2. 中国农业大学资源与环境学院, 国家农业绿色发展研究院, 北京 100193;
3. 广西农业科学院甘蔗研究所, 广西甘蔗遗传改良重点实验室, 农业农村部甘蔗生物技术与遗传改良重点实验室(广西), 南宁 530007;
4. 昆士兰大学, 昆士兰农业和食品创新联盟, 澳大利亚圣卢西亚 4067
2. College of Resources and Environmental Science, National Academy of Agriculture Green Development, China Agricultural University, Beijing 100193, China;
3. Key Laboratory of Sugarcane Biotechnology and Genetic Improvement (Guangxi), Ministry of Agriculture and Rural Affairs; Guangxi Key Laboratory of Sugarcane Genetic Improvement, Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning 530007, China;
4. Queensland Alliance for Agriculture and Food Innovation, University of Queensland, St Lucia 4067, Australia
土壤侵蚀,尤其水力侵蚀会引起坡耕地土壤退化、农业生产力下降以及农业面源污染等生态环境问题[1]。近年来,随着肉蛋奶需求激增,畜牧业扩张,畜禽粪便作为有机肥部分替代化肥成为实现化肥减施与促进区域种养循环的重要战略[2]。然而,粪便中残留着大部分难以被动物完全吸收利用的兽用抗生素,经粪肥施用的方式进入农田,加剧了土壤中抗生素累积与迁移风险(达到μg·kg–1或mg·kg–1级水平)[3-4]。坡耕地施用有机肥后,降雨诱发的水蚀在养分流失与抗生素扩散方面发挥着主要作用[4-5]。抗生素改变了农田土壤和侵蚀沉积物中碳(C)和氮(N)的水平,进一步影响了农田和水生生态系统的演变进程和方向[6-7]。
水蚀对于土壤养分与污染物迁移的影响主要由地表径流对土壤颗粒的物理搬运、土壤粒径的再分配以及土壤吸附共同导致的[8]。研究表明,径流和泥沙输移对山坡养分流失均有重要贡献,部分C、N等养分以溶解态的形式随径流输出;因土壤细颗粒易受径流优先流动的影响,另一部分则被侵蚀土壤颗粒吸附,以颗粒态的形式随沉积物进入径流[9-10]。土壤微生物作为维持土壤功能和服务(如C、N等养分循环)的核心,其活动状态深刻影响着土壤健康[11]。然而,水蚀过程不仅直接抑制了微生物活性,降低了土壤微生物生物量,还间接阻碍了土壤表层C、N的封存[12-13]。农业系统中,有机肥的广泛应用虽促进了外源有机物质的积累,却也引发了抗生素在土壤中的富集问题[8,14]。这种抗生素污染对土壤C、N循环构成了复杂影响,既通过改变微生物群落结构与功能来干扰C、N的转化和稳定性,又随水蚀迁移,对径流和沉积物中的C、N构成潜在威胁[15]。具体而言,抗生素与水蚀之间的叠加效应可能表现为:抗生素削弱了土壤微生物对C、N的固定和转化能力,导致更多的C、N以易流失形态存在;同时,在水蚀作用下加剧了这些土壤颗粒和可溶态C、N的流失。有研究表明,抗生素残留对参与土壤C、N循环的微生物群体造成持续性负面效应,包括减少特定微生物种群数量、破坏功能微生物群落结构与多样性等,最终影响碳矿化、氮硝化和反硝化等关键过程[16-17]。考虑到抗生素对土壤细菌的重要作用,采用细菌定量和高通量测序等方法成为评估其对土壤及沉积物中微生物群落影响的重要手段。同时,微生物生物量碳氮(MBC和MBN)作为土壤活性微生物量的关键指标,对于理解土壤微生物动态至关重要。此外,大部分抗生素会残留在土壤中或被降解,其余部分会在降雨条件下,受水蚀驱动并以溶解态、胶体结合态或颗粒吸附态等形式随径流迁移至地表水及地下水系统[18]。土壤中外源有机物质的增加还进一步调控了抗生素的吸附-解吸行为,对其迁移路径产生潜在影响[19-20]。因此,在有机肥替代背景下,抗生素与水蚀对土壤C、N流失的叠加效应是一个复杂而重要的研究领域,值得深入探究。
坡耕地作为全球耕地的重要组成部分,正面临着严峻的水蚀挑战[21]。特别是中国西南山地紫色土区域,既是重要的蔬果生产和畜禽养殖基地,也是水蚀高发风险区[22]。鉴于C、N和抗生素在水蚀过程中的复杂相互作用,本研究通过室内坡地模拟人工降雨试验,旨在:(1)分析施用添加抗生素的有机肥对水土流失的影响;(2)探讨抗生素随水蚀的迁移规律;(3)揭示抗生素在水蚀作用下对C、N流失的复杂影响。研究结果可为三峡库区水环境保护与农业绿色发展的双重需求提供理论依据。
1 材料与方法 1.1 供试土壤本试验土壤采自中国重庆市北碚区某紫色土坡耕地,土壤类型为紫色土。去除土壤表面生长的植物以及其他杂物后,共采集了约7 t的耕层(0~20 cm)土壤。同时,基于“S型”多点随机采样法收集原始土样,参考土壤农化分析方法[23]测定了其理化性质(表 1)。
|
|
表 1 供试土壤理化性质 Table 1 Physico-chemical properties of the experimental soils |
本试验设置了5组施肥处理(图 1),每组重复3次:(1)CK,不施肥对照;(2)NP,单施化肥;(3)OMNP,有机肥替代30%化肥磷;(4)OMNPT,有机肥替代30%化肥磷加土霉素(OTC);(5)OMNPQ,有机肥替代30%化肥磷加恩诺沙星(ENR)。
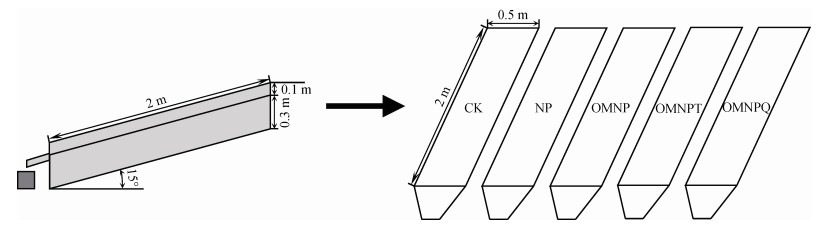
|
注:CK:不施肥对照;NP:单施化肥;OMNP:有机肥替代30%化肥磷;OMNPT:有机肥替代30%化肥磷加土霉素(OTC);OMNPQ:有机肥替代30%化肥磷加恩诺沙星(ENR)。下同。 Note: CK: No fertilization control; NP: Only chemical fertilizer; OMNP: Organic fertilizer substituting 30% chemical phosphorus fertilizer; OMNPT: Oxytetracycline(OTC)contaminated organic fertilizer substituting 30% chemical phosphorus fertilizer; OMNPQ: Enrofloxacin(ENR)contaminated organic fertilizer substituting 30% chemical phosphorus fertilizer. The same as below. 图 1 试验装置示意图 Fig. 1 Schematic diagram of experimental equipment |
根据当地农业委员会对夏玉米(Zea mays L.)施肥建议,氮肥和磷肥施用量分别为270 kg·hm–2的尿素(N≥46%)和105 kg·hm–2的过磷酸钙(P2O5≥12%)。有机肥采用鸡粪源有机肥,施用量为1 500 kg·hm–2,其中有机质>50.0%,N和P2O5含量分别为2.3%和2.1%。选用在我国畜禽粪便、农田土壤以及水体中,抗生素检出率和检出量高的四环素类(OTC)和喹诺酮类抗生素(ENR)为研究对象[3,7],浓度设定环境相关浓度(5 mg·kg–1)。将抗生素先与有机肥均匀混合,混合好后与化肥分别均匀撒播于处理组地块的表面,然后使用长柄园艺工具将其与土壤均匀混合,密封遮盖两周左右后装槽。
