盐渍土是一系列受盐碱成分作用的,包括不同程度盐化和碱化的各种类型土壤的统称,也称为盐碱土[1]。土壤盐渍化因其高盐渗透作用,腐蚀植被根系,影响农作物吸收水分,还会导致土壤理化性质恶化,影响农作物生长,是目前农业生产以及生态环境面临的世界性难题之一[2]。目前,盐碱地改良方法主要有物理改良、化学改良和生物改良[3]。虽然在盐碱土改良方面,物理和化学手段已经取得了很大的成就,但其工作量大、成本高等缺陷也不容忽视[4],而生物改良技术因具有高效、环保、经济效益高等优点[5],近年来得到了广泛关注。
已有相关研究利用微藻或细菌来改良盐渍化土壤,结果表明,微藻和细菌可通过产生胞外聚合物与盐离子螯合[6],从而有效降低土壤盐分。利用微生物改良盐渍化土壤虽然是一种绿色可持续的有效方法,但单一施用微藻或细菌对土壤的改良效果有限[7],此外,微藻在农业上的应用还具有生长周期长、生产效率低等问题[8]。自然环境中藻菌之间存在直接或间接的相互作用,对藻菌的逆境抵御和生理功能等方面具有重要影响,因而微藻-细菌共生在环境修复中的应用往往优于单一微生物的应用。周璇等[9]发现某些产VB12细菌在与莱茵衣藻(Chlamydomonas reinhardtii)共生时,可以显著地增强衣藻的耐热能力。张波等[10]探究藻菌共生体系对造纸废水的处理效果时发现,水单胞菌(Aquimonas sp.)与棕鞭藻(Ochromonas sp.)藻菌共生体系对造纸废水中化学需氧量(chemical oxygen demand,COD)、总氮量(total nitrogen,TN)、总磷量(total phosphorus,TP)及色度的去除效果显著优于纯藻和纯菌处理体系,去除率最高分别达75.67%、35.19%、90.2% 和93.45%。张慧洁等[11]研究发现相较于单一微藻或细菌,巨大芽孢杆菌(Bacillus megaterium)和小球藻(Chlorella vulgaris)配施处理更有利于改善土壤微生物群落结构,刺激土壤微生物活性提高土壤磷有效性。由此可见,微生物之间互惠互利的共生关系可以进一步增强各自的能力,尽管如此,国内外的研究大多局限于单一微藻或细菌,利用藻菌联合作用改良盐渍化土壤的相关研究较少。
因此,本研究从华北平原盐渍化土壤中分离筛选得到耐盐藻菌组合,利用液相实验,分析其对盐胁迫的响应。通过向盐渍化土壤表层接种耐盐藻菌组合,进一步探究藻菌联合对盐渍化土壤的生物改良作用。研究结果将为盐渍化土壤治理和修复提供新的改良途径和理论依据。
1 材料与方法 1.1 盐渍化土壤藻株和菌株的分离与培养从河北省沧州市(37°49′~38°10′N,116°32′~117°01′E)盐渍化土壤表面采集样品,将10 g土壤样品置于灭菌后的锥形瓶中,加入BG-11液体培养基,置于24 ℃、光照强度为8 000 Lux、光/暗比为16/8的光照培养箱中富集培养[12]。待土壤样品表面明显变绿后,用平板稀释涂布法将藻液涂布至固体BG-11培养基上进行分离、纯化,得到单种藻细胞。继续用液体BG-11培养基扩大培养,显微观察藻细胞形态,得到了5株纯种的藻体。镜检显示藻1为真核藻类,其余4种藻株均为原核藻类。
称取土壤样品10 g,加入无菌水,制成10–1、10–2、10–3和10–4的土壤样品稀释液。分别吸取200 μL的稀释液加入无菌LB平板中,涂布均匀后倒置于37 ℃的培养箱。待长出菌落后,挑取单菌落接种到新的培养基中培养,待菌苔长出后,检查其形态特征是否一致,若发现有杂菌,则再进行一次分离和纯化[13]。经过3次的分离和纯化后,获得了10种菌株。
1.2 藻株和菌株耐盐预筛选及耐盐藻菌组合的筛选(1)藻株的耐盐筛选。将从盐渍化土壤中分离得到的5种藻株的富集培养液经离心收集后,接种至NaCl浓度为5 g·L–1的BG-11液体培养基中培养7 d。以OD680 nm表征藻株的生物量[14],通过比较盐胁迫下5种藻株的生物量及比生长率变化,筛选出藻1、藻2和藻5作为耐盐藻株材料。
