2. 中国科学院亚热带农业生态研究所亚热带农业生态过程重点实验室, 长沙 410125;
3. 广西自治区重点实验室, 中国科学院环江喀斯特生态系统观测研究站, 广西环江 547100
2. Key Laboratory of Agro-Ecological Processes in Subtropical Region, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, China;
3. Guangxi Key Laboratory of Karst Ecological Processes and Services, Huanjiang Observation and Research Station for Karst Ecosystems, Chinese Academy of Science, Huanjiang 547100, Guangxi, China
土壤是陆地生态系统最大的碳库,土壤碳库的微量变化均会引起大气中二氧化碳(CO2)浓度的剧烈变化[1]。尽管土壤微生物量仅占土壤有机质库的1%~3%,但微生物通过氧化与还原过程和生长与死亡过程显著影响土壤有机碳(SOC)的形成与稳定性[2-3]。微生物群落的生理代谢特征,如生长速率、呼吸速率、微生物生物量碳利用效率(CUE)和周转时间对SOC的形成与稳定起至关重要的作用[4-5]。土壤底物质量和数量、微生物生物量和群落组成、酶活性以及pH均会显著影响土壤微生物生物量碳代谢特征[6-8]。通常而言,细菌偏好高质量的底物,而真菌偏好低质量的底物,因此,底物质量通过改变微生物群落组成而进一步影响微生物生物量碳代谢特征[9]。如在土壤有机质含量丰富的土壤中,微生物主要以真菌为主,由于真菌生物量的C:N较大,因此其CUE大于以细菌为主的土壤[10]。但也有研究认为,较高的土壤pH和氮素有效性会促进细菌生长,从而提高CUE[11]。此外,土壤质地和金属矿物也会影响底物可利用性以及酶或微生物活性,进而影响微生物生物量碳代谢特征[12-14]。高黏粒或铁铝氧化物含量有利于保护土壤有机质免受微生物分解,从而降低微生物生长速率和CUE [13,15-16]。但也有研究认为,黏粒含量不影响CUE,但却显著影响微生物周转速率[17]。这些不同的结果表明,目前对微生物生物量碳代谢特征的驱动因子尚未有统一结论,需要进一步深入探讨。
我国喀斯特地区横跨中国西南部的8个省份,是世界上最大的喀斯特地区之一。贵州省位于西南喀斯特的中心地带,喀斯特地区占据全省面积的62%[18]。其中以石灰岩为主的喀斯特土壤具有土壤pH和钙、镁含量相对较高的特点,而以碎屑岩为主的非喀斯特土壤则具有土层较厚、土壤pH呈酸性、土壤铁铝含量高等特点[19]。岩性会在很大程度上影响土壤的理化性质[20]、植被生长[21]以及微生物群落、组成和功能的差异[22]。不同岩性土壤中铁铝氧化及交换性钙(Ca)、镁(Mg)含量也会影响微生物生物量碳代谢,相对于铁铝氧化物,较高的交换性Ca、Mg含量会促进微生物代谢熵[23]。不同岩性会导致相关微生物的组成和多样性的差异,也会显著影响微生物生物量碳代谢的差异[24-25]。然而,石灰岩发育的土壤和碎屑岩发育的土壤微生物生物量碳代谢特征的差异及其原因如何?目前仍尚不明确。
本研究假设石灰岩发育的土壤高pH会促进微生物生长和提高CUE,而碎屑岩发育的土壤较高的铁铝含量会限制底物的可利用性进而抑制微生物生长和降低CUE。基于此,本研究选取以石灰岩发育的喀斯特森林土壤和碎屑岩发育的非喀斯特森林土壤,分析土壤基础理化性质、有机质矿物保护特性、酶活性和微生物群落组成,明确不同岩性下土壤微生物生物量碳代谢特征的差异及其原因,阐明不同岩性对土壤微生物生物量碳代谢的调控机制。研究结果为深入理解不同母岩发育的森林土壤有机碳库的差异提供理论依据。
1 材料与方法 1.1 研究区概况研究区位于贵州省绥阳县宽阔水保护区的林区(28°14′24″N,107°11′24″E)。该区的平均海拔为1 300~1 350 m,为典型的亚热带湿润季风气候,年平均降水量为1 160 mm,年平均气温为14℃。该地区以众多的喀斯特地质构造为特征,喀斯特地区母岩以石灰岩为主,同一地区非喀斯特地区母岩以碎屑岩为主。森林植被为典型亚热带常绿落叶阔叶混交林,根据联合国粮农组织/联合国教科文组织的分类体系,石灰岩之上为钙质岩石土(钙质土壤),碎屑岩之上为铁铝土(红壤)。
1.2 土壤样品采集2018年9月,在两种母岩发育的森林土壤区域分别选择了5个样地,每个样地面积为20 m × 20 m,每个样地植被覆盖度相似、林木年龄相近(60 ±5年);每个样地采用“网格”型布设10个采样点,采集0~15 cm表层土壤,10个采样点均匀混合1个土壤样本(去除可见的有机残体),两种母岩发育土壤分别采集5个土壤样本(作为5次复重),共计10个土壤样本。随后将采集的土壤运回实验室处理,新鲜的土壤被筛分(2 mm)并均质化,去除细根和其他植物碎片。将土壤样品分为三部分,其中一部分新鲜土壤样品立即放入4℃冰箱中,用于土壤微生物生物量碳(MBC)、微生物生物量氮(MBN)、微生物生物量磷(MBP)和酶活性分析;另一部分放入–20℃冰箱中,用于磷脂脂肪酸(PLFA)提取以及微生物代谢指标的测定;其余部分进行风干,用于测定其他理化性质,如pH、有机碳(SOC)、全氮(TN)、全磷(TP)、有机质分组、土壤质地、金属铁铝氧化物和交换性Ca、Mg等。