1.3 室内人工降雨过程室内模拟降雨试验于2022年7月在中国重庆市北碚区西南大学水土保持模拟降雨大厅(29°48′42′′N,106°24′20′′E)进行,使用由中国科学院水土保持研究所和水利部所研发的侧喷式自动人工降雨装置。设计了三个自制的可变坡钢制径流槽(长2 m ×宽0.5 m ×高0.3 m)(图 1),随机排列。根据重庆不同时期暴雨的强度和频率[24-25],降雨强度及其持续时间分别设定为90 mm·h–1和60 min。由于重庆紫色土坡耕地主要集中在5°~20°之间,且处于15°时极易发生水土及养分流失[22],故选择该区域的典型坡度15°作为本试验径流槽的坡度。每场降雨事件均展开持续30 min且雨强为30 mm·h–1的预降雨,以润湿径流槽内的试验土壤材料,确保初始土壤含水量基本保持一致。用塑料布遮盖并放置24 h后,必须进行持续5 min的2~3次降雨强度的校准。当最终测量的降雨强度在90 ± 3 mm·h–1范围内且降雨均匀性达到>80%时,即可掀开塑料布并将径流槽的坡度调至15°,从而展开正式的人工暴雨试验。在降雨过程中,分别记录降雨开始和初始产流时间。径流一旦产生,立刻用塑料桶接取径流和沉积物混样。在前10 min内每隔2 min接一次样,然后每5 min接一次直至降雨结束。
1.4 样品采集与处理试验前,收集用于人工降雨的雨水与有机肥分别扣除其养分与抗生素的背景值。降雨结束后,测量并记录每个塑料桶中浑水的体积。然后将其充分搅拌均匀,用550 mL聚乙烯瓶(含抗生素处理组用500 mL棕色玻璃瓶)迅速从每个桶中取样,每桶取两瓶,密封、标记后带回实验室。其中,一瓶用于测定沉积物量(存于4 ℃冰箱);另一瓶用于测定养分或抗生素浓度(存于–80 ℃冰箱)。各塑料桶中剩余的浑水样需静置24 h后倒出上清液,然后取出沉积物,部分储存在–80 ℃冰箱中,另一部分风干、研磨、过筛,待测。
1.5 样品分析测试径流样:浑水样总量与沉积物量的差值得到径流量。采用碱性过硫酸钾消解—紫外分光光度计比色法测定浑水样的全氮(TN)。浑水样过0.45 μm微孔纤维滤膜后,采用TOC分析仪测定溶解性有机碳(DOC),紫外分光光度计比色法测定硝态氮(NO3–-N),靛酚蓝比色法测定铵态氮(NH4+-N),液相色谱—质谱仪测定抗生素。
沉积物样:采用烘干—称重体积法按比例计算测定沉积物量[26]。采用TOC分析仪测定DOC,湿筛分离法测定颗粒态有机碳(POC)和矿物结合态有机碳(MAOC),紫外分光光度计比色法测定NO3–-N,靛酚蓝比色法测定NH4+-N,氯仿熏蒸法提取—碳氮分析仪测定微生物生物量碳(MBC)和微生物生物量氮(MBN)[27],液相色谱—质谱仪测定抗生素,实时荧光定量(qPCR)分析测定细菌丰度,高通量测序(Illumina测序)测定微生物群落的物种组成。
(1)抗生素提取和分析。径流样:采集的浑水样通过0.45 μm微孔纤维过滤膜后,加入150 mg抗坏血酸和0.25 g乙二胺四乙酸二钠(Na2EDTA),用盐酸溶液调节每个样品pH至2。用Oasis®HLB(500 mg、6 cc)固相萃取柱提取水样中的抗生素。5 mL甲醇、5 mL Milli-Q水和5 mL Milli-Q水先活化萃取柱,水样以10~15 mL·min–1的流速全部通过萃取柱后,用5 mL Milli-Q水淋洗小柱,再抽真空干燥10 min,然后加入10 mL甲醇洗脱,并收集洗脱液。洗脱液经氮吹仪浓缩至接近干燥,用甲醇溶解定容至1.0 mL,过0.22 μm有机滤膜后待测。
沉积物和土样:收集的沉积物样和土样冷冻干燥后,委托科学指南针—杭州研趣信息技术有限公司(中国浙江杭州)进一步提取和分析。取5 g于50 mL离心管中,加入0.4 g Na2EDTA。每个样品用10 mL乙腈-磷酸盐缓冲溶液(v/v=1:1)提取。对样品进行震荡(2 min)、超声处理(20 min)和离心(10 min),以上操作重复2次,合并上清液。然后蒸发,加入15 mL Milli-Q水。将其先过Oasis®MAX(500 mg,6 cc)去除杂质,后续同径流样的前处理步骤过Oasis®HLB(500 mg、6 cc)洗脱,氮吹干燥、定容、过滤后待测。
仪器分析:采用液相色谱-质谱仪(LC-MS,岛津LCMS8050)对目标抗生素进行定量。使用分析柱AQ-C18(GL Sciences InertSustain,50 mm × 2.1 mm,1.9 μm)分离抗生素化合物。流动相A为0.1%甲酸水,流动相B为0.1%甲酸-甲醇/乙腈(v/v=1︰1)。该质谱仪是在电喷雾源(ESI)正负电离模式下进行的。OTC和ENR在水中的检出限均为1 ng·L–1,回收率分别为87.7%、63%;在沉积物和土壤中的检出限分别为0.04 ng·g–1、0.5 ng·g–1,回收率分别为84.2%、62.4%。工作校准曲线的相关系数(R2)高于0.99。
(2)细菌总数绝对定量与微生物群落组成。DNA提取:使用MJ-soil Soil DNA Extraction Kit试剂盒(Yuhua,Shanghai,China)并按照制造商的说明书分别提取沉积物和土壤(0.5 g)的总细菌群基因组DNA。然后用1%(w/v)琼脂糖凝胶电泳检测DNA的完整性[28],再用NanoDrop2000分光光度计(Thermo Fisher Scientific,USA))测定DNA的浓度和纯度。检测质量合格的沉积物和土壤的DNA样品送至美吉生物医药科技有限公司(Shanghai Majorbio Bio-pharm Technology Co.,Ltd.)进行qPCR扩增和Illumina测序。
qPCR扩增:以上述提取的DNA为模板,使用引物Eub338_Eub806(F端序列:~5′-ACTCCTACG GGAGGCAGCAG-3′ R端序列:5′-GGACTACHVG GGTWTCTAAT-3′)对沉积物和土壤中细菌16S rRNA基因V3~V4可变区进行qPCR扩增[27]。扩增反应体系包含Taq Plus Master Mix(2 X)缓冲液10 μL、前后引物(5 μM)各0.8 μL、模板DNA 1 μL和ddH2O 7.4 μL,总体积20 μL。反应条件为95 ℃预变性5 min、35个循环(95 ℃变性30 s,58 ℃退火30 s,72 ℃延伸1 min)。每个样本3次重复。使用含细菌16S rRNA基因的重组pMD18-T载体作为标准质粒,然后计算出标准质粒的拷贝数,10倍梯度稀释构建好的各质粒,90 µL稀释液+ 10 µL质粒,一般做4~6个点,通过预实验分别选取16S标准品的10–2倍~10–7倍稀释液用于制备标准曲线。在ABI 7300型荧光定量PCR仪(Applied Biosystems,USA)上进行qPCR分析。qPCR反应体系包含5 μL ChamQ SYBR Color qPCR Master Mix(2 X)、0.4 μL上游引物F(5 μM)、0.4 μL下游引物R(5 μM)、0.2 μL ROX Reference Dye 1(50 X)、1 μL模板DNA和3 μL ddH2O,总体积10 μL。循环条件为95 ℃预变性3 min、40个循环(95 ℃变性5 s、58 ℃退火30 s、72 ℃延伸1 min)。