(2)菌株的耐盐筛选。将盐渍化土壤中分离得到的10种菌株经富集培养后离心收集,接种至NaCl浓度为20 g·L–1的BG-11液体培养基中培养7 d。以OD600 nm表征菌株的生物量[15],通过比较盐胁迫下10种菌株的生物量及比生长率变化,筛选出菌1、菌5、菌6和菌8作为耐盐菌株材料。
(3)耐盐藻菌组合的筛选。耐盐藻株和耐盐菌株分别组成藻菌组合,接种至BG-11液体培养基中培养7 d。采用丙酮提取—紫外分光光度法测定藻株光合色素含量:取培养液5 mL,离心后去上清,加入90%的丙酮进行提取,测定663 nm、645 nm和450 nm下的OD值:
| $\text{叶绿素a含量}^{[16]}\left(\mathrm{mg} \cdot \mathrm{L}^{-1}\right)=\left(12.72 \times \mathrm{OD}_{663 \mathrm{~nm}}-2.7 \times \mathrm{OD}_{645 \mathrm{~nm}}\right) \times V_t / V $ | (1) |
| $\text{类胡萝卜素含量}^{[17]}\left(\mathrm{mg} \cdot \mathrm{L}^{-1}\right)=4.1 \times \mathrm{OD}_{450 \mathrm{~nm}}-0.0435 \times\text{叶绿素a含量} $ | (2) |
式中,Vt为提取后定容的体积,V为提取所用的藻液体积。
通过比较各藻菌组合中藻株的光合色素含量变化,分别筛选与藻1、藻2和藻5联合耐盐能力最强的菌株,组成耐盐藻菌组合。
1.3 藻菌组合对盐胁迫的响应将3种耐盐藻菌组合接种至盐浓度为0、5、10、15和20 g·L–1的BG-11液体培养基中培养10 d。采用离心法分级提取胞外聚合物(EPS)。取5 mL培养液,10 000 r·min–1离心15 min,用0.45 μm微孔滤膜抽滤得到滤液,滤液通过苯酚-硫酸法测定EPS含量[18]。用多参数水质分析仪(YSI Pro Professional Plus,USA)测定培养液的盐度。
1.4 藻菌组合对盐渍化土壤的改良作用盐渍化土壤样品自然风干后,过0.78 mm筛网去除枯枝落叶及大颗粒杂质备用。设置空白对照组、微藻、细菌和藻菌组合处理组。定时用等量去离子水灌溉土壤使土壤含水率保持在40%左右,培养30 d后,测定土壤理化性质。采用碱性过硫酸钾氧化—紫外分光光度法[19]测定土壤总氮含量,苯酚-硫酸法测定土壤EPS含量。土壤样品烘干后按照土︰水=1︰5水土浸出比,制备土壤浸出液,用多参数水质分析仪测定土壤浸出液盐度和pH。
1.5 藻株和菌株基因组DNA的提取与鉴定采用SDS碱法提取藻株基因组DNA[20];使用碱-SDS-煮沸法提取菌株基因组DNA[21]。藻1引物序列为真核生物通用引物:18S-F(5′ACCTGG TTGATCCTGCCAGT3′)和18S-R(5′TCACCTA CGGAAACCTTGT3′)[20];菌8引物序列为细菌通用引物:27F(5′AGAGTTTGATCCTGGCTCA3′)和1492R(5′GGTTACCTTGTTACGACTT3′)[22]。由生工生物技术有限公司合成。PCR反应体系为50 μL,其中含有引物(20 pmol)各1 μL,基因组DNA 1 μL,10×PCR Buffer 5 μL,dNTP(MgCl2)4 μL,Taq酶0.3 μL,其余以ddH2O补足。反应条件为:95 ℃、5 min;30个循环:94 ℃、30 s,50 ℃、1 min,72 ℃、1 min30 s;72 ℃延伸、10 min;4 ℃保存。对扩增产物用2%的琼脂糖凝胶电泳检测,在凝胶成像仪(培清JS-680B,上海)上观察,检测目的条带,并将目的产物送生工公司测序。利用BLAST在线工具与NCBI数据库的模式物种序列进行同源性比对,下载模式物种序列,采用MEGA(MEGA.11,Kumar Lab)软件,利用CLUSTALW对所有序列进行多序列的比对,再用系统邻接法(Neighbor-joining,NJ),设置100次的Bootstrap检验各分支的置信度,其他参数均为默认值,构建系统进化树,对藻株和菌株进行鉴定。