1.3 测定项目与方法(1) 土壤基本理化性质的测定。土壤pH采用电位法测定(土/水为1︰2.5 w/v)(FE28-Standard,瑞士);SOC采用重铬酸钾容量法测定;TN采用元素分析仪(Elementar wario MAX cube,德国)测定;TP采用H2SO4 + HClO4溶液消化钒钼黄比色法测定;土壤可溶性有机碳(DOC)采用0.5 mol·L–1 K2SO4溶液浸提,湿氧化法-有机碳分析仪(TOC-Vwp,德国)测定;土壤粒径采用激光粒度仪(Mastersizer 2000,英国)测定;MBC、MBN采用氯仿熏蒸–0.5 mol·L–1 K2SO4溶液浸提,湿氧化法–有机碳分析仪(TOC-Vwp,德国)测定,MBP采用氯仿熏蒸–0.5 mol·L–1 NaHCO3浸提,AA3(AatoAnalyzer,德国)流动分析测定,其含量等于熏蒸与未熏蒸样本的差值,再分别除以转化系数,其中,MBC、MBN和MBP的转化系数分别为0.45、0.45和0.40。
(2) 土壤有机质矿物保护指标的测定。利用铁(Fe)、铝(Al)氧化物和交换性Ca、Mg含量来表征有机质矿物保护特性[26]。采用柠檬酸–碳酸氢钠–二亚硫酸钠测定游离态Fe、Al氧化物((Fe+Al)d),采用草酸萃取法测定非晶态Fe、Al氧化物((Fe+Al)o);用乙酸铵提取可交换性Ca、Mg,浸提后的溶液均使用电感耦合等离子发射光谱仪测定(ICP-OES,美国)。
(3) 土壤有机质物理分组及金属结合态有机碳测定。用0.5%的六偏磷酸钠作为分散剂,用恒温振荡器振荡18 h,震荡后将土壤悬浊液立即过53 μm筛,筛分得到 < 53 μm矿物结合态有机质[27],60℃烘干研磨过100目筛后,用0.5 mol·L–1 Na2SO4浸提,后用总有机碳分析仪(TOC-Vwp,日本)测定DOC浓度,得到的含量为Ca、Mg结合态有机碳(OC-Ca/ Mg);残余土壤烘干研磨过100目筛后,一式两份,一份加柠檬酸–碳酸氢钠–二亚硫酸钠,另一份加碳酸氢钠–氯化钠,均采用1.0 mol·L–1氯化钠溶液冲洗,然后在60℃的烘箱中干燥以进行有机碳分析,差值即为铁铝结合态有机碳(OC-Fe/Al)[28]。
(4) 土壤酶活性的测定。参照Saiya-Cork等[29]的方法测定β-葡萄糖苷酶(BG)、β-木糖苷酶(BX)、α-葡萄糖苷酶(AG)、纤维生物糖甘酶(CBH)、β-乙酰葡糖胺糖苷酶(NAG)、亮氨酸氨基肽酶(LAP)、酸性磷酸酶(AP)以及两种氧化酶:酚氧化酶(PPO)和过氧化氢酶(PER)活性。具体方法如下:取1 g新鲜土壤,加入125 mL的三水合乙酸钠缓冲液(50 mmol·L–1),用磁力搅拌器连续搅拌10 min使其均质化,后用移液器取200 μL移于96孔黑色微孔板。用4-甲基羟基香豆素(MUB)作为标准物测定水解酶活性(LAP采用7-氨基-4-甲基香豆素(AMC)为标准物),不同胞外酶加入对应的底物,微平板置于黑暗环境下经过25 ℃恒温4 h培养后,加入10 μL 1 mol·L–1 NaOH终止反应,用多功能酶标仪(Infinite M200 PRO,瑞士)在365 nm和450 nm激发波长测定水解酶的荧光度。PPO和PER测定用DOPA和0.3% H2O2做底物,置于20 ℃黑暗条件下分别培养24 h和18 h,酶活性用微孔板分光光度计测定460 nm处吸光值。酶活性表示为nmol·g–1·h–1。
(5) 土壤磷脂脂肪酸的提取。土壤微生物生物量采用磷脂脂肪酸(PLFA)方法测定,根据调整后的Bligh-Dyer[30]方法提取。首先称取2~3 g冷冻干燥后的土于50 mL玻璃离心管中,按顺序加入柠檬酸缓冲液、氯仿和甲醇(体积比0.8︰1︰2),震荡离心后收集下层氯仿相,依次加入氯仿、丙酮和甲醇,溶液通过SPE柱萃取获得磷脂,最后依次定量加入甲醇甲苯混合液(体积比1︰1),氢氧化钾–甲醇溶液(0.2 mol·L–1)、正己烷氯仿混合液(体积比4︰1)、醋酸溶液(0.3 mol·L–1)和去离子水,收集上层溶液,氮气吹干后溶于正己烷,转移至棕色进样瓶中,采用稳定同位素质谱仪(Delta V Adwantage,Themo Fisher,美国)。i15︰0、a15:0、i16︰0、i17︰0指示革兰氏阳性菌(G+);C16︰1ω7c、C18︰1ω7c指示革兰氏阴性菌(G–);16︰0-10Me指示放线菌;指示真菌(F)的脂肪酸标记物为C18︰2ω6c、C18︰1ω9c;革兰氏阳性菌、阴性菌和放线菌的总和为细菌(B)[31]。用F︰B以及G+︰G–表征微生物群落组成。
(6) 土壤微生物生物量碳代谢特征的测定。将0.4 g鲜土经预培养48 h之后加入2 mL螺帽冻存管中。用移液枪在其中一组加入97 atom% 18O标记水,使最终土壤水中的18O丰度达到20%,并保持60%田间持水量;另一组加入同样体积的未标记超纯水,作为自然丰度。将2 mL冻存管中的土壤样品在涡旋仪上轻轻混匀,然后打开螺帽,并将其立即转移至50 mL培养瓶中密封,在25 ℃条件下培养24 h。在结束之后,抽取30 mL气体,注入已经用氦气清洗过的12 mL顶空瓶中,用气相色谱仪GC-6890(7890 A,Agilent Technologies,美国)测定CO2浓度。然后取出2 mL冻存管、盖上螺帽,保存于–80 ℃用于DNA提取(DNeasy® PowerSoil® Pro Kit)。使用微孔板分光光度计(®M200,奥地利Tecan)定量DNA浓度。将30 μL DNA加入到银胶囊中,然后在60℃的烘箱中干燥过夜以除去任何水。随后使用通过稳定同位素比质谱仪(MAT253,Thermo Fisher,美国)偶联的高温元素分析仪(HT-EA Thermo Fisher,美国)测量18O丰度和总O含量[32]。
微生物生长速率(Cgrowth,μg·g–1·d–1,以C计)表示为:
