison of reactive intermediates concentration between paddy water(PW)and natural surface water(NSW) |
稻田上覆水中自由基等活性组分的产生主要来源于DOM、硝酸盐和亚硝酸盐等光敏物质。因此,光敏物质浓度或结构的变化,均会对上覆水中活性组分的形成产生较大影响。稻田耕作区的土壤类型决定了上覆水中陆源性有机质的组成,李飞跃等[42]研究表明,pH较低的南方稻田土壤中,DOM中的脂肪族、高度不饱和酚类化合物等活性成分含量更高;而在pH相对较高的稻田土壤中,多酚(来自植物)等组分含量较高[43],DOM组分的差异将影响上覆水中活性组分的形成。土壤中铁锰矿物的含量也对光敏物质的性质有重要影响,研究发现稻田DOM的化学多样性随着铁络合指数的增加而降低,金属矿物对有机质的络合作用选择性地捕获了富含羟基和羧基的化合物,抑制了土壤有机质的溶出[43]。因此,在富含铁矿物的稻田土壤上覆水中,光敏物质相对较少,活性组分产率也更低。
化肥和有机肥的施用也会对DOM、硝酸盐和亚硝酸盐等光敏物质有显著影响[5,44]。Li等[43]研究发现,频繁施用氮肥导致稻田土壤C/N比下降和土壤酸化,降低了稻田有机质多样性。此外,大量的无效氮加剧了生物反硝化过程,增加了上覆水中的亚硝酸盐和硝酸盐含量,能够促进•OH的产生[4]。不同有机肥添加对上覆水的光化学活性影响差异较大,并且秸秆还田措施增加了上覆水中DOM的浓度,其中木质素和类蛋白质成分的增加尤为显著,从而导致上覆水中自由基等活性组分浓度增加了16.8倍[38,41]。与之相反的是,生物质炭虽然会溶出部分有机质,但其并未对上覆水中的自由基产率有显著影响。这可能因为生物质炭对土壤养分起到保护作用,降低了土壤中碳氮元素向水体的迁移[45]。
除光敏物质外,太阳辐射也直接影响上覆水中自由基的光产率,辐射强度与自由基的浓度呈现出线性正相关。Liu等[13]进行的稻田原位自由基捕获实验表明,正午时分自由基的稳态浓度最高,并向日出和日落两侧递减,这主要与太阳高度角和方位角的日变化有关,正午时分到达地面的垂直太阳辐射强度最大,因此原位测得的自由基浓度也越高。此外,南北方稻田的光照强度也存在巨大差异,Wu等[46]研究表明,春分日时赤道上溶解性有机质的自由基光产量约为北纬50度地区(相当于中国的黑龙江地区)的2倍,因为高纬度地区太阳高度角更低,到达地面的垂直太阳辐射强度更低。因此,相比于南方地区,东北地区稻田上覆水中的光敏物质更多,但受限于太阳辐射强度的限制,其原位自由基产率可能要低于南方地区。
2.4 上覆水光致活性组分对污染物非生物转化贡献稻田生态系统面临着多种污染风险,例如受污染的灌溉水、农药的过度使用和部分耕作地区土壤重金属本底值超标。上覆水中产生了大量具有高化学反应活性的活性组分,有利于砷和多种有机污染物的快速转化。Liu等[38]研究表明,稻田上覆水产生的•OH、1O2和H2O2加速了三价砷的氧化,使其转化为毒性更低、迁移性更差的五价砷,降低了砷的生态风险。Carena等[29]研究了稻田常用除草剂敌稗在上覆水中的光转化,发现•OH及其衍生自由基CO3•–在敌稗的光转化中起了重要作用,敌稗与•OH的反应速率高达7.0×109 mol·L–1,而3CDOM*对稻田水中灭草松的光转化具有重要贡献,反应产物主要为羟基化产物[3]。研究表明,3CDOM*和•OH是稻田上覆水中咪唑乙烟酸间接光转化的主要驱动力,其能够将咪唑乙烟酸降解为毒性降低的小分子有机物[33]。Xia等[47]研究了上覆水中的富里酸对五种多环芳烃(苊、氟、菲、氟蒽和芘)光降解的影响,发现低浓度富里酸显著加速了多环芳烃的降解,其中•OH对多环芳烃的降解起主导作用,贡献约为33%~69%;而当富里酸浓度较高时,由于其光屏蔽效应,抑制了多环芳烃的光降解速率。Tian等[48]发现稻田中藻源性有机质也具有较高的光化学活性,其产生的3CDOM*加速了盐酸四环素的光降解。Zeng等[41]探究了除草剂水解产物二氯苯酚在稻田上覆水中的光转化,发现自由基过程显著加速了二氯苯酚的降解,3CDOM*和•OH在其降解中的贡献分别约占72%和28%,值得注意的是,•OH诱导的加羟基过程降低了产物毒性,而3CDOM*诱导的二聚化产物毒性相比于母体有所提升,存在更高的生态风险。综上所述,稻田上覆水光致活性组分对有机污染物的转化具有重要贡献,其不仅影响污染物的降解速率,而且对污染物降解中间产物、途径及其潜在的毒性具有重要影响。