1.6 数据分析数据采用均值±标准偏差表示,采用SPSS 26(IBM Inc.,Chicago,IL,美国)进行独立样本T检验、方差齐性检验和单因素方差分析(ANOVA),P < 0.05被认为具有统计学意义,使用Origin 2021(Origin Lab Inc.,Northampton,MA,美国)作图。
2 结果 2.1 耐盐藻菌组合的筛选为了初步筛选最优的耐盐组合,对不同藻菌组合的微藻光合色素含量进行测定。结果表明,在不同组合藻1的叶绿素a和类胡萝卜素含量呈现出不同的变化趋势,藻1菌1组合中藻1的光合色素含量呈波动状态,藻1菌5、藻1菌6和藻1菌8组合中藻1的光合色素含量均呈上升趋势,其中藻1菌8组合的光合色素含量增势最为明显,叶绿素a和类胡萝卜素含量分别增长了15.54倍和8.65倍(图 1a和图 1d)。藻2也呈现出不同的变化趋势,藻2菌5、藻2菌6、藻2菌8组合光合色素含量均呈波动状态,藻2菌1组合光合色素含量增长稳定,叶绿素a和类胡萝卜素含量分别增长了1.43倍和1.13倍(图 1b和图 1e)。藻5在4种组合的叶绿素a含量均稳定上升,藻5菌1、藻5菌5、藻5菌6组合类胡萝卜素含量增长趋势较为波动,其中藻5菌8组合光合色素含量增长稳定,叶绿素a和类胡萝卜素含量分别增长了10.83倍和2.75倍(图 1c和图 1f)。综上,共筛选得到藻1菌8、藻2菌1和藻5菌8三种藻菌组合。
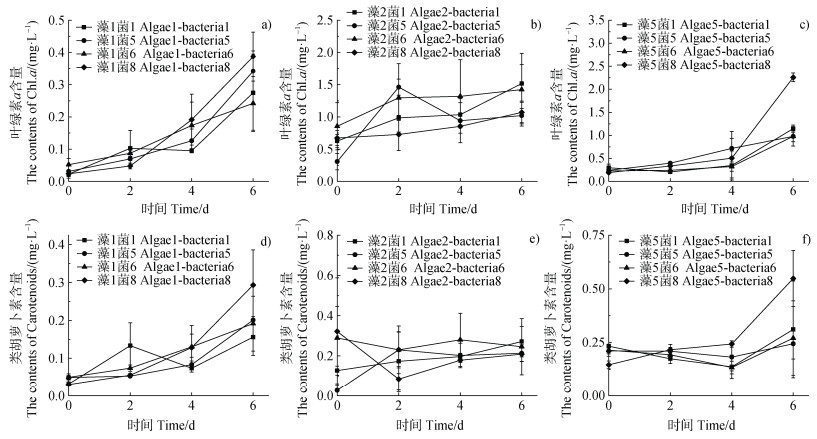
|
注:a),b)和c)分别为藻1,2和5与菌1,5,6和8组合的叶绿素a含量;d),e)和f)分别为藻1,2和5与菌1,5,6和8组合的类胡萝卜素含量。 Note: a), b)and c)represent Chl.a contents of algae 1, 2 and 5 with bacteria 1, 5, 6 and 8, respectively; d), e)and f)represent carotenoids contents of algae 1, 2 and 5 with bacteria 1, 5, 6 and 8, respectively. 图 1 藻菌组合光合色素的含量 Fig. 1 The contents of photosynthetic pigments in different algae-bacterial consortia |
为了探究藻菌联合对盐胁迫的响应,对各盐浓度下不同藻菌组合EPS分泌量进行测定。结果表明,在不同浓度盐胁迫下,各处理组EPS分泌量呈现不同的变化。藻1在20 g·L–1 NaCl处理下EPS分泌量显著增加,增加了98.46%(P < 0.05,图 2a);菌8在15 g·L–1 NaCl处理下EPS分泌量显著增加,增加了24.73%(P < 0.05,图 2b);藻1菌8组合在15和20 g·L–1 NaCl处理下EPS分泌量均显著增加,分别增加了44.56%和43.19%(P < 0.05,图 2c)。在各浓度盐胁迫下,藻2和菌1的EPS分泌量均显著增加(P < 0.05,图 2d和图 2e);在5和10 g·L–1 NaCl处理下,藻2菌1组合EPS分泌量显著增加,而在高盐处理下其EPS分泌量无显著变化(P < 0.05,图 2f)。藻5在5和10 g·L–1 NaCl处理下EPS分泌量显著增加,分别增加了25.68%、26.87%(P < 0.05,图 2g);而菌8及藻5菌8处理组均出现了EPS分泌量显著下降的情况(P < 0.05,图 2h和图 2i)。
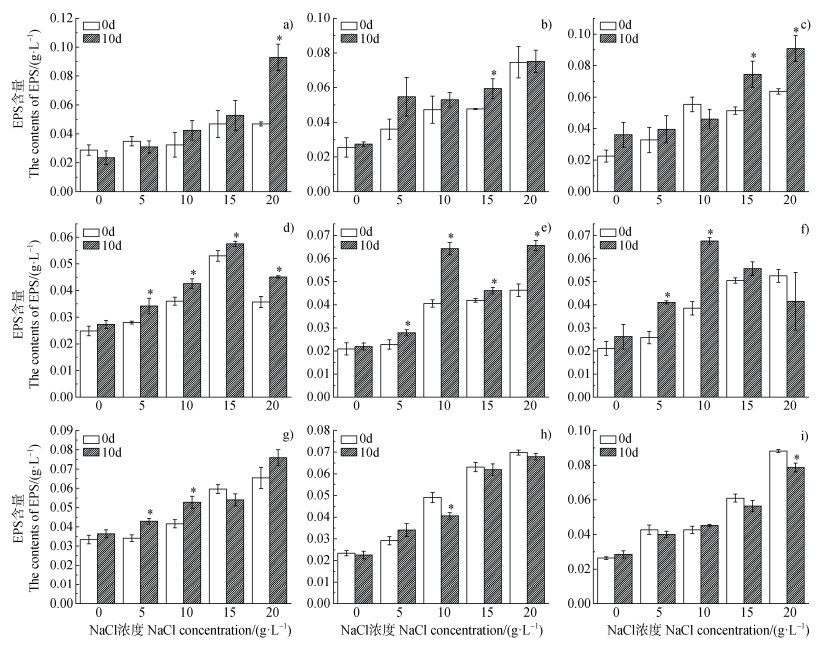
|