| $ {C}_{\text{growth}}= \frac{{f}_{\text{DNA}}\times {\text{DNA}}_{\text{produced}}\times 1000}{DW\times t} $ | (1) |
式中,DW为土壤干质量(g);t为培养时间(h);fDNA为转换因子,即微生物生物量碳(mg·kg–1)与DNA浓度的比值(μg·g–1)。
微生物呼吸速率(Crespiration,μg·g–1·d–1,以C计)表示为:
| $ {C_{{\text{respiration}}}} = \frac{{{D_{{\text{C}}{{\text{O}}_2}}}}}{{DW \times t}} \times \frac{{p \times n}}{{R \times T}} \times {V_{hs}} \times 1\;000 $ | (2) |
式中,p为大气压力(kPa);n为C元素的分子质量(12.01 g·mol–1);R为理想气体常数(8.314 J· mol–1·K–1);T为气体的绝对温度(295.15 K);Vhs为培养瓶的体积(L);
微生物生物量碳利用效率(CUE)表示为:
| $ {\text{CUE}} = \frac{{{C_{{\text{growth}}}}}}{{{C_{{\text{growth}}}} + {C_{{\text{respiration}}}}}} $ | (3) |
式中,各项物理意义同前。
微生物量生物量碳周转时间(Tturnover,d)表示为:
| $ T_{\text {turnover }}=\frac{\mathrm{MBC}}{C_{\text {growth }}} $ | (4) |
式中,Cgrowth同式(1);MBC为微生物生物量碳(mg·kg–1,以C计)。
1.4 数理统计方法SPSS 25.0进行独立样本T检验比较两种母岩发育土壤的理化性质、矿物保护性质、酶活性、微生物生物量和群落组成以及微生物生物量碳代谢之间的差异(P < 0.05)。采用相关性分析(Correlation analysis)明确土壤基础理化性质、矿物保护、酶活性、微生物生物量和群落组成对微生物呼吸、生长和CUE的影响,并用Origin 2019图表进行绘制。用R4.2.2的“corrplot”包进行相关性分析并绘制热图。
2 结果 2.1 土壤基础理化特征由表 1可以看出,两种母岩发育土壤pH、SOC、TN和DOC存在显著差异,其中,碎屑岩发育土壤pH较石灰屑岩发育土壤显著降低31.3%,但SOC、TN和DOC却分别较石灰岩发育土壤显著增加60.8%、41.5%和344.9%。两种母岩发育土壤TP含量差异并不显著。碎屑岩发育土壤的C︰P和N︰P较石灰岩发育土壤显著提高42.6%和26.3%,而C︰N差异并不显著。石灰岩发育土壤的0.05~0.002mm粒径含量显著高于碎屑岩发育土壤,而 < 0.002 mm粒径和 > 0.05 mm粒径含量却相反。
|
|
表 1 试验土壤基本理化性质 Table 1 Basic properties of the tested soils |
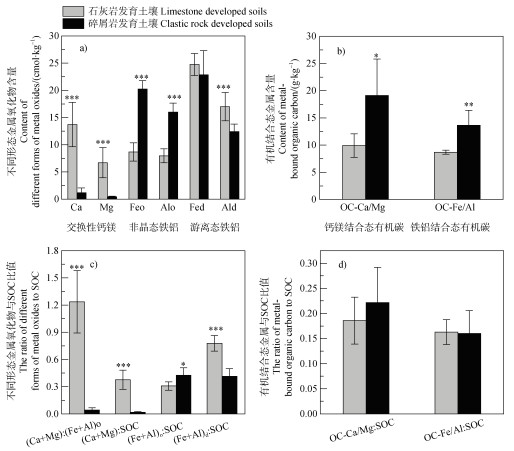
碎屑岩发育土壤交换性Ca、Mg以及游离态Al(Al)d含量较石灰岩发育土壤显著降低91.3%、93.8%和26.9%,但非晶态铁(Fe)o、非晶态铝(Al)o较石灰岩发育土壤显著升高了133.3%和101.1%(图 1)。碎屑岩发育土壤的交换性Ca、Mg(Ca+Mg)与(Fe+Al)o、交换性Ca、Mg(Ca+Mg)与SOC和(Fe+Al)d与SOC的比值分别较石灰岩发育土壤显著降低96.5%、95.2%和46.9%(图 1),碎屑岩发育土壤的(Fe+Al)o与SOC的比值、OC-Ca/Mg和OC-Fe/Al却较石灰岩发育土壤显著提高了38.4%、93.1%和57.1%(图 1)。
|
注:“*”、“**”和“***”分别表示两种母岩发育土壤在P < 0.05、P < 0.01和P < 0.001水平上差异显著。下同。 Note: "*", "**" and"***"indicate that soils developed from different parent rocks are significantly different at the P < 0.05, 0.01 and P < 0.001 levels, respectively. The same as below. 图 1 不同母岩发育土壤有机质矿物保护特性 Fig. 1 Mineral protection of soils with different lithological development |
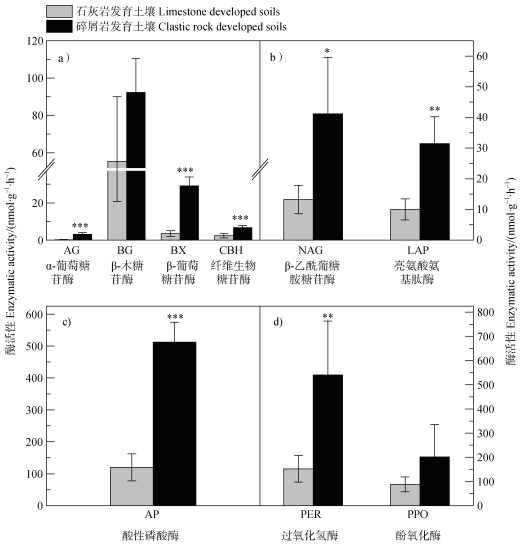
石灰岩发育土壤碳循环相关酶(AG、BX、CBH)、氮循环相关酶(NAG、LAP)和磷循环相关酶活性(AP)均显著低于碎屑岩发育土壤(图 2),分别降低了91.2%、87.6%、63.8%、67.9%、68.3%和76.7%。石灰岩和碎屑岩发育土壤过氧化氢酶(PER)存在显著差异,石灰岩发育土壤较碎屑岩发育土壤显著降低了71.8%(图 2),而酚氧化酶(PPO)无显著差异。
|
图 2 两种母岩发育土壤的酶活性 Fig. 2 Enzyme activity of soils developed from two different parent rocks |