Illumina测序:使用2%琼脂糖凝胶回收PCR产物,利用AxyPrep DNA Gel Extraction Kit(Axygen Biosciences,Union City,CA,USA)进行纯化,Tris-HCl洗脱,2%琼脂糖电泳检测[29]。利用Qubit4.0(thermofisher,USA)进行检测定量。根据PCR文库构建标准操作规程将纯化后的扩增片段构建illumina文库。利用Illumina公司的Miseq平台进行PE300测序。使用fastp软件对原始fastq数据进行质控。使用Vsearch软件(version 2.22.1)基于97%的相似度对序列进行OTU聚类,随后利用RDP classifier(version 2.13)对OTU序列进行物种分类学注释。获得分类信息,并统计计算每个分类水平下每个样本的群落组成。
1.6 数据分析使用Excel 2016和Origin Pro 8.5分别进行数据统计和图表绘制。基于SPSS 23.0软件进行单因素方差—多重比较分析和Pearson相关分析,P<0.05的结果被认为是显著的,而P>0.05的结果则被认为是不显著的。所有数据均表示为平均值±标准差。
2 结果 2.1 有机肥部分替代化肥对产流和产沙的影响坡面平均产流率、平均含沙量以及累积径流量、累积沉积物量均在NP处最高(1.43 L·min–1、46.88 g·L–1、78.02 L、3.2 kg),在CK处最低(1.12 L·min–1、31.31 g·L–1、64.94 L、1.86 kg)(图 2)。与NP相比,有机肥的投入(OMNP、OMNPT和OMNPQ)导致平均产流率、平均含沙量以及累积径流量、累积沉积物量分别显著减少了11.91%~14.64%、13.01%~15.96%、9.99%~11.49%和17.37%~20.41%(P<0.05);然而,OMNP、OMNPT和OMNPQ之间不存在显著性差异(P>0.05)。这说明施用有机肥能够缓解水土流失,但施用含抗生素有机肥对水土流失的减少效益不明显。
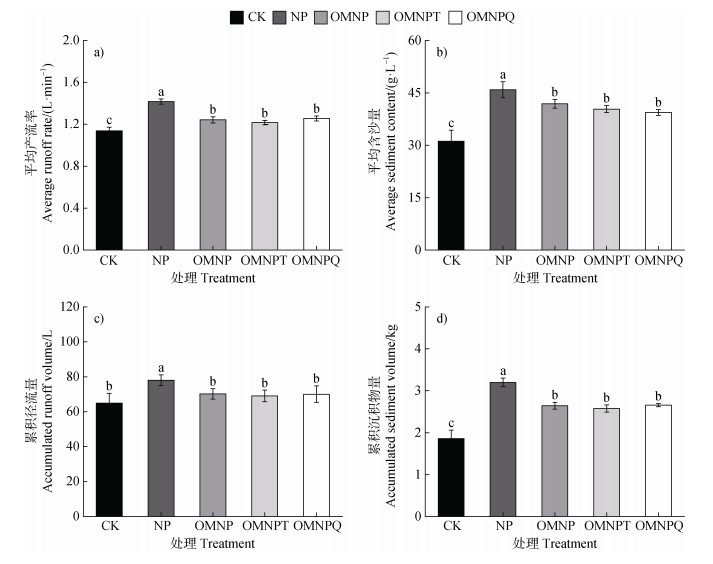
|
注:基于单因素方差分析,不同小写字母表示每组处理之间的差异显著(P<0.05)。下同。 Based on one-way ANOVA, different lowercase letters indicate significant differences among the five treatments(P < 0.05). The same as below. 图 2 不同处理下平均产流率(a)、平均含沙量(b)、累积径流量(c)和累积沉积物量(d)的比较 Fig. 2 Comparison of average runoff rate(a), average sediment content(b), cumulative runoff volume(c)and cumulative sediment volume(d)under different treatments |
土壤中抗生素的残留浓度范围为25.55~32.30 μg·kg–1,其中OTC(32.30 μg·kg–1)的残留浓度显著高于ENR(25.55 μg·kg–1)(P<0.05)(图 3a),残留量分别为11.43 mg和8.97 mg。径流中,OTC损失量高达178.60 μg,损失率为1.56%;而ENR损失量仅为2.40 μg,损失率极低,为0.03%;沉积物中,OTC损失量和损失率分别降至59.19 μg和0.52%;相反,ENR损失量和损失率分别显著增至79.18 μg和0.88%(P<0.05)(图 3b)。这表明OTC主要通过径流流失,而ENR则更易在沉积物中累积。
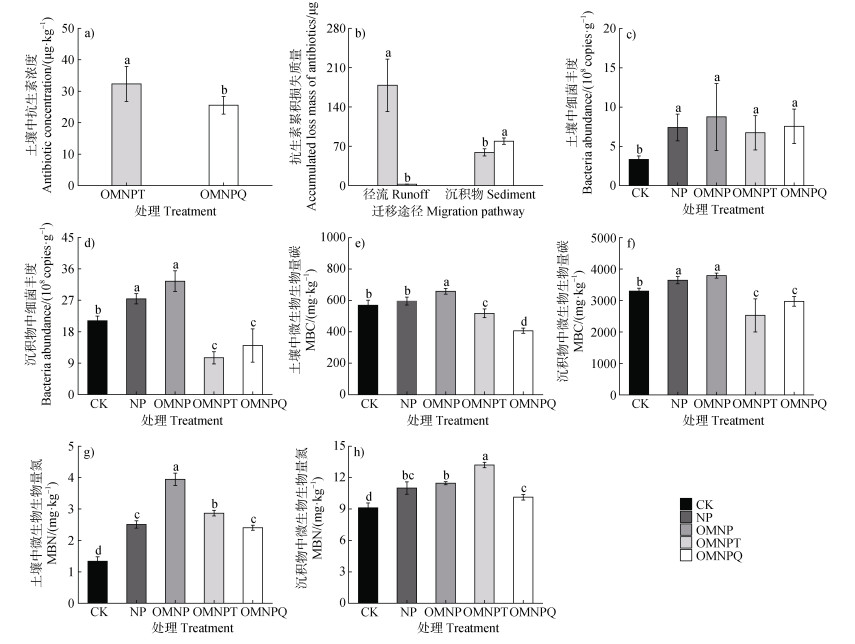
|
注:MBC:微生物生物量碳;MBN:微生物生物量氮。下同。 Note: MBC: microbial biomass carbon; MBN: microbial biomass nitrogen. The same as below. 图 3 未侵蚀土壤中抗生素浓度(a),径流和沉积物中抗生素的累积损失质量(b),以及不同处理下未受侵蚀土壤中(c,e,g)和沉积物中(d,f,h)的细菌丰度、MBC和MBN的比较 Fig. 3 Comparison of antibiotic concentrations in uneroded soil(a), accumulative mass loss of antibiotics in runoff and sediment(b), and bacteria abundance, MBC and MBN in uneroded soil(c, e, g)and sediment(d, f, h)under different treatments |
如图 3c、图 3e和图 3g,在未受侵蚀土壤中,细菌丰度和MBN在CK处最低(3.33×108 copies·g–1、1.34 mg·kg–1),MBC含量在OMNPQ处最低(407.01 mg·kg–1),但它们均在OMNP处最高(8.75× 108 copies·g–1、658.34 mg·kg–1、3.95 mg·kg–1)。NP、OMNP、OMNPT和OMNPQ之间的细菌丰度没有显著性差异(P>0.05)。OMNPT和OMNPQ的MBC含量相比其他处理分别显著降低了9.03%~21.38%和28.46%~38.18%(P<0.05);其MBN含量相比OMNP分别显著降低了27.32%和39.09%,相比CK又分别显著增加了114.38%和79.67%(P<0.05)。如图 3d、图 3f和图 3h,在沉积物中,细菌丰度和MBC含量均在OMNP处最高(3.25×109 copies·g–1、3 793 mg·kg–1),在OMNPT处最低(1.05×109 copies·g–1、2 484. mg·kg–1);OMNPT有MBN含量的最大值(13.21 mg·kg–1),而CK有其最小值(9.12 mg·kg–1)。与其他处理相比,OMNPT和OMNPQ的细菌丰度分别显著降低了50.12%~67.52%和33.59%~56.76%,其MBC含量分别显著降低了23.31%~33.35%和9.46%~21.31%(P<0.05)。OMNPT的MBN含量较其他处理显著提高了15.22%~44.86%(P<0.05);OMNPQ的MBN含量较OMNP显著降低了11.65%,较CK显著提高了11.08%(P<0.05)。
2.3 抗生素随水蚀过程对C、N流失的影响如图 4a,TOC和MAOC的损失通量均在NP处最高(434.67 kg·hm–2、365.59 kg·hm–2),DOC损失通量在OMNP处最高(12.55 kg·hm–2),POC损失通量在OMNPT处最高(58.79 kg·hm–2),它们均在CK处最低(204.17 kg·hm–2、172.11 kg·hm–2、8.52 kg·hm–2、23.54 kg·hm–2)。与NP相比,有机肥的投入均导致TOC和MAOC的损失通量分别显著减少了8.94%~11.54%和11.23%~13.97%(P<0.05)。OMNP、OMNPT和OMNPQ之间TOC、DOC、POC和MAOC的损失通量没有显著性差异(P>0.05)。
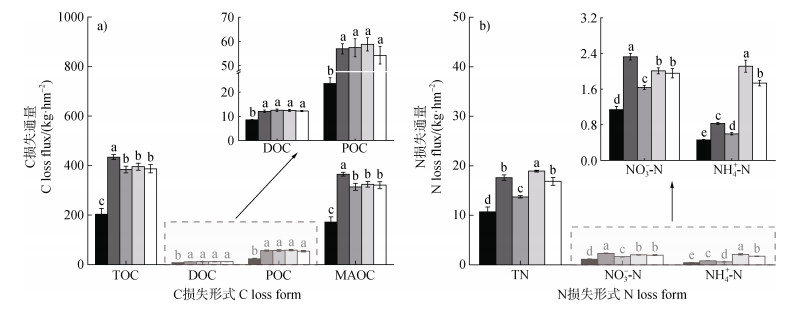
|