注:a),b)和c)分别表示藻1,菌8和藻1菌8联合处理;d),e)和f)分别表示藻2,菌1和藻2菌1联合处理;g),h)和i)分别表示藻5,菌8和藻5菌8联合处理;* 表示P < 0.05。 Note:a),b)and c)represent the treatment of algae-1,bacteria-8 and algae1-bacteria8 consortium,respectively;d),e)and f)represent the treatment of algae-2,bacteria-1 and algae2-bacteria1 consortium,respectively;g),h)and i)represent the treatment of algae-5,bacteria-8 and algae5-bacteria8 consortium,respectively;* represents P < 0.05. 图 2 培养液中微生物分泌的胞外聚合物(EPS)的变化 Fig. 2 Changes of EPS secreted by microorganisms in culture medium |
图 3显示,在不同盐浓度梯度下,各处理组的可溶性盐含量均显著下降(P < 0.05)。在5、10、15和20 g·L–1 NaCl浓度下,藻菌联合及藻处理组可溶性盐含量均显著低于菌处理组(P < 0.05,图 3)。此外,在藻1菌8处理中,藻菌联合处理组在20 g·L–1 NaCl浓度下可溶性盐含量显著低于藻处理组(P < 0.05,图 3a)。各藻菌联合处理组在各盐度下的去盐结果表明,在10 g·L–1 NaCl浓度下,藻1菌8和藻2菌1联合处理组的去盐率均显著高于藻5菌8处理组,去盐率分别为57.57%和57.29%(P < 0.05,图 4);在15 g·L–1 NaCl浓度下,藻1菌8联合处理组的去盐率显著高于藻2菌1和藻5菌8处理组,去盐率达57.89%(P < 0.05,图 4);在20 g·L–1 NaCl浓度下,藻1菌8和藻5菌8联合处理组的去盐率均显著高于藻2菌1处理组,去盐率分别为57.55%、57.79%(P < 0.05,图 4)。由此,藻1菌8组合在高盐环境中表现出稳定高效的去盐能力,筛选其开展后续的试验。
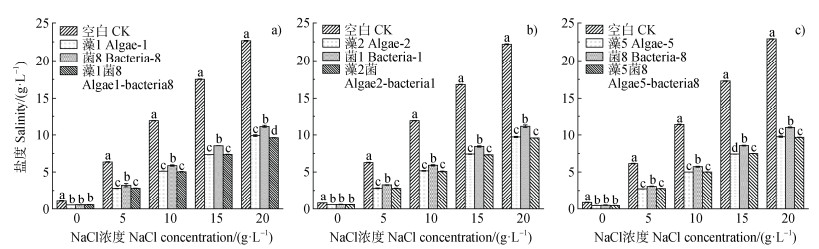
|
注:a)表示藻1,菌8和藻1菌8联合处理;b)表示藻2,菌1和藻2菌1联合处理;c)表示藻5,菌8和藻5菌8联合处理;不同字母表示不同处理间的显著性差异(P < 0.05)。Note:a)represents the treatment of algae-1,bacteria-8 and algae1-bacteria8 consortium,respectively;b)represents the treatment of algae-2,bacteria-1 and algae2-bacteria1 consortium,respectively;c)represents the treatment of algae-5,bacteria-8 and algae5-bacteria8 consortium,respectively;different letters represent significant differences between treatments at the 0.05 level. 图 3 不同藻菌处理对液态环境中可溶性盐含量的影响 Fig. 3 Effects of different algal and bacterial treatments on soluble salt content in liquid environment |
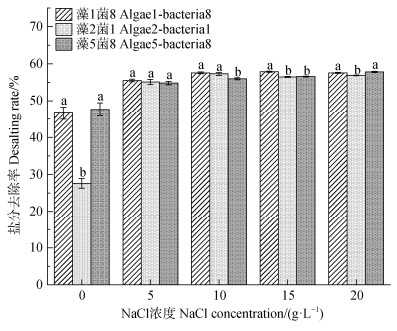
|
注:不同字母表示不同处理间的显著性差异(P < 0.05)。 Note: different letters represent significant differences between treatments at the 0.05 level. 图 4 不同藻菌组合盐分去除率的比较 Fig. 4 Comparison of desalting rate at different algae-bacterial consortia |