两种母岩发育土壤的MBC和MBP均存在显著差异(表 2),其中,碎屑岩发育土壤的MBC较石灰岩发育土壤提高32.9%,而MBP却降低了37.9%。碎屑岩发育土壤的F︰B和G+︰G–显著高于石灰岩发育土壤。两种母岩发育土壤的PLFA总量、真菌、细菌含量以及G+、放线菌含量并无显著差异。两种母岩发育土壤微生物群落组成有显著差异,其中碎屑岩发育土壤F︰B和G+︰G–比值显著大于石灰岩发育土壤。
|
|
表 2 两种母岩发育土壤微生物生物量和PLFA指标 Table 2 Microbial biomass and PLFA of soils with different lithological development |
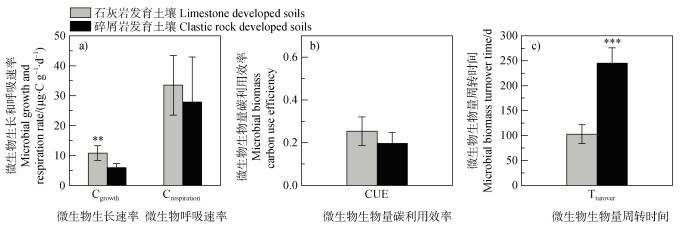
如图 3所示,石灰岩发育土壤与碎屑岩发育土壤的微生物生长速率存在显著差异,且较碎屑岩发育土壤显著升高了80.6%(P < 0.01)。但石灰岩发育土壤较碎屑岩发育土壤的微生物生物量周转时间显著降低了58.1%(P < 0.001)。而微生物呼吸速率和CUE在两种土壤之间差异并不显著(P > 0.05)。
|
图 3 不同母岩发育土壤微生物生物量碳代谢指标 Fig. 3 Indicators of microbial C metabolism in soils with different lithological development |
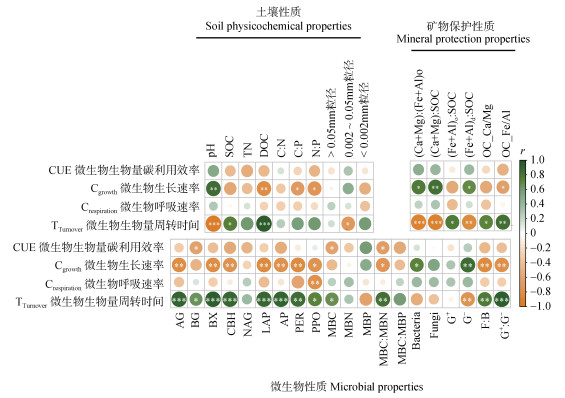
通过相关性分析可以看出(图 4),土壤CUE仅与MBC和MBC︰MBN呈显著负相关;微生物呼吸速率仅与PPO活性呈显著负相关;土壤微生物生长速率与土壤pH、(Ca+Mg)︰(Fe+Al)o、(Ca+Mg)︰SOC、(Fe+Al)d︰SOC呈显著正相关,而与DOC、C︰P、N︰P以及OC-Fe/Al呈显著负相关关系。微生物生长速率除与细菌和革兰氏阴性细菌呈显著正相关外,与其他大多数微生物性质均呈显著负相关。微生物量周转时间与土壤pH、(Ca+Mg)︰(Fe+Al)o、(Ca+Mg)︰SOC、(Fe+Al)d︰SOC均呈显著负相关,而与SOC、DOC、粉粒含量以及OC-Ca/Mg和OC-Fe/Al呈显著正相关性。微生物量周转时间除与革兰氏阴性细菌呈显著负相关外,与其他大多数微生物性质均呈显著正相关性(P < 0.05)。
|
图 4 土壤微生物生物量碳代谢指标与土壤性质、矿物保护性质和微生物性质之间的相关性 Fig. 4 The Spearman's correlations between microbial biomass C metabolism(growth, respiration, CUE and turnover time)with soil physiochemical, mineral protection and microbial properties |
不同母岩发育土壤的性质,如pH、SOC、养分等会表现出不同程度的差异[33]。石灰岩发育土壤pH较高,碎屑岩发育土壤pH较低,这主要是因为与石灰岩发育土壤相比碎屑岩发育土壤中含有较高的碳酸根和钙离子[34]。土壤有机质与矿物之间的相互作用被认为是控制土壤有机质稳定性的关键机制[35]。本研究结果表明,石灰岩发育土壤中交换性Ca、Mg含量较高,表明石灰岩发育土壤中的Ca、Mg保护作用更强,碎屑岩发育土壤中Fe、Al含量高,表明碎屑岩发育土壤中的Fe、Al保护作用更强。Ca通过促进有机质和矿物表面之间Ca2+桥的形成来促进有机质的稳定[36]。一方面能够限制底物的可获取性从而抑制微生物活性,另一方面通过吸附复杂有机质降解的胞外酶降低其活性,从而减少有机质的分解[37]。然而很多研究表明Fe、Al矿物对于SOC的保护作用更强[18,25,38]。段勋等[39]研究表明,Fe介导的SOC固持机制主要取决于自身的矿物学特性,能够通过吸附、络合、共沉淀和夹层复合等方式形成Fe结合态有机碳,从而对SOC起到直接的矿物保护作用。
岩性的差异也会造成微生物生物量、酶活性和群落组成的差异。碎屑岩发育土壤的MBC含量较高,可能是因为其DOC含量较高,微生物可直接利用。此外,研究表明MBC与土壤碳、氮含量存在显著正相关关系,即MBC与土壤中可利用的碳、氮的变化同步[40]。本研究发现碎屑岩发育土壤碳、氮、磷循环相关的酶活性均显著高于石灰岩发育土壤,这表明碎屑岩发育土壤具有更高的微生物活性,这可能是由于碎屑岩发育土壤具有更高的碳可利用性会刺激微生物胞外酶的分泌[41]。本研究中碎屑岩发育的土壤C︰P和N︰P均显著高于石灰岩发育土壤,而MBP却显著低于石灰岩发育土壤,说明相对于石灰岩发育土壤,碎屑岩发育土壤中的磷相对受限,这与Chen等[42]在非喀斯特森林土壤的磷限制程度较喀斯特森林土壤更为明显的研究结果相一致。相对于G+,G–更易受养分有效性所约束[43],因此,碎屑岩发育土壤中磷有效性较低,导致G–相对较低,G+︰G–较大。
3.2 两种母岩发育的森林土壤微生物生物量碳代谢差异的可能原因微生物代谢特征,如生长、呼吸、CUE和周转时间通常受土壤pH、质地、计量学、酶活性和微生物群落组成所影响[12,44-47]。本研究中,石灰岩发育土壤pH显著高于碎屑岩发育土壤,这可能会导致微生物群落组成发生改变,因为随pH升高,细菌和G–相对丰度会随之增加[48-49],这与本研究结果一致。由于相对于G+和真菌,G–和细菌偏好利用来源于植物的易利用SOC[9,43],同时,高土壤pH有利于细菌生长[11]。因此,石灰岩发育土壤较高的微生物生长和周转速率可能是由于高pH导致的群落改变所致。此外,养分限制也会影响微生物生长代谢,如氮添加会显著促进真菌生长,而磷会增加细菌生长,说明氮和磷分别是真菌和细菌生长的限制养分[50-51]。本研究结果发现,石灰岩发育土壤C︰P和N︰P显著低于碎屑岩发育土壤,说明碎屑岩发育土壤更受磷限制(表 2),这可能会导致细菌生长受限,因此进一步降低微生物生长速率和周转速率。从酶活性来看,碎屑岩发育土壤碳氮磷获取酶活性显著低于石灰岩发育土壤,这表明碎屑岩土壤微生物更受资源限制。由于资源限制会显著降低土壤微生物生长[52-54],这可能是碎屑岩发育土壤微生物生长低于石灰岩发育土壤的另一原因。