3 研究展望稻田上覆水的光学过程中产生了3CDOM*、1O2和•OH等活性组分,其对污染物转化和物质循环有重要作用,但是该反应过程在过去研究中并未受到充分重视。直至近两年,部分研究团队开始了关于稻田自由基等活性组分的研究,涉及了稻田上覆水自由基的产生机制及其在水稻生长周期内的变化、不同土壤改良措施等对自由基产生的影响。并系统探讨了光照上覆水产自由基过程对稻田砷、二氯苯酚等农药水解产物和其他有机污染物的转化。但目前相关研究并不系统,并未形成对上覆水自由基过程的系统性认识,后续研究应围绕上覆水活性组分的通量、时空分布规律等,聚焦全球尺度下稻田光致活性组分形成过程、不同土壤类型和改良措施等对活性组分的影响,活性组分对污染物转化、碳氮物质循环的影响及其内在机制等,达成稻田上覆水活性组分形成及环境效应理论的系统认知。此外,当前研究仅关注了部分污染物(如砷和农药)在稻田中的降解转化,后续研究应不仅仅关注这些污染物本身的降解,更应关注污染物降解产物的毒性及其生态风险。还应关注和加强稻田光致活性组分对一些新兴污染物如新型农药、抗生素、抗性基因和微塑料等在稻田系统中的转化过程研究。此外,关于活性组分对稻田有机碳、氮等元素转化贡献与机制方面尚缺乏研究,应该进一步关注活性组分对稻田甲烷、二氧化碳和氮氧化物等温室气体排放的影响,为实现农田生态系统的固碳减排提供支撑。
| [1] |
Vione D. A critical view of the application of the APEX software(aqueous photochemistry of environmentally-occurring xenobiotics)to predict photoreaction kinetics in surface freshwaters[J]. Molecules, 2019, 25(1): 9. DOI:10.3390/molecules25010009
(  0) 0) |
| [2] |
Goldstone J V, Pullin M J, Bertilsson S, et al. Reactions of hydroxyl radical with humic substances: Bleaching, mineralization, and production of bioavailable carbon substrates[J]. Environmental Science & Technology, 2002, 36(3): 364-372.
(  0) 0) |
| [3] |
Carena L, Fabbri D, Passananti M, et al. The role of direct photolysis in the photodegradation of the herbicide bentazone in natural surface waters[J]. Chemosphere, 2020, 246: 125705. DOI:10.1016/j.chemosphere.2019.125705
(  0) 0) |
| [4] |
Zeng Y, Fang G D, Fu Q L, et al. Photochemical characterization of paddy water during rice cultivation: Formation of reactive intermediates for As(III)oxidation[J]. Water Research, 2021, 206: 117721. DOI:10.1016/j.watres.2021.117721
(  0) 0) |
| [5] |
Zhong S X, He H F, Chen Z L, et al. Advancement in study on biochemical behavior of arsenic in flooded paddy soil (In Chinese)[J]. Acta Pedologica Sinica, 2018, 55(1): 1-17. [钟松雄, 何宏飞, 陈志良, 等. 水淹条件下水稻土中砷的生物化学行为研究进展[J]. 土壤学报, 2018, 55(1): 1-17.]
(  0) 0) |
| [6] |
Fu Q L, Fujii M, Ma R. Development of a gaussian-based alignment algorithm for the ultrahigh-resolution mass spectra of dissolved organic matter[J]. Analytical Chemistry, 2023, 95(5): 2796-2803. DOI:10.1021/acs.analchem.2c04113
(  0) 0) |
| [7] |
Wasswa J, Driscoll C T, Zeng T. Photochemical characterization of surface waters from lakes in the adirondack region of New York[J]. Environmental Science & Technology, 2020, 54(17): 10654-10667.