通过测序比对,结果表明,藻1与Borodinellopsis sp. QY-2024a(PP544782.1)高度相似,序列相似性可达99.94%,结合其形态学特性,鉴定其属于绿球藻目中的Borodinellopsis sp.[23](图 5a和图 5b)。菌8与Bacillus sp. hb11(KF863811.1)高度相似,序列相似性可达99.92%,结合其形态学特性,鉴定其属于芽孢杆菌属(Bacillus)(图 5c和图 5d)。
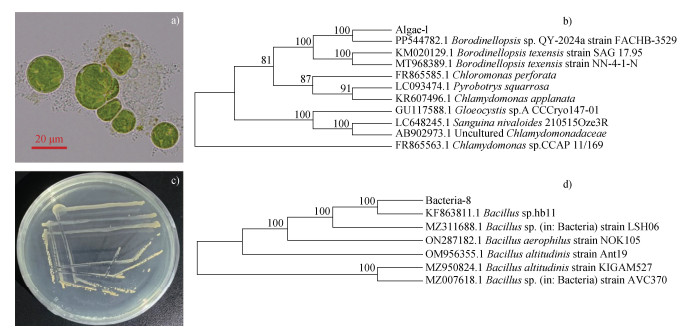
|
注:a)表示藻1显微镜检照片;b)表示藻1的系统进化树;c)表示菌8的形态特征;d)表示菌8的系统进化树;各分支上的数值为Bootstrap值。Note:a)represents the microscopic image of algae-1;b)represents the phylogenetic tree of algae-1;c)represents the morphological characteristics of bacteria-8;d)represents the phylogenetic tree of bacteria-8;the values on each branch represent Bootstrap values. 图 5 藻1和菌8形态及系统邻接进化树 Fig. 5 Morphology and neighbor-joining phylogenetic tree of algae-1 and bacteria-8 |
将藻1、菌8及藻1菌8组合接种至盐渍化土壤中,探讨其对土壤理化性质的影响。结果显示,单独接种菌8的土壤pH为7.44,显著低于初始值和空白组,其他处理组均无显著变化(P < 0.05,图 6a)。藻1、菌8和藻1菌8处理组土壤浸出液盐度较空白均显著下降,分别降低了5.10%、3.45%和7.00%,其中藻1菌8的土壤浸出液盐度显著低于单独接种藻或菌的土壤(P < 0.05,图 6b)。藻1、菌8及藻菌联合处理组土壤EPS含量均显著增加,分别增加了51.72%、8.20%和185.88%(P < 0.05,图 6c)。藻菌联合处理组土壤中的全氮含量显著高于空白含量,提高了55.33%,而接种单一藻或菌的土壤全氮含量无显著变化(P < 0.05,图 6d)。
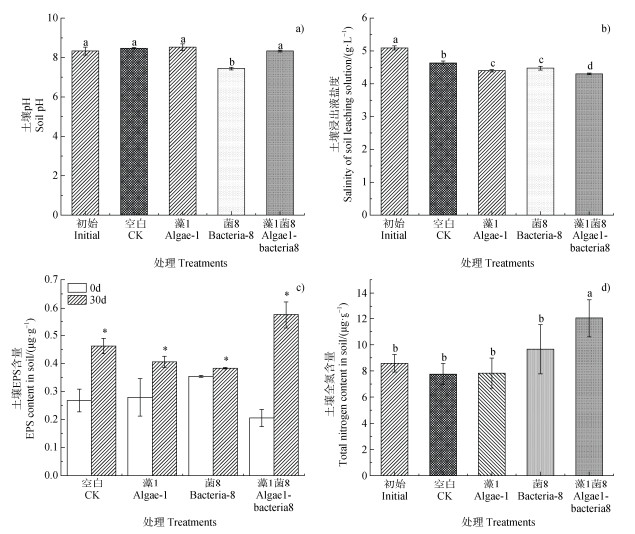
|
注:不同字母表示不同处理间的显著性差异(P < 0.05),* 表示P < 0.05。Note:different letters represent significant difference between treatments at the 0.05 level,* represents P < 0.05. 图 6 藻1、菌8及联合作用下土壤理化性质的变化 Fig. 6 Changes of soil physicochemical properties at treatments of algae-1, bacteria-8, and their consortium |
盐胁迫下微藻和细菌分泌的EPS,一方面可作为渗透保护屏障,保护细胞免受外界高渗环境的影响[24],提高其对高盐环境的适应能力;另一方面,其能与盐离子发生反应,降低液态环境中盐分含量[25]。本研究发现,在高盐环境中,微藻和细菌能分泌较高浓度的EPS以降低盐分的胁迫,这与段会杰[7]的研究结果一致。此外,本研究还发现,藻1菌8组合在20 g·L–1 NaCl浓度下的去盐效果优于单独的藻或菌。该结果支持了先前的研究,如:Gu等[26]发现藻类可通过光合作用为细菌提供额外的有机碳源、生长因子、O2等,促进其生长;张哲[27]发现细菌呼吸以及将大分子有机物水解产生的CO2能供微藻进行光合作用,在盐胁迫下有效促进微藻的生长。这表明藻1菌8联合,可能通过调节有机碳源和光合作用来提高二者在高盐环境中的活性。