土壤中短续态Fe、Al矿物含量越高,将会导致微生物生长和呼吸受限,这是由于矿物保护一方面可以影响微生物群落组成,另一方面能够约束微生物生长与移动以及底物的可获得性[18]。本研究发现,碎屑岩发育土壤中短续态Fe、Al矿物含量显著高于石灰岩发育土壤,从而导致微生物生长受限。粉黏粒(< 0.05 mm粒径)丰富的土壤一方面能够为微生物提供生长场所(特别是细菌),使其能够更有效地利用外源碳,从而促进微生物生长[44,55];另一方面,粉黏粒丰富的土壤往往有着更高的土壤pH[55]。相对于碎屑岩发育土壤,石灰岩发育土壤粉黏粒含量更高,这也会导致其微生物生长速率更高。为了保持微生物自身养分计量学的平衡,土壤微生物生物量CUE随土壤C︰N升高而降低[46,56-57]。而本研究中两种土壤C︰N比无显著差异,这可能是导致CUE差异在两种母岩之间差异不显著的重要原因(图 3)。
4 结论通过对采自于贵州喀斯特区域的两种不同母岩发育土壤的微生物生物量碳代谢特征研究表明,石灰岩发育土壤微生物生长速率和微生物量周转速率高于碎屑岩发育土壤。这主要是由于石灰岩发育土壤pH更高、较弱的非晶态铁铝矿物保护作用、更低的微生物资源限制以及较大的细菌(特别是革兰氏阴性细菌)生物量所致。因此,鉴于微生物生物量碳代谢特征是直接决定土壤矿物结合态有机碳的主要驱动因素,所以在全球变化背景下评估SOC形成和稳定性方面必须要考虑岩性调控因素。
| [1] |
Karhu K, Alaei S, Li J, et al. Microbial carbon use efficiency and priming of soil organic matter mineralization by glucose additions in boreal forest soils with different C: N ratios[J]. Soil Biology and Biochemistry, 2022, 167: 108615. DOI:10.1016/j.soilbio.2022.108615
(  0) 0) |
| [2] |
Gunina A, Kuzyakov Y. From energy to(soil organic)matter[J]. Global Change Biology, 2022, 28(7): 2169-2182. DOI:10.1111/gcb.16071
(  0) 0) |
| [3] |
Camenzind T, Mason-Jones K, Mansour I, et al. Formation of necromass-derived soil organic carbon determined by microbial death pathways[J]. Nature Geoscience, 2023, 16(2): 115-122. DOI:10.1038/s41561-022-01100-3
(  0) 0) |
| [4] |
Schimel J. Modeling ecosystem-scale carbon dynamics in soil: The microbial dimension[J]. Soil Biology and Biochemistry, 2023, 178: 108948. DOI:10.1016/j.soilbio.2023.108948
(  0) 0) |
| [5] |
Peng T, Ma S L, Ma C X, et al. Effects of long-term monocropping on soil microbial metabolic activity and diversity in topsoil and subsoil horizons of Lycium barbarum fields (In Chinese)[J]. Acta Prataculturae Sinica, 2023, 32(1): 89-98. [彭彤, 马少兰, 马彩霞, 等. 长期单作对枸杞园不同土层土壤微生物代谢活性和多样性的影响[J]. 草业学报, 2023, 32(1): 89-98.]
(  0) 0) |
| [6] |
Schimel J, Weintraub M N, Moorhead D. Estimating microbial carbon use efficiency in soil: Isotope-based and enzyme-based methods measure fundamentally different aspects of microbial resource use[J]. Soil Biology and Biochemistry, 2022, 169: 108677. DOI:10.1016/j.soilbio.2022.108677
(  0) 0) |
| [7] |