注:TOC:总有机碳;DOC:溶解性有机碳;POC:颗粒态有机碳;MAOC:矿物结合态有机碳;TN:全氮;NO3–-N:硝态氮;NH4+-N:铵态氮。下同。 Note: TOC: Total organic carbon; DOC: Dissolved organic carbon; POC: Particulate organic carbon; MAOC: Mineral-associated organic carbon; TN: Total nitrogen; NO3–-N: Nitrate nitrogen; NH4+-N: Ammonium nitrogen. The same as below. 图 4 不同处理下各种形式的C(a)和N(b)的损失通量 Fig. 4 Loss fluxes of various forms of C(a)and N(b)under different treatments |
如图 4b,TN和NH4+-N的损失通量在OMNPT处最高(18.91 kg·hm–2、2.11 kg·hm–2),NO3–-N损失通量在NP处最高(2.33 kg·hm–2),它们均在CK处最低(10.68 kg·hm–2、0.46 kg·hm–2、1.14 kg·hm–2)。与NP相比,OMNP的TN、NO3–-N和NH4+-N的损失通量均分别显著减少了22.00%、29.68%和27.81%,OMNPT的TN损失通量显著增加了7.43%,OMNPT和OMNPQ的NO3–-N损失通量均分别显著减少了13.66%和15.99%,OMNPT和OMNPQ的NH4+-N损失通量均分别增加了155.09%和109.41%(P<0.05)。与OMNP相比,OMNPT和OMNPQ的TN、NO3–-N和NH4+-N的损失通量均分别显著增加了22.77%~37.73%、19.46%~22.77%和190.10%~253.38%(P<0.05)。OMNPQ的TN和NH4+-N的损失通量均较OMNPT分别显著减少了10.86%和17.91%(P<0.05),两者之间NO3–-N损失通量却不存在显著性差异(P>0.05)。这些结果表明施用有机肥具有减轻C、N养分流失的效果,而施用含抗生素有机肥会加剧TN、NO3–-N和NH4+-N流失的风险。
如图 5a和图 5b,在径流中,NO3–-N损失浓度和NH4+-N损失浓度均随产流率的增加而减少。其中,OMNPT处理减少得最快,NO3–-N和NH4+-N均随产流率每增加1 L·min–1其对应的损失浓度分别减少2.81 mg·L–1和3.99 mg·L–1;OMNP处理减少得最慢,NO3–-N和NH4+-N均随产流率每增加1 L·min–1其对应的损失浓度分别减少1.79 mg·L–1和1.07 mg·L–1。如图 5c和图 5d,在沉积物中,NO3–-N损失浓度和NH4+-N损失浓度均随含沙量的增加而增加。其中,CK处理的NO3–-N损失浓度增加得最快,OMNPT处理的NH4+-N损失浓度增加得最快,均随含沙量每增加1 g·L –1其对应的损失浓度分别增加5.56 mg·kg–1和6.85 mg·kg –1;OMNP处理的NO3–-N损失浓度增加得最慢,NP处理的NH4+-N损失浓度增加得最慢,均随含沙量每增加1 g·L –1其对应的损失浓度分别增加15.58 mg·kg –1和5.85 mg·kg –1。这些结果说明产流率和含沙量均显著影响NO3–-N和NH4+-N的损失浓度,且施用含抗生素有机肥尤其施用含OTC有机肥的影响大于不含抗生素施肥。

|
图 5 不同施肥处理下NO3–-N和NH4+-N的浓度与产流率(a,b)和含沙量(c,d)的关系 Fig. 5 Correlation of NO3−-N and NH4+-N concentrations with runoff rate(a, b)and sediment content(c, d)under different treatments |
通过Pearson相关分析来解释不同抗生素处理下土壤侵蚀、沉积物中的微生物、土壤C和N的损失与抗生素浓度之间的相关关系(图 6)。如图 6,沉积物中抗生素浓度与产流率、细菌丰度和MBC均呈负相关关系,与含沙量以及MBN、TOC、DOC、POC、MAOC、TN、NO3–-N和NH4+-N的损失浓度均呈正相关关系。同时,不同抗生素对土壤C、N流失的影响存在差异,OTC浓度与各形态C、N的损失浓度仅呈正相关关系,而ENR浓度与TOC和NO3–-N的损失浓度均呈显著正相关关系(P<0.05)。这些结果说明,抗生素在沉积物中的暴露对微生物产生抑制作用,对C、N的流失产生促进作用。

|
注:*表示在P<0.05水平显著相关,**表示在P<0.01水平极显著相关。 Note: * indicates a significant correlation at the P < 0.05 level, and ** indicates a highly significant correlation at the P < 0.01 level. 图 6 土壤侵蚀、沉积物中微生物及C和N的损失浓度与抗生素浓度之间的相关性 Fig. 6 Correlation among soil erosion, microorganisms and C and N loss concentrations in sediment, and antibiotic concentration |
在本研究中,施用有机肥可以有效降低坡面由于水蚀带来的水土流失风险(图 2)。这与Shi等[29]观察到的结果相似,有机肥替代20%化肥N可以降低7.13%径流和35.59%泥沙的产生。这一结果可能与施用有机肥改善了土壤结构和土壤性质有关[30]。已有研究表明,施用有机肥不仅通过降低土壤容重和增加土壤孔隙度来增强渗透,还促进了水稳定性团聚体的形成,导致土壤抗侵蚀能力的增强,从而减少了径流的生成以及土壤颗粒的剥离和运移[31-32]。
施用粪肥是土壤中抗生素残留的主要来源,其占比超过50%[3,33]。在本研究中,施用含抗生素有机肥后(OMNPT和OMNPQ),OTC更易在土壤中富集(图 3a)。同样,前人多项研究也证实,相比其他抗生素,四环素类抗生素尤其是OTC在土壤中的污染水平更高[4,34]。这可能与四环素类抗生素在土壤中的较强的持久性有关,同时,OTC的结构相对稳定且生物降解低[35-36]。此外,有研究表明,施用粪肥后,约0.13%抗生素经径流和侵蚀沉积物途径迁移[4]。因此,施用粪肥可能会给农田和水体带来抗生素污染风险。地表径流和颗粒搬运过程是抗生素从农田分散到周围水环境的重要途径[37]。降雨初期,雨滴飞溅剥离了坡地表层疏松土壤颗粒,部分吸附在悬浮土壤颗粒上的抗生素受雨水冲刷被径流挟带走,而部分具有水溶性的抗生素通过扰动和再溶解释放到径流中并随之迁移[38-39]。然而,持续的降雨事件产生更多的径流会稀释抗生素浓度,导致降雨后期抗生素随地表径流和沉积物的迁移过程受到限制[40]。
四环素类抗生素和喹诺酮类抗生素均具有较长的半衰期和较高的流动性,而ENR的半衰期大于OTC[41-42]。ENR在水中受光解而被消散的速率相对较快,然而,沉积物这种类似黑暗的底栖环境阻碍了OTC和ENR的光解[35,43]。这些可能解释了在本研究中,沉积物中均检测出OTC和ENR的原因。此外,本研究还发现OTC以径流输出为主,而ENR以沉积物输出为主(图 3b),其原因主要与抗生素的吸附常数(OTC的吸附能力较弱于ENR)[35-36]以及侵蚀对土壤环境条件(含水量、有机质含量等)的改变[44]有关。同样,在四川延亭丘陵农业集水区[45]和东部长三角典型丘陵区[4]等其他地区也观察到OTC通过径流运输的损失较ENR多。然而,在英国约克韦尔伯恩的一个农场报道称地表径流造成的OTC损失小于ENR[46]。这种差异可能是所使用的肥料的类型及其施用量造成的,不同类型肥料中的抗生素成分复杂且残留水平各异[47]。此外,抗生素的径流损失还受土壤类型的影响,例如四环素类抗生素对酸性和黏粒比例高的土壤具有强的吸附亲和力[35]。相似的是,长江中游流域的报告也观察到OTC和ENR在沉积物剖面中具有最广泛的潜在风险[48]。在过去的二十年里,在土壤中积累大量的抗生素经暴雨事件后,导致抗生素污染在径流和沉积物中频繁发生,从而对地下水和周边湖泊、河流等水生生态系统构成潜在威胁,并可能进一步威胁人类健康[49-50]。