3.2 藻菌联合对盐渍化土壤理化性质的影响土壤pH直接关系到土壤肥力,进而影响作物生长。适量的碱性可以提高土壤中的有效养分含量,有利于作物的生长和发育[28],然而过高的碱性也可能导致土壤中的某些营养元素变得不可利用,从而限制作物的生长[29]。本研究发现单独接种菌8的土壤pH显著降低,这支持了唐岷宸等[30]的研究。他们发现某些芽孢杆菌能够通过代谢产生多种有机酸如乙酸、草酸等,由此,推测有机酸的分泌可能是菌8降低土壤pH的原因之一。同时,本研究表明单独接种藻1土壤pH无显著变化,这与崔丽洋等[25]的研究结果一致,说明单独应用微藻对土壤酸碱性的改良效果不明显。尽管多项研究表明藻菌联合应用会使土壤pH升高[7-8],但本研究发现接种藻菌组合的土壤pH无显著变化,这表明本研究筛选得到的藻菌组合不会加重盐渍化土壤的碱性,使得利用其改良盐渍化土壤更具可行性。
土壤盐度过高会限制植物的吸水和蒸腾,降低土壤肥力,导致农作物减产[31]。结果表明,藻1、菌8和藻1菌8联合均能够降低土壤盐度,起到改善土壤性质的作用,并且藻菌联合对盐渍化土壤的改良效果优于单独应用藻或菌的效果。Arp等[6]同样发现微藻和细菌可以通过直接吸收或分泌EPS等物质固定土壤中的盐分,从而降低土壤盐度,此外,微藻和细菌还能分泌促生激素[7]并为彼此提供营养物质[32]。因此在藻菌联合应用中,二者间的相互作用可以促进彼此的生长及生物活性,提高微藻和细菌的降盐能力。
微生物产生的EPS与土壤结皮、土壤团聚体稳定性等性质密切相关[33]。Mishra和Jha[34]证实,EPS中的部分官能团如-OH、-COOH、-NH2和-C=O等能络合盐离子,一定程度上能够限制土壤中盐分的迁移,减少盐分对微生物和植物的影响。此外,EPS能够通过静电引力和范德华力包被在土壤颗粒外表,通过增加土壤颗粒的黏结性来促进团聚体的形成[35],土壤团聚体的形成和稳定可以进一步促进土壤孔隙结构的发育,改善土壤结构[36],增加土壤有机质含量,提高土壤肥力[37]。在本研究结果中,藻1、菌8及藻菌联合处理组土壤EPS含量均显著增加,表明接种微藻或细菌能够起到改善土壤性质的作用。
盐渍化土壤中氮含量较低[7],不利于植物的吸收和利用。本研究发现,接种单一藻或菌的土壤全氮含量无明显变化,而接种藻菌组合的土壤全氮含量显著升高。吴志宇[38]、胡应平[39]等研究表明,某些芽孢杆菌具有一定的固氮能力,由此,推测在藻菌联合应用中,藻1和菌8间的相互作用提高了菌8的固氮能力,从而增加土壤含氮量,改善土壤肥力。
4 结论从华北地区盐渍化土壤中分离纯化得到5种藻株和10种菌株,并进行耐盐初筛及共生能力复筛,再通过探究藻菌组合对盐胁迫的生理生化响应进一步筛选出藻1菌8为最优组合。经鉴定,藻1为Borodinellopsis sp.,菌8为芽孢杆菌属(Bacillus)。在盐胁迫下,Borodinellopsis、芽孢杆菌及藻菌组合均表现出较强的耐盐能力及促生物质分泌能力。高盐环境中,藻菌联合相较于单独的藻或菌,对溶液中可溶性盐的去除效果更佳。此外,藻菌组合在高盐胁迫下胞外聚合物分泌量显著增加,其抵抗盐胁迫的能力较强。将Borodinellopsis、芽孢杆菌及藻菌组合分别接种于盐渍化土壤表面,结果发现,藻菌联合可有效降低土壤盐度,增加土壤胞外聚合物含量,提高土壤全氮含量,且相较于单一应用微藻或细菌改良,藻菌联合对盐渍化土壤的改良效果更加明显。
| [1] |
Wang Z Q. Salt-affected soils of China (In Chinese). Beijing: Science Press, 1993. [王遵亲. 中国盐渍土[M]. 北京: 科学出版社, 1993.]
(  0) 0) |
| [2] |