Kästner M, Miltner A, Thiele-Bruhn S, et al. Microbial necromass in soils-Linking microbes to soil processes and carbon turnover[J]. Frontiers in Environmental Science, 2021, 9: 756378. DOI:10.3389/fenvs.2021.756378
(  0) 0) |
| [8] |
Kallenbach C M, Frey S D, Grandy A S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls[J]. Nature Communications, 2016, 7(1): 1-10.
(  0) 0) |
| [9] |
Xu X F, Wang N N, Lipson D, et al. Microbial macroecology: In search of mechanisms governing microbial biogeographic patterns[J]. Global Ecology and Biogeography, 2020, 29(11): 1870-1886. DOI:10.1111/geb.13162
(  0) 0) |
| [10] |
Gavazov K, Canarini A, Jassey V E J, et al. Plant-microbial linkages underpin carbon sequestration in contrasting mountain tundra vegetation types[J]. Soil Biology and Biochemistry, 2022, 165: 108530. DOI:10.1016/j.soilbio.2021.108530
(  0) 0) |
| [11] |
Silva-Sánchez A, Soares M, Rousk J. Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality[J]. Soil Biology and Biochemistry, 2019, 134: 25-35. DOI:10.1016/j.soilbio.2019.03.008
(  0) 0) |
| [12] |
Kleber M, Bourg I C, Coward E K, et al. Dynamic interactions at the mineral–organic matter interface[J]. Nature Reviews Earth & Environment, 2021, 2(6): 402-421.
(  0) 0) |
| [13] |
Wilhelm R C, Lynch L, Webster T M, et al. Susceptibility of new soil organic carbon to mineralization during dry-wet cycling in soils from contrasting ends of a precipitation gradient[J]. Soil Biology and Biochemistry, 2022, 169: 108681. DOI:10.1016/j.soilbio.2022.108681
(  0) 0) |
| [14] |
Hartmann M, Six J. Soil structure and microbiome functions in agroecosystems[J]. Nature Reviews Earth & Environment, 2023, 4(1): 4-18.
(  0) 0) |
| [15] |
Sokol N W, Sanderman J, Bradford M A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry[J]. Global Change Biology, 2019, 25(1): 12-24. DOI:10.1111/gcb.14482
(  0) 0) |
| [16] |
Finley B K, Mau R L, Hayer M, et al. Soil minerals affect taxon-specific bacterial growth[J]. The ISME Journal, 2022, 16(5): 1318-1326. DOI:10.1038/s41396-021-01162-y
(  0) 0) |
| [17] |
Wang C, Qu L R, Yang L M, et al. Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon[J]. Global Change Biology, 2021, 27(10): 2039-2048. DOI:10.1111/gcb.15550
(  0) 0) |
| [18] |
Wang S J, Li R L, Sun C X, et al. How types of carbonate rock assemblages constrain the distribution of Karst rocky desertified land in Guizhou Province, PR China: Phenomena and mechanisms[J]. Land Degradation & Development, 2004, 15(2): 123-131.