3.2 抗生素暴露对侵蚀过程中C、N流失的影响侵蚀过程本身对微生物具有负面影响,而土壤或沉积物中抗生素的存在会进一步灭杀或抑制微生物,如限制细菌的生长、破坏微生物群落结构及其多样性等[12,44]。因此,在本研究中,施用含抗生素有机肥(包括OMNPT和OMNPQ)较OMNP显著降低了土壤中MBC和MBN的含量以及沉积物中细菌丰度和MBC含量,然而,OMNPT显著增加了沉积物中MBN含量,OMNPQ显著降低了沉积物中MBN含量(图 3d~图 3h)。
本研究中,施用含抗生素有机肥对TOC、DOC、POC和MAOC的流失的影响与施用不含抗生素有机肥间差异不显著(图 4a)。不同抗生素浓度可能对土壤C循环过程存在不同的影响。研究表明,低浓度抗生素对土壤DOC含量没有明显影响,但DOC含量随着抗生素浓度增加而显著增加[51]。在本研究中,抗生素浓度与各有机碳组分浓度之间存在正相关关系,但无显著性(0.38≤R2≤0.67,P>0.05);其中,与TOC浓度之间具有显著正相关关系(P<0.05)(图 6)。这与Guo等[52]的研究结果相似,四环素类抗生素(四环素和OTC)与有机碳(TOC和DOC)浓度呈显著的正相关性(P<0.05,0.46≤R2≤0.90)。通常,抗生素与土壤有机物之间的相互作用主要通过吸附作用来完成。据报道,OTC更倾向吸附在具有较大的比表面积、孔隙结构和阳离子交换量的颗粒态有机物(POM)上[53]。此外,DOC也可以作为抗生素迁移的媒介,又因其具有强吸附在土壤胶体或颗粒表面的能力,导致抗生素的毒性增强,对微生物产生胁迫,进一步抑制微生物利用DOC以及对有机碳的矿化,最终引发DOC的累积[51]等。
抗生素的存在加剧了水蚀过程中N的流失主要是由于低浓度或高浓度的抗生素残留可能刺激或抑制N循环过程,如硝化、反硝化或硝酸盐还原铵等过程[16,54]。本研究中,抗生素导致土壤N损失的增加,其中施用含OTC有机肥处理下NH4+-N损失的增加程度高于NO3–-N(图 4b)。有研究表明,OTC的暴露抑制了氨氧化细菌(AOB)和硝化细菌,降低了铵盐消耗率17.65%~50.86%,导致了铵盐的积累[55]。本研究中抗生素也导致NO3–-N损失量的增加,与施用不含抗生素有机肥相比,提高了19.46%~22.77%(图 4b)。原因可能是OTC残留抑制了参与硝化过程和反硝化过程的细菌,导致铵盐和硝酸盐的积累[56],从而增加了其径流损失;此外,更多的OTC暴露于径流和沉积物中,导致它对参与反硝化过程的微生物、相关酶活性的抑制作用更大,引起硝酸盐的积累增加[57]。
侵蚀过程也改变了土壤的C、N循环过程。水蚀产生的沉积物为反硝化过程提供了一个低氧或厌氧的环境,其微位点和底物的存在又刺激了细菌在沉积物表面的富集,并促进了反硝化细菌和功能基因的丰度的增加,还通过吸附降低了抗生素的选择压力,从而缓解了抗生素对反硝化速率的抑制[15,58]。根据未侵蚀土壤和侵蚀沉积物的微生物群落物种组成的结果(图 7),施用含抗生素有机肥(包括OMNPT和OMNPQ)提高了变形菌门(Proteobacteria)和放线菌门(Actinobacteriota)的相对丰度,降低了硝化螺旋菌门(Nitrospirota)的相对丰度。此外,变形菌门和放线菌门对反硝化过程有重要作用,硝化螺旋菌门含有丰富的硝化功能菌属。有研究发现,TN含量与硝化速率和反硝化速率均具有负相关性[59]。因此,当抗生素暴露于沉积物时,会抑制硝化和反硝化过程,导致NO3–-N和NH4+-N的积累,并最终引起TN含量的增加。抗生素通过改变未侵蚀土壤和侵蚀沉积物中微生物群落的物种组成(图 7),影响了由侵蚀过程引起的土壤N流失。这些原因可能解释了在本研究中,施用含抗生素有机肥较施用不含抗生素有机肥均显著增加了TN、NO3–-N和NH4+-N的流失风险(图 4b)。
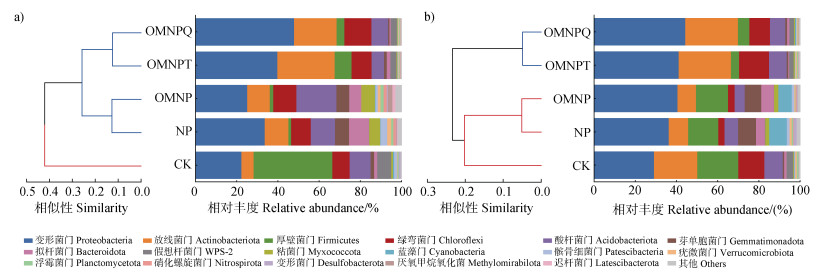
|
图 7 不同处理下土壤(a)和沉积物(b)中微生物在门水平的相似树(左)和群落物种组成(右) Fig. 7 Similarity tree(left)and community composition(right)of microorganisms in soil(a)and sediment(b)at the phylum level under different treatments |
与CK相比,NP、OMNP、OMNPT和OMNPQ导致沉积物中TOC损失通量分别提高了112.90%、88.33%、93.86%和89.85%,TN损失通量分别增加了64.85%、28.59%、77.10%和57.87%(图 8)。造成这种差异的原因一部分是施肥带来了外源有机质和氮等养分物质,增加了土壤中C、N的含量,另外一部分原因则来自水蚀对于土壤C、N的富集效应作用[60-61]。与CK相比,NP、OMNP、OMNPT和OMNPQ导致土壤中SOC含量分别增加了4.15 g·kg–1、9.38 g·kg–1、7.71 g·kg–1和6.60 g·kg–1,TN含量分别增加了0.11 g·kg–1、0.22 g·kg–1、0.27 g·kg–1和0.26 g·kg–1;同时,水蚀对侵蚀沉积物中TOC和TN的富集贡献为71.81%和96.68%(NP),66.64%和77.26%(OMNP),78.01%和83.80%(OMNPT),以及78.12%和80.55%(OMNPQ)(图 8)。与OMNP相比,OMNPT和OMNPQ分别增加了土壤TOC(0.80%~2.94%)和TN(22.77%~37.73%)的流失通量(图 8)。

|
图 8 与CK相比,水蚀与施肥对土壤和侵蚀沉积物TOC和TN的影响 Fig. 8 Compared with CK, the effects of water erosion and fertilization on soil and sediment TOC and TN |
本研究探讨分析了有机肥部分替代化肥背景下,紫色土坡耕地受水力侵蚀驱动下的抗生素迁移及其对土壤C、N流失特性的叠加影响。施用有机肥能够显著降低紫色土坡面由于水力侵蚀带来的水土流失风险。施用含抗生素有机肥对土壤C流失的影响不显著,但会显著加剧土壤N流失。与单施化肥和施用不含抗生素有机肥相比,施用含抗生素有机肥增加了TN和NH4+-N的损失通量。此外,沉积物中抗生素浓度与各形态C、N的损失浓度呈正相关性。施用含抗生素有机肥对微生物群落的物种组成有显著影响,抗生素可以使有机肥配施中C、N流失通量增加0.80%~2.94%和TN22.77%~37.73%。由于三峡库区畜禽废弃物的负荷大以及有机肥替代化肥的持续推广,可能导致越来越多来自农业地区的N进入水体,使新的农业复合污染物的面源污染问题更加凸显。因此,针对三峡库区及类似农业区域面临的农业复合污染物的面源污染问题,需要采取一系列综合措施来有效管理和控制氮素(N)及其他污染物的排放,以保障水体的生态环境安全。
| [1] |
Borrelli P, Robinson D A, Panagos P, et al. Land use and climate change impacts on global soil erosion by water(2015-2070)[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(36): 21994-22001.