(  0) 0) |
| [8] |
Wang H, Han M Q, Wang M, et al. Microheterogeneous triplet oxidation of hydrophobic organic contaminants in dissolved black carbon solutions under simulated solar irradiation[J]. Environmental Science & Technology, 2022, 56(20): 14574-14584.
(  0) 0) |
| [9] |
Wasswa J, Driscoll C T, Zeng T. Contrasting impacts of photochemical and microbial processing on the photoreactivity of dissolved organic matter in an adirondack lake watershed[J]. Environmental Science & Technology, 2022, 56(3): 1688-1701.
(  0) 0) |
| [10] |
Sha H T, Yan S W, Deng Y, et al. Photosensitized transformation of hydrogen peroxide in dissolved organic matter solutions under simulated solar irradiation[J]. Environmental Science & Technology, 2022, 56(19): 14080-14090.
(  0) 0) |
| [11] |
Yan S W, Sun J Q, Sha H T, et al. Microheterogeneous distribution of hydroxyl radicals in illuminated dissolved organic matter solutions[J]. Environmental Science & Technology, 2021, 55(15): 10524-10533.
(  0) 0) |
| [12] |
Wang Z, Lv J T, Zhang S H, et al. Interfacial molecular fractionation on ferrihydrite reduces the photochemical reactivity of dissolved organic matter[J]. Environmental Science & Technology, 2021, 55(3): 1769-1778.
(  0) 0) |
| [13] |
Liu S C, Tan M X, Huang D Y, et al. Dynamic changes of reactive oxygen species in paddy overlying water: Mechanisms and implications[J]. Journal of Soils and Sediments, 2022, 22(6): 1746-1760. DOI:10.1007/s11368-022-03195-y
(  0) 0) |
| [14] |
Appiani E, Mcneill K. Photochemical production of singlet oxygen from particulate organic matter[J]. Environmental Science & Technology, 2015, 49(6): 3514-3522.
(  0) 0) |
| [15] |
Fabbri D, Minella M, Maurino V, et al. Photochemical transformation of phenylurea herbicides in surface waters: A model assessment of persistence, and implications for the possible generation of hazardous intermediates[J]. Chemosphere, 2015, 119: 601-607. DOI:10.1016/j.chemosphere.2014.07.034
(  0) 0) |
| [16] |
de Laurentiis E, Prasse C, Ternes T A, et al. Assessing the photochemical transformation pathways of acetaminophen relevant to surface waters: Transformation kinetics, intermediates, and modelling[J]. Water Research, 2014, 53: 235-48. DOI:10.1016/j.watres.2014.01.016
(  0) 0) |
| [17] |
Vione D, Fabbri D, Minella M, et al. Effects of the antioxidant moieties of dissolved organic matter on triplet-sensitized phototransformation processes: Implications for the photochemical modeling of sulfadiazine[J]. Water Research, 2018, 128: 38-48. DOI:10.1016/j.watres.2017.10.020
(  0) 0) |
| [18] |
Bianco A, Fabbri D, Minella M, et al. New insights into the environmental photochemistry of 5-chloro-2-(2, 4-dichlorophenoxy)phenol(triclosan): Reconsidering the importance of indirect photoreactions[J]. Water Research, 2015, 72: 271-80. DOI:10.1016/j.watres.2014.07.036
(  0) 0) |
| [19] |
Minella M, Giannakis S, Mazzavillani A, et al. Phototransformation of acesulfame K in surface waters: Comparison of two techniques for the measurement of the second-order rate constants of indirect photodegradation, and modelling of photoreaction kinetics[J]. Chemosphere, 2017, 186: 185-192. DOI:10.1016/j.chemosphere.2017.07.128
(  0) 0) |
| [20] |
Escalada J P, Pajares A, Gianotti J, et al. Photosensitized degradation in water of the phenolic pesticides bromoxynil and dichlorophen in the presence of riboflavin, as a model of their natural photodecomposition in the environment[J]. Journal of Hazardous Materials, 2011, 186(1): 466-472. DOI:10.1016/j.jhazmat.2010.11.026
(  0) 0) |
| [21] |
Zhou M Z, Cang L. Progress of chemical oxidation mechanism of peroxymonosulfate and its application in remediation of organic contaminated soil (In Chinese)[J]. Soils, 2022, 54(4): 653-666. [周明珠, 仓龙. 过一硫酸盐的化学氧化机理及在有机污染土壤修复中应用研究进展[J]. 土壤, 2022, 54(4): 653-666.]