(  0) 0) |
| [19] |
Hu P L, Zhang W, Chen H S, et al. Lithologic control of microbial-derived carbon in forest soils[J]. Soil Biology and Biochemistry, 2022, 167: 108600. DOI:10.1016/j.soilbio.2022.108600
(  0) 0) |
| [20] |
Hahm W J, Riebe C S, Lukens C E, et al. Bedrock composition regulates mountain ecosystems and landscape evolution[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(9): 3338-3343.
(  0) 0) |
| [21] |
Jiang Z H, Liu H Y, Wang H Y, et al. Bedrock geochemistry influences vegetation growth by regulating the regolith water holding capacity[J]. Nature Communications, 2020, 11(1): 1-9. DOI:10.1038/s41467-019-13993-7
(  0) 0) |
| [22] |
Weemstra M, Peay K G, Davies S J, et al. Lithological constraints on resource economies shape the mycorrhizal composition of a Bornean rain forest[J]. New Phytologist, 2020, 228(1): 253-268. DOI:10.1111/nph.16672
(  0) 0) |
| [23] |
Possinger A R, Weiglein TL, Bowman M M, et al. Climate effects on subsoil carbon loss mediated by soil chemistry[J]. Environmental Science & Technology, 2021, 55(23): 16224-16235.
(  0) 0) |
| [24] |
Brandt L, Stache F, Poll C, et al. Mineral type and land-use intensity control composition and functions of microorganisms colonizing pristine minerals in grassland soils[J]. Soil Biology and Biochemistry, 2023, 182: 109037. DOI:10.1016/j.soilbio.2023.109037
(  0) 0) |
| [25] |
Stone B W G, Dijkstra P, Finley B K, et al. Life history strategies among soil bacteria-Dichotomy for few, continuum for many[J]. The ISME Journal, 2023, 17(4): 611-619. DOI:10.1038/s41396-022-01354-0
(  0) 0) |
| [26] |
Chen L Y, Liu L, Qin S Q, et al. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nature Communications, 2019, 10: 5112.
(  0) 0) |
| [27] |
Cotrufo M F, Ranalli M G, Haddix M L, et al. Soil carbon storage informed by particulate and mineral-associated organic matter[J]. Nature Geoscience, 2019, 12(12): 989-994. DOI:10.1038/s41561-019-0484-6
(  0) 0) |
| [28] |
Wang S M, Jia Y F, Liu T, et al. Delineating the role of calcium in the large-scale distribution of metal-bound organic carbon in soils[J]. Geophysical Research Letters, 2021, 48(10): 11-21.
(  0) 0) |
| [29] |
Saiya-Cork K R, Sinsabaugh R L, Zak D R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil[J]. Soil Biology and Biochemistry, 2002, 34(9): 1309-1315. DOI:10.1016/S0038-0717(02)00074-3
(  0) 0) |
| [30] |
Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification[J]. Canadian Journal of Biochemistry and Physiology, 1959, 37(8): 911-917. DOI:10.1139/o59-099
(  0) 0) |
| [31] |
Guo J F, Yang Z J, Lin C F, et al. Conversion of a natural evergreen broadleaved forest into coniferous plantations in a subtropical area: Effects on composition of soil microbial communities and soil respiration[J]. Biology and Fertility of Soils, 2016, 52(6): 799-809. DOI:10.1007/s00374-016-1120-x
(  0) 0) |
| [32] |
Zheng Q, Hu Y T, Zhang S S, et al. Growth explains microbial carbon use efficiency across soils differing in land use and geology[J]. Soil Biology and Biochemistry, 2019, 128: 45-55. DOI:10.1016/j.soilbio.2018.10.006
(  0) 0) |
| [33] |
Wilson S G, Dahlgren R A, Margenot A J, et al. Expanding the Paradigm: The influence of climate and lithology on soil phosphorus. Geoderma, 2022, 421: 115809.
(  0) 0) |
| [34] |
Zhu D N, Zou S Z, Zhou C S, et al. Desorption characteristics and hysteresis of adsorbed cadmium in calcareous soils on Karst area (In Chinese)[J]. Environmental Chemistry, 2016, 35(7): 1407-1414. [朱丹尼, 邹胜章, 周长松, 等. 岩溶区石灰性土壤对Cd2+吸附的解吸特性及滞后效应[J]. 环境化学, 2016, 35(7): 1407-1414.]
(  0) 0) |
| [35] |
Schrumpf M, Kaiser K, Guggenberger G, et al. Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals[J]. Biogeosciences, 2013, 10(3): 1675-1691. DOI:10.5194/bg-10-1675-2013
(  0) 0) |
| [36] |
Rowley M C, Grand S, Adatte T, et al. A cascading influence of calcium carbonate on the biogeochemistry and pedogenic trajectories of subalpine soils, Switzerland[J]. Geoderma, 2020, 361: 114065. DOI:10.1016/j.geoderma.2019.114065
(  0) 0) |
| [37] |
Qin S Q, Kou D, Mao C, et al. Temperature sensitivity of permafrost carbon release mediated by mineral and microbial properties[J]. Science Advances, 2021, 7(32): 11-21.
(  0) 0) |
| [38] |
Ye C L, Chen D M, Hall S J, et al. Reconciling multiple impacts of nitrogen enrichment on soil carbon: Plant, microbial and geochemical controls[J]. Ecology Letters, 2018, 21(8): 1162-1173. DOI:10.1111/ele.13083
(  0) 0) |
| [39] |
Duan X, Li Z, Liu M, et al. Research progress on iron-mediated soil organic carbon fixation and mineralization (In Chinese)[J]. Advances in Earth Science, 2022, 37(2): 202-211. [段勋, 李哲, 刘淼, 等. 铁介导的土壤有机碳固持和矿化研究进展[J]. 地球科学进展, 2022, 37(2): 202-211.]