(  0) 0) |
| [2] |
Zhang T, Hou Y, Meng T, et al. Replacing synthetic fertilizer by manure requires adjusted technology and incentives: A farm survey across China[J]. Resources, Conservation and Recycling, 2021, 168: 105301. DOI:10.1016/j.resconrec.2020.105301
(  0) 0) |
| [3] |
Zhang Y, Cheng D M, Xie J, et al. Impacts of farmland application of antibiotic—contaminated manures on the occurrence of antibiotic residues and antibiotic resistance genes in soil: A meta-analysis study[J]. Chemosphere, 2022, 300: 134529. DOI:10.1016/j.chemosphere.2022.134529
(  0) 0) |
| [4] |
Zhao F K, Chen L D, Yen H, et al. Multimedia mass balance approach to characterizing the transport potential of antibiotics in soil–plant systems following manure application[J]. Journal of Hazardous Materials, 2020, 393: 122363. DOI:10.1016/j.jhazmat.2020.122363
(  0) 0) |
| [5] |
Wang S, Feng X J, Wang Y D, et al. Characteristics of nitrogen loss in sloping farmland with purple soil in southWestern China during maize(Zea mays L.)growth stages[J]. Catena, 2019, 182: 104169. DOI:10.1016/j.catena.2019.104169
(  0) 0) |
| [6] |
Deng L Z, Sun T Y, Fei K, et al. Coupling loss characteristics of N-P-C through runoff and sediment in the hilly region of SE China under simulated rainfall[J]. Environmental Science and Pollution Research International, 2021, 28(28): 37204-37216. DOI:10.1007/s11356-021-13186-0
(  0) 0) |
| [7] |
Lyu J, Yang L S, Zhang L, et al. Antibiotics in soil and water in China–A systematic review and source analysis[J]. Environmental Pollution, 2020, 266: 115147. DOI:10.1016/j.envpol.2020.115147
(  0) 0) |
| [8] |
Holz M, Augustin J. Erosion effects on soil carbon and nitrogen dynamics on cultivated slopes: A meta-analysis[J]. Geoderma, 2021, 397: 115045. DOI:10.1016/j.geoderma.2021.115045
(  0) 0) |
| [9] |
Wang L H, Yen H, Wang X, et al. Deposition- and transport-dominated erosion regime effects on the loss of dissolved and sediment-bound organic carbon: Evaluation in a cultivated soil with laboratory rainfall simulations[J]. Science of the Total Environment, 2021, 750: 141717. DOI:10.1016/j.scitotenv.2020.141717
(  0) 0) |
| [10] |
Wu L, Yen H, Ma X Y. Effects of particulate fractions on critical slope and critical rainfall intensity for runoff phosphorus from bare loessial soil[J]. Catena, 2021, 196: 104935. DOI:10.1016/j.catena.2020.104935
(  0) 0) |
| [11] |
Kong Y L, Qin H, Zhu C Q, et al. Research progress on the mechanism by which soil microorganisms affect soil health (In Chinese)[J]. Acta Pedologica Sinica, 2024, 61(2): 331-347. [孔亚丽, 秦华, 朱春权, 等. 土壤微生物影响土壤健康的作用机制研究进展[J]. 土壤学报, 2024, 61(2): 331-347.]
(  0) 0) |
| [12] |
Qiu L P, Zhang Q, Zhu H S, et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality[J]. The ISME Journal, 2021, 15(8): 2474-2489. DOI:10.1038/s41396-021-00913-1
(  0) 0) |
| [13] |
Yang Q S, Yang W, Peng J, et al. Response of soil microbial diversity and community structure to erosion-deposition in slope farmland in typical black soil area of northeast China (In Chinese)[J]. Acta Pedologica Sinica, 2024, 61(6): 1741-1754. DOI:10.11766/trxb202308150325 [杨青松, 杨伟, 彭珏, 等. 典型黑土区坡耕地土壤微生物多样性及群落结构对侵蚀-沉积的响应[J]. 土壤学报, 2024, 61(6): 1741-1754.]