(  0) 0) |
| [22] |
Remucal C K, McNeill K. Photosensitized amino acid degradation in the presence of riboflavin and its derivatives[J]. Environmental Science & Technology, 2011, 45(12): 5230-7.
(  0) 0) |
| [23] |
Zhang Y T, Zhang R C, Li S L, et al. Photo-ammonification of low molecular weight dissolved organic nitrogen by direct and indirect photolysis[J]. Science of the Total Environment, 2021, 764: 142930. DOI:10.1016/j.scitotenv.2020.142930
(  0) 0) |
| [24] |
Wang X L, Wu G H, Fang G D, et al. Transition metal activated persulfate for environmental remediation: A review (In Chinese)[J]. Journal of Ecology and Rural Environment, 2021, 37(2): 145-154. [王肖磊, 吴根华, 方国东, 等. 过渡金属活化过硫酸盐在环境修复领域的研究进展[J]. 生态与农村环境学报, 2021, 37(2): 145-154.]
(  0) 0) |
| [25] |
Ward C P, Cory R M. Complete and partial photooxidation of dissolved organic matter draining permafrost soils[J]. Environmental Science & Technology, 2016, 50(7): 3545-53.
(  0) 0) |
| [26] |
Marchetti G, Minella M, Maurino V, et al. Photochemical transformation of atrazine and formation of photointermediates under conditions relevant to sunlit surface waters: laboratory measures and modelling[J]. Water Research, 2013, 47(16): 6211-22. DOI:10.1016/j.watres.2013.07.038
(  0) 0) |
| [27] |
Avetta P, Marchetti G, Minella M, et al. Phototransformation pathways of the fungicide dimethomorph((E, Z)4-[3-(4-chlorophenyl)-3-(3, 4-dimethoxyphenyl)-1-oxo-2-propenyl]morpholine), relevant to sunlit surface waters[J]. Science of the Total Environment, 2014, 500/501: 351-360. DOI:10.1016/j.scitotenv.2014.08.067
(  0) 0) |
| [28] |
Carena L, Vione D. Modelling the photochemistry of imazethapyr in rice paddy water[J]. Science of the Total Environment, 2018, 644: 1391-1398. DOI:10.1016/j.scitotenv.2018.06.324
(  0) 0) |
| [29] |
Carena L, Minella M, Barsotti F, et al. Phototransformation of the herbicide propanil in paddy field water[J]. Environmental Science & Technology, 2017, 51(5): 2695-2704.
(  0) 0) |
| [30] |
Fabbri D, Minella M, Maurino V, et al. A model assessment of the importance of direct photolysis in the photo-fate of cephalosporins in surface waters: Possible formation of toxic intermediates[J]. Chemosphere, 2015, 134: 452-8. DOI:10.1016/j.chemosphere.2015.04.102
(  0) 0) |
| [31] |
de Laurentiis E, Chiron S, Kouras-Hadef S, et al. Photochemical fate of carbamazepine in surface freshwaters: Laboratory measures and modeling[J]. Environmental Science & Technology, 2012, 46(15): 8164-73.
(  0) 0) |
| [32] |
Avetta P, Fabbri D, Minella M, et al. Assessing the phototransformation of diclofenac, clofibric acid and naproxen in surface waters: Model predictions and comparison with field data[J]. Water Research, 2016, 105: 383-394. DOI:10.1016/j.watres.2016.08.058
(  0) 0) |
| [33] |
Fabbri D, Maurino V, Minella M, et al. Modelling the photochemical attenuation pathways of the fibrate drug gemfibrozil in surface waters[J]. Chemosphere, 2017, 170: 124-133. DOI:10.1016/j.chemosphere.2016.11.135
(  0) 0) |
| [34] |
Vione D, Maddigapu P R, de Laurentiis E, et al. Modelling the photochemical fate of ibuprofen in surface waters[J]. Water Research, 2011, 45(20): 6725-6736. DOI:10.1016/j.watres.2011.10.014
(  0) 0) |
| [35] |
Gornik T, Vozic A, Heath E, et al. Determination and photodegradation of sertraline residues in aqueous environment[J]. Environmental Pollution, 2020, 256: 113431. DOI:10.1016/j.envpol.2019.113431
(  0) 0) |
| [36] |
Song X N, Yu T, Zhang Y, et al. Distribution characterization and source analysis of dissolved organic matters in Taihu Lake using three dimensional fluorescence excitation-emission matrix (In Chinese)[J]. Acta Scientiae Circumstantiae, 2010, 30(11): 2321-2331. [宋晓娜, 于涛, 张远, 等. 利用三维荧光技术分析太湖水体溶解性有机质的分布特征及来源[J]. 环境科学学报, 2010, 30(11): 2321-2331.]