(  0) 0) |
| [40] |
Wang W H, Yu Y N, Xie J Q, et al. Accumulation and stoichiometric characteristics of soil carbon, nitrogen and phosphorus in different afforestation models in south subtropical region of China (In Chinese)[J]. Acta Ecologica Sinica, 2023, 43(5): 1793-1803. [王薇菡, 虞依娜, 谢嘉淇, 等. 中国南亚热带不同造林模式碳汇林土壤碳、氮、磷的积累及化学计量特征[J]. 生态学报, 2023, 43(5): 1793-1803.]
(  0) 0) |
| [41] |
孙彩丽, 王艺伟, 王从军, 等. 喀斯特山区土地利用方式转变对土壤酶活性及其化学计量特征的影响[J]. 生态学报, 2021, 41(10): 4140-4149.
(  0) 0) |
| [42] |
Chen H, Li D J, Xiao K C, et al. Soil microbial processes and resource limitation in Karst and non-Karst forests[J]. Functional Ecology, 2018, 32(5): 1400-1409. DOI:10.1111/1365-2435.13069
(  0) 0) |
| [43] |
Fanin N, Kardol P, Farrell M, et al. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils[J]. Soil Biology and Biochemistry, 2019, 128: 111-114. DOI:10.1016/j.soilbio.2018.10.010
(  0) 0) |
| [44] |
Islam M R, Singh B, Dijkstra F A. Microbial carbon use efficiency of glucose varies with soil clay content: A meta-analysis[J]. Applied Soil Ecology, 2023, 181: 104636. DOI:10.1016/j.apsoil.2022.104636
(  0) 0) |
| [45] |
Jones D L, Cooledge E C, Hoyle F C, et al. pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities[J]. Soil Biology and Biochemistry, 2019, 138: 107584. DOI:10.1016/j.soilbio.2019.107584
(  0) 0) |
| [46] |
Mganga K Z, Sietio O M, Meyer N, et al. Microbial carbon use efficiency along an altitudinal gradient. Soil Biology and Biochemistry, 2022, 173: 108799.
(  0) 0) |
| [47] |
Pold G, Kwiatkowski B L, Rastetter E B, et al. Sporadic P limitation constrains microbial growth and facilitates SOM accumulation in the stoichiometrically coupled, acclimating microbe–plant–soil model[J]. Soil Biology and Biochemistry, 2022, 165: 108489. DOI:10.1016/j.soilbio.2021.108489
(  0) 0) |
| [48] |
Yang T H, Li X J, Hu B, et al. Soil microbial biomass and community composition along a latitudinal gradient in the arid valleys of southwest China[J]. Geoderma, 2022, 413: 115750. DOI:10.1016/j.geoderma.2022.115750
(  0) 0) |
| [49] |
Zheng H F, Vesterdal L, Schmidt I K, et al. Ecoenzymatic stoichiometry can reflect microbial resource limitation, substrate quality, or both in forest soils[J]. Soil Biology and Biochemistry, 2022, 167: 108613. DOI:10.1016/j.soilbio.2022.108613
(  0) 0) |
| [50] |
Kamble P N, Bååth E. Comparison of fungal and bacterial growth after alleviating induced N-limitation in soil[J]. Soil Biology and Biochemistry, 2016, 103: 97-105. DOI:10.1016/j.soilbio.2016.08.015
(  0) 0) |
| [51] |
Nottingham A T, Hicks L C, Ccahuana A J Q, et al. Nutrient limitations to bacterial and fungal growth during cellulose decomposition in tropical forest soils[J]. Biology and Fertility of Soils, 2018, 54(2): 219-228. DOI:10.1007/s00374-017-1247-4
(  0) 0) |
| [52] |
Feng X H, Qin S Q, Zhang D Y, et al. Nitrogen input enhances microbial carbon use efficiency by altering plant-microbe-mineral interactions[J]. Global Change Biology, 2022, 28(16): 4845-4860. DOI:10.1111/gcb.16229
(  0) 0) |
| [53] |
Johnston E R, Kim M, Hatt J K, et al. Phosphate addition increases tropical forest soil respiration primarily by deconstraining microbial population growth[J]. Soil Biology and Biochemistry, 2019, 130: 43-54. DOI:10.1016/j.soilbio.2018.11.026
(  0) 0) |
| [54] |
Morris K A, Richter A, Migliavacca M, et al. Growth of soil microbes is not limited by the availability of nitrogen and phosphorus in a Mediterranean oak-savanna[J]. Soil Biology and Biochemistry, 2022, 169: 108680. DOI:10.1016/j.soilbio.2022.108680
(  0) 0) |
| [55] |
Angst G, Pokorný J, Mueller C W, et al. Soil texture affects the coupling of litter decomposition and soil organic matter formation[J]. Soil Biology and Biochemistry, 2021, 159: 108302. DOI:10.1016/j.soilbio.2021.108302
(  0) 0) |
| [56] |
Khan K S, Joergensen R G. Stoichiometry of the soil microbial biomass in response to amendments with varying C/N/P/S ratios[J]. Biology and Fertility of Soils, 2019, 55(3): 265-274. DOI:10.1007/s00374-019-01346-x
(  0) 0) |
| [57] |
Sinsabaugh R L, Turner B L, Talbot J M, et al. Stoichiometry of microbial carbon use efficiency in soils[J]. Ecological Monographs, 2016, 86(2): 172-189. DOI:10.1890/15-2110.1
(  0) 0) |
 2024, Vol. 61
2024, Vol. 61