(  0) 0) |
| [14] |
Wang S P, Guo S F, Zhai L M, et al. Comprehensive effects of integrated management on reducing nitrogen and phosphorus loss under legume-rice rotations[J]. Journal of Cleaner Production, 2022, 361: 132031. DOI:10.1016/j.jclepro.2022.132031
(  0) 0) |
| [15] |
Zhang R J, Xu X M, Jia D T, et al. Sediments alleviate the inhibition effects of antibiotics on denitrification: Functional gene, microbial community, and antibiotic resistance gene analysis[J]. Science of the Total Environment, 2022, 804: 150092. DOI:10.1016/j.scitotenv.2021.150092
(  0) 0) |
| [16] |
Ghirardini A, Grillini V, Verlicchi P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure - Environmental risk due to antibiotics after application to soil[J]. Science of the Total Environment, 2020, 707: 136118. DOI:10.1016/j.scitotenv.2019.136118
(  0) 0) |
| [17] |
Shawver S, Wepking C, Ishii S, et al. Application of manure from cattle administered antibiotics has sustained multi-year impacts on soil resistome and microbial community structure[J]. Soil Biology and Biochemistry, 2021, 157: 108252. DOI:10.1016/j.soilbio.2021.108252
(  0) 0) |
| [18] |
Spielmeyer A, Höper H, Hamscher G. Long-term monitoring of sulfonamide leaching from manure amended soil into groundwater[J]. Chemosphere, 2017, 177: 232-238. DOI:10.1016/j.chemosphere.2017.03.020
(  0) 0) |
| [19] |
Álvarez-Esmorís C, Rodríguez-López L, Núñez-Delgado A, et al. Influence of pH on the adsorption-desorption of doxycycline, enrofloxacin, and sulfamethoxypyridazine in soils with variable surface charge[J]. Environmental Research, 2022, 214: 114071. DOI:10.1016/j.envres.2022.114071
(  0) 0) |
| [20] |
Zeng Q T, Sun J T, Zhu L Z. Occurrence and distribution of antibiotics and resistance genes in greenhouse and open-field agricultural soils in China[J]. Chemosphere, 2019, 224: 900-909. DOI:10.1016/j.chemosphere.2019.02.167
(  0) 0) |
| [21] |
Han Z, Zhong S Q, Ni J P, et al. Estimation of soil erosion to define the slope length of newly reconstructed gentle-slope lands in hilly mountainous regions[J]. Scientific Reports, 2019, 9(1): 4676. DOI:10.1038/s41598-019-41405-9
(  0) 0) |
| [22] |
Ma X, Li Y, Li B L, et al. Nitrogen and phosphorus losses by runoff erosion: Field data monitored under natural rainfall in Three Gorges Reservoir Area, China[J]. Catena, 2016, 147: 797-808. DOI:10.1016/j.catena.2016.09.004
(  0) 0) |
| [23] |
Bao S D. Soil and agricultural chemistry analysis (In Chinese). 3rd ed[M]. Beijing: China Agriculture Press, 2000. [鲍士旦. 土壤农化分析[M]. 3版. 北京: 中国农业出版社, 2000.]
(  0) 0) |
| [24] |
Liu Y J, Hu J M, Wang T W, et al. Effects of vegetation cover and road-concentrated flow on hillslope erosion in rainfall and scouring simulation tests in the Three Gorges Reservoir Area, China[J]. Catena, 2016, 136: 108-117. DOI:10.1016/j.catena.2015.06.006
(  0) 0) |
| [25] |
Wang Y, Zhang J H, Zhang Z H, et al. Impact of tillage erosion on water erosion in a hilly landscape[J]. Science of the Total Environment, 2016, 551: 522-532.
(  0) 0) |
| [26] |
Soil and Water Conservation Monitoring Center(SWCMC)of the Ministry of Water Resources of the People's Republic of China. Handbook of soil and water conservation monitoring for runoff plots and small watersheds, Edition No. 1. 3 . Beijing: China Water Power Press, 2015.
(  0) 0) |
| [27] |
Xu N, Tan G C, Wang H Y, et al. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure[J]. European Journal of Soil Biology, 2016, 74: 1-8. DOI:10.1016/j.ejsobi.2016.02.004
(  0) 0) |
| [28] |
Liu L, Ma Y L, Yue F X, et al. Effects of biochar on nitrogen transformation functional genes abundances, arbuscular mycorrhizal fungi and N2O emission of rainfed maize season in cinnamon soil (In Chinese)[J]. Acta Ecologica Sinica, 2021, 41(7): 2803-2815. [刘领, 马宜林, 悦飞雪, 等. 生物炭对褐土旱地玉米季氮转化功能基因、丛枝菌根真菌及N2O释放的影响[J]. 生态学报, 2021, 41(7): 2803-2815.]
(  0) 0) |
| [29] |
Shi Y L, Zhang Q W, Liu X R, et al. Organic manure input and straw cover improved the community structure of nitrogen cycle function microorganism driven by water erosion[J]. International Soil and Water Conservation Research, 2022, 10(1): 129-142. DOI:10.1016/j.iswcr.2021.03.005
(  0) 0) |
| [30] |
Du Y D, Cui B J, Zhang Q, et al. Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis[J]. Catena, 2020, 193: 104617. DOI:10.1016/j.catena.2020.104617
(  0) 0) |
| [31] |
Ostermann A, Mortimer P E, Huang R C, et al. Symbiotic nitrogen fixation in soil contaminated with the veterinary antibiotics oxytetracycline and sulfamethazine[J]. Journal of Environmental Quality, 2019, 48(4): 1067-1073. DOI:10.2134/jeq2019.01.0021
(  0) 0) |
| [32] |
Zhang B B, Yan S H, Li B, et al. Combining organic and chemical fertilizer plus water-saving system reduces environmental impacts and improves apple yield in rainfed apple orchards[J]. Agricultural Water Management, 2023, 288: 108482. DOI:10.1016/j.agwat.2023.108482
(  0) 0) |
| [33] |
Zhou X, Wang J, Lu C, et al. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment[J]. Chemosphere, 2020, 255: 127006. DOI:10.1016/j.chemosphere.2020.127006
(  0) 0) |
| [34] |
Wang Y T, Wang Y H, Shao T J, et al. Antibiotics and microplastics in manure and surrounding soil of farms in the Loess Plateau: Occurrence and correlation[J]. Journal of Hazardous Materials, 2024, 465: 133434. DOI:10.1016/j.jhazmat.2024.133434
(  0) 0) |
| [35] |
Pan M, Chu L M. Adsorption and degradation of five selected antibiotics in agricultural soil[J]. Science of the Total Environment, 2016, 545: 48-56.
(  0) 0) |
| [36] |
Pan M, Chu L M. Fate of antibiotics in soil and their uptake by edible crops[J]. Science of the Total Environment, 2017, 599: 500-512.
(  0) 0) |
| [37] |
Dolliver H, Gupta S. Antibiotic losses in leaching and surface runoff from manure-amended agricultural land[J]. Journal of Environmental Quality, 2008, 37(3): 1227-1237. DOI:10.2134/jeq2007.0392
(  0) 0) |
| [38] |
Lu J, Zheng F L, Li G F, et al. The effects of raindrop impact and runoff detachment on hillslope soil erosion and soil aggregate loss in the Mollisol region of Northeast China[J]. Soil and Tillage Research, 2016, 161: 79-85. DOI:10.1016/j.still.2016.04.002
(  0) 0) |
| [39] |
Zhi D, Yang D X, Zheng Y X, et al. Current progress in the adsorption, transport and biodegradation of antibiotics in soil[J]. Journal of Environmental Management, 2019, 251: 109598. DOI:10.1016/j.jenvman.2019.109598
(  0) 0) |
| [40] |
Joy S R, Bartelt-Hunt S L, Snow D D, et al. Fate and transport of antimicrobials and antimicrobial resistance genes in soil and runoff following land application of swine manure slurry[J]. Environmental Science & Technology, 2013, 47(21): 12081-12088.