(  0) 0) |
| [37] |
Yoshinaga I, Miura A, Hitomi T, et al. Runoff nitrogen from a large sized paddy field during a crop period[J]. Agricultural Water Management, 2007, 87(2): 217-222. DOI:10.1016/j.agwat.2006.06.020
(  0) 0) |
| [38] |
Liu S C, Tan M X, Ge L Q, et al. Photooxidation mechanism of As(III)by straw-derived dissolved organic matter[J]. Science of the Total Environment, 2021, 757: 144049. DOI:10.1016/j.scitotenv.2020.144049
(  0) 0) |
| [39] |
Xiong L, Liu X Y, Vinci G, et al. Molecular changes of soil organic matter induced by root exudates in a rice paddy under CO2 enrichment and warming of canopy air[J]. Soil Biology and Biochemistry, 2019, 137: 107544. DOI:10.1016/j.soilbio.2019.107544
(  0) 0) |
| [40] |
Guan L Z, Jiang X N, Zhang G C, et al. Effects of rice straw-derived biochar on organic carbon activity in coastal saline paddy soil (In Chinese)[J]. Soils, 2019, 51(1): 205-209. [关连珠, 姜雪楠, 张广才, 等. 添加稻草生物质炭对滨海水稻土有机质活性的影响[J]. 土壤, 2019, 51(1): 205-209.]
(  0) 0) |
| [41] |
Zeng Y, Fang G D, Fu Q L, et al. Mechanistic study of the effects of agricultural amendments on photochemical processes in paddy water during rice growth[J]. Environmental Science & Technology, 2022, 56(7): 4221-4230.
(  0) 0) |
| [42] |
Li F Y, Gui X Y, Xu J H, et al. Spectral analysis of dissolved organic matter from biochar (In Chinese)[J]. Spectroscopy and Spectral Analysis, 2019, 39(11): 3475-3481. [李飞跃, 桂向阳, 许吉宏, 等. 生物炭中溶解性有机质的光谱分析[J]. 光谱学与光谱分析, 2019, 39(11): 3475-3481.]
(  0) 0) |
| [43] |
Li X M, Sun G X, Chen S C, et al. Molecular chemodiversity of dissolved organic matter in paddy soils[J]. Environmental Science & Technology, 2018, 52(3): 963-971.
(  0) 0) |
| [44] |
Liao C L, Li L N, Xie L H, et al. Effect of increased or decreased application of organic manure on aggregates stability and soil cement in red paddy soil (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(4): 978-988. [廖超林, 黎丽娜, 谢丽华, 等. 增减施有机肥对红壤性水稻土团聚体稳定性及胶结物的影响[J]. 土壤学报, 2021, 58(4): 978-988.]
(  0) 0) |
| [45] |
Zheng X, Li X, Singh B P, et al. Biochar protects hydrophilic dissolved organic matter against mineralization and enhances its microbial carbon use efficiency[J]. Science of the Total Environment, 2021, 795: 148793. DOI:10.1016/j.scitotenv.2021.148793
(  0) 0) |
| [46] |
Wu B B, Liu T, Wang Y L, et al. High sample throughput LED reactor for facile characterization of the quantum yield spectrum of photochemically produced reactive intermediates[J]. Environmental Science & Technology, 2021, 55(23): 16204-16214.
(  0) 0) |
| [47] |
Xia X, Li G, Yang Z, et al. Effects of fulvic acid concentration and origin on photodegradation of polycyclic aromatic hydrocarbons in aqueous solution: Importance of active oxygen[J]. Environmental Pollution, 2009, 157(4): 1352-1359. DOI:10.1016/j.envpol.2008.11.039
(  0) 0) |
| [48] |
Tian Y J, Wei L X, Yin Z, et al. Photosensitization mechanism of algogenic extracellular organic matters(EOMs)in the photo-transformation of chlortetracycline: Role of chemical constituents and structure[J]. Water Research, 2019, 164: 114940. DOI:10.1016/j.watres.2019.114940
(  0) 0) |
 2024, Vol. 61
2024, Vol. 61