Chen L, Amina H J, Sun Y Y. Study on the present situation of soil salinization and its rapid detection method (In Chinese)[J]. Xinjiang Youse Jinshu, 2024, 47(3): 90-91. [陈林, 阿米娜·胡吉, 孙阳阳. 土壤盐渍化现状和快速检测方法研究[J]. 新疆有色金属, 2024, 47(3): 90-91.]
(  0) 0) |
| [3] |
Gu W T, Dong X C, Li W J, et al. Research progress in amelioration of saline-alkali soil (In Chinese)[J]. Journal of Anhui Agricultural Sciences, 2014, 42(6): 1620-1623. [顾文婷, 董喜存, 李文建, 等. 盐渍化土壤改良的研究进展[J]. 安徽农业科学, 2014, 42(6): 1620-1623.]
(  0) 0) |
| [4] |
Tejada M, Garcia C, Gonzalez J L, et al. Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil[J]. Soil Biology and Biochemistry, 2006, 38(6): 1413-1421. DOI:10.1016/j.soilbio.2005.10.017
(  0) 0) |
| [5] |
Li M, Sun Z J, Chen W M, et al. Summary of microbial community and the effects of the biological amelioration on saline soil in Ningxia (In Chinese)[J]. Journal of Anhui Agricultural Sciences, 2014, 42(4): 1042—1044, 1046. [李明, 孙兆军, 陈卫民, 等. 宁夏地区盐渍化土壤微生物群落的研究现状及生物改良对其的影响[J]. 安徽农业科学, 2014, 42(4): 1042—1044, 1046.]
(  0) 0) |
| [6] |
Arp G, Reimer A, Reitner J. Calcification in cyanobacterial biofilms of alkaline salt lakes[J]. European Journal of Phycology, 1999, 34(4): 393-403. DOI:10.1080/09670269910001736452
(  0) 0) |
| [7] |
Duan H J. Effect and mechanism of bacteria-microalgae collaboration on the improvement of the Yellow River Delta soil[D]. Jinan: Qilu University of Technology, 2023.[段会杰. 菌藻协同对黄河三角洲滨海盐碱土壤改良效果及机理研究[D]. 济南: 齐鲁工业大学, 2023.]
(  0) 0) |
| [8] |
Yu X Y. Culture of Chlorella pyrenoidosa based on symbiosis of algae and bacteria and its application in improving continuous cropping soil obstacles[D]. Shijiazhuang: Hebei University of Science and Technology, 2022.[于枭燕. 基于藻菌共生的蛋白核小球藻培养及其在连作土壤障碍改良上的应用[D]. 石家庄: 河北科技大学, 2022.]