(  0) 0) |
| [41] |
Lin Y X, Xu S, Li J. Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles[J]. Chemical Engineering Journal, 2013, 225: 679-685. DOI:10.1016/j.cej.2013.03.104
(  0) 0) |
| [42] |
Liu X W, Lü K, Deng C X, et al. Persistence and migration of tetracycline, sulfonamide, fluoroquinolone, and macrolide antibiotics in streams using a simulated hydrodynamic system[J]. Environmental Pollution, 2019, 252: 1532-1538. DOI:10.1016/j.envpol.2019.06.095
(  0) 0) |
| [43] |
Rico A, Dimitrov M R, Van Wijngaarden R P A, et al. Effects of the antibiotic enrofloxacin on the ecology of tropical eutrophic freshwater microcosms[J]. Aquatic Toxicology, 2014, 147: 92-104. DOI:10.1016/j.aquatox.2013.12.008
(  0) 0) |
| [44] |
Liu X Y, Zhang J Q, Huang W, et al. Occurrence characteristics and influencing factors of antibiotics in soils of China: A review (In Chinese)[J]. Soils, 2021, 53(4): 672-681. [刘欣雨, 张建强, 黄雯, 等. 中国土壤中抗生素赋存特征与影响因素研究进展[J]. 土壤, 2021, 53(4): 672-681.]
(  0) 0) |
| [45] |
Liu X Y, Gu X Y, Liu C, et al. Field migration of veterinary antibiotics via surface runoff from chicken-raising orchard in responding to natural rainfalls[J]. Science of the Total Environment, 2024, 909: 168527. DOI:10.1016/j.scitotenv.2023.168527
(  0) 0) |
| [46] |
Nightingale J, Carter L, Sinclair C J, et al. Influence of manure application method on veterinary medicine losses to water[J]. Journal of Environmental Management, 2023, 334: 117361. DOI:10.1016/j.jenvman.2023.117361
(  0) 0) |
| [47] |
Wang H, Chu Y X, Fang C R. Occurrence of veterinary antibiotics in swine manure from large-scale feedlots in Zhejiang Province, China[J]. Bulletin of Environmental Contamination and Toxicology, 2017, 98(4): 472-477. DOI:10.1007/s00128-017-2052-3
(  0) 0) |
| [48] |
Wang C, Mao Y J, Zhou W Q, et al. Inhomogeneous antibiotic distribution in sediment profiles in anthropogenically impacted lakes: Source apportionment, fate drivers, and risk assessment[J]. Journal of Environmental Management, 2023, 341: 118048. DOI:10.1016/j.jenvman.2023.118048
(  0) 0) |
| [49] |
Huang X Y, Chen C Y, Zeng Q Y, et al. Field study on loss of tetracycline antibiotics from manure-applied soil and their risk assessment in regional water environment of Guangzhou, China[J]. Science of the Total Environment, 2022, 827: 154273. DOI:10.1016/j.scitotenv.2022.154273
(  0) 0) |
| [50] |
Zhang Z B, Duan Y P, Zhang Z J, et al. Multimedia fate model and risk assessment of typical antibiotics in the integrated demonstration zone of the Yangtze River Delta, China[J]. Science of the Total Environment, 2022, 805: 150258. DOI:10.1016/j.scitotenv.2021.150258
(  0) 0) |
| [51] |
Ling D, Li T, Zhang S R, et al. Effects of exogenetic oxytetracycline and sulfamethazine on soil labile organic carbon contents (In Chinese)[J]. Journal of Agro-Environment Science, 2015, 34(2): 297-302. [凌德, 李婷, 张世熔, 等. 外源土霉素和磺胺二甲嘧啶对土壤活性有机碳含量的影响[J]. 农业环境科学学报, 2015, 34(2): 297-302.]
(  0) 0) |
| [52] |
Guo X Y, Feng C H, Gu E X, et al. Spatial distribution, source apportionment and risk assessment of antibiotics in the surface water and sediments of the Yangtze Estuary[J]. Science of the Total Environment, 2019, 671: 548-557. DOI:10.1016/j.scitotenv.2019.03.393
(  0) 0) |
| [53] |
Liu X H, Zhang H B, Luo Y M, et al. Sorption of oxytetracycline in particulate organic matter in soils and sediments: Roles of pH, ionic strength and temperature[J]. Science of the Total Environment, 2020, 714: 136628. DOI:10.1016/j.scitotenv.2020.136628
(  0) 0) |
| [54] |
Chen J G, Zhu B, Zhang Y H. A meta-analysis on the responses of soil microbial biomass and community structure to antibiotics[J]. Applied Soil Ecology, 2023, 184: 104786. DOI:10.1016/j.apsoil.2022.104786
(  0) 0) |
| [55] |
Langbehn R K, Michels C, Soares H M. Tetracyclines lead to ammonium accumulation during nitrification process[J]. Journal of Environmental Science and Health, 2020, 55(8): 1021-1031. DOI:10.1080/10934529.2020.1765642
(  0) 0) |
| [56] |
Yin G Y, Hou L J, Liu M, et al. Effects of multiple antibiotics exposure on denitrification process in the Yangtze Estuary sediments[J]. Chemosphere, 2017, 171: 118-125. DOI:10.1016/j.chemosphere.2016.12.068
(  0) 0) |
| [57] |
Chen H, Liu S, Xu X R, et al. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure[J]. Marine Pollution Bulletin, 2015, 90(1/2): 181-187.
(  0) 0) |
| [58] |
Lin Y B, Qin Y Q, Ma S Y, et al. Temporal and spatial variation and environmental response of denitrification and anaerobic ammonium oxidation rates in mangrove sediments (In Chinese)[J]. Journal of Tropical Biology, 2023, 14(1): 129-135. [林雨彬, 秦永强, 马思远, 等. 红树林沉积物反硝化和厌氧氨氧化速率的时空变化及环境响应[J]. 热带生物学报, 2023, 14(1): 129-135.]
(  0) 0) |
| [59] |
Jin Z F, Gong J L, Shi Y L, et al. Nitrate source identification and nitrification-denitrification at the sediment-water interface (In Chinese)[J]. Environmental Science, 2017, 38(4): 1423-1430. [金赞芳, 龚嘉临, 施伊丽, 等. 沉积物-水界面氮的源解析和硝化反硝化[J]. 环境科学, 2017, 38(4): 1423-1430.]
(  0) 0) |
| [60] |
Shao F F, Wu J H, Li Y C. Establishment and validation of the model for prediction of nutrient loss with runoff on bare slopes in the Loess Plateau (In Chinese)[J]. Acta Pedologica Sinica, 2021, 58(4): 900-910. DOI:10.11766/trxb202002240069 [邵凡凡, 吴军虎, 李玉晨. 黄土区裸露坡地径流养分流失模型的建立与验证[J]. 土壤学报, 2021, 58(4): 900-910.]
(  0) 0) |
| [61] |
Liu L, Cui J F, Yang H W, et al. Effects of exogenous organic materials on soil nutrients and organic carbon components in the hilly region of central Sichuan, China (In Chinese)[J]. Soils, 2023, 55(5): 991-1000. [刘玲, 崔俊芳, 杨红薇, 等. 长期外源有机物料添加对川中丘陵区农田土壤养分和有机碳组分的影响[J]. 土壤, 2023, 55(5): 991-1000.]
(  0) 0) |
 2025, Vol. 62
2025, Vol. 62