(  0) 0) |
| [9] |
Zhou X, Li J Y, Wu G, et al. The roles of vitamin B12 in the interaction between bacteria and eukaryotic algae (In Chinese)[J]. Microbiology China, 2015, 42(5): 913-919. [周璇, 李军英, 伍刚, 等. 维生素B12在细菌与真核藻类相互作用中的功能研究进展[J]. 微生物学通报, 2015, 42(5): 913-919.]
(  0) 0) |
| [10] |
Zhang B, Chen J C, Cui M Y, et al. Construction of microalgae-bacteria symbiosis system and the treatment effect on papermaking wastewater (In Chinese)[J]. Science Technology and Engineering, 2021, 21(3): 1211-1216. [张波, 陈佳琛, 崔梦瑶, 等. 藻菌共生体系的建立及其对造纸废水的处理效果[J]. 科学技术与工程, 2021, 21(3): 1211-1216.]
(  0) 0) |
| [11] |
Zhang H J, Liu J Z, Wu Y H. Effects of combined application of algae and bacteria on paddy soil phosphorus availability and microbial community (In Chinese)[J]. Acta Pedologica Sinica, 2022, 59(5): 1369-1377. DOI:10.11766/trxb202103240159 [张慧洁, 刘俊琢, 吴永红. 藻、菌配合施用对水稻土磷有效性及微生物群落的影响[J]. 土壤学报, 2022, 59(5): 1369-1377.]
(  0) 0) |
| [12] |
Zhang C, Yu X W, Liu W J, et al. Study on screening of a salt-tolerant soil microalgae and its physiological responses under salt stress (In Chinese)[J]. Journal of Shaanxi University of Science & Technology, 2022, 40(3): 27-32. [张超, 喻先伟, 刘文建, 等. 一株耐盐土壤微藻的筛选及其耐盐特性研究[J]. 陕西科技大学学报, 2022, 40(3): 27-32.]
(  0) 0) |
| [13] |
Wu Q F, Fu L, Lu Z F. Purification and molecular identification experiments of microbe in soil (In Chinese)[J]. Journal of Anyang Institute of Technology, 2016, 15(4): 27-29. [吴秋芳, 付亮, 路志芳. 土壤微生物分离纯化和分子鉴定实验研究[J]. 安阳工学院学报, 2016, 15(4): 27-29.]
(  0) 0) |
| [14] |
Prajapati S K, Kumar P, Malik A, et al. Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: A closed loop bioenergy generation process[J]. Bioresource Technology, 2014, 158: 174-180. DOI:10.1016/j.biortech.2014.02.023
(  0) 0) |
| [15] |
Liu G H, Lin Y Z, Liu B, et al. Study on rapid detection method of genus Bacillus cell concentration (In Chinese)[J]. Chinese Agricultural Science Bulletin, 2012, 28(24): 213-217. [刘国红, 林营志, 刘波, 等. 芽胞杆菌菌体浓度的快速检测方法研究[J]. 中国农学通报, 2012, 28(24): 213-217.]
(  0) 0) |
| [16] |
Nusch E A. Comparison of different methods for chlorophyll and phaeopigment determination[J]. Archive für Hydrobiologie-Beiheft Ergebnisse der Limnologie, 1980, 14: 14-36.
(  0) 0) |
| [17] |
Du Y M, Zhang H B, Wang X L, et al. Determination of total carotenoids in tobacco by photometer (In Chinese)[J]. Chinese Tobacco Science, 2003, 24(3): 28-29. [杜咏梅, 张怀宝, 王晓玲, 等. 光度法测定烟草中总类胡萝卜素的方法研究[J]. 中国烟草科学, 2003, 24(3): 28-29.]
(  0) 0) |
| [18] |
Masuko T, Minami A, Iwasaki N, et al. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format[J]. Analytical Biochemistry, 2005, 339(1): 69-72. DOI:10.1016/j.ab.2004.12.001
(  0) 0) |
| [19] |
Gan C F, Tan C J, Ma J Q. Determination of nitrogen in starch phosphate by alkaline potassium persulphate oxidation-ultraviolet spectrophotometry (In Chinese)[J]. Contemporary Chemical Industry, 2011, 40(1): 106-109. [甘春芳, 谭超娟, 马建强. 碱性过硫酸钾氧化-紫外分光光度法测定淀粉磷酸酯中氮含量[J]. 当代化工, 2011, 40(1): 106-109.]
(  0) 0) |
| [20] |
Shen X. Preliminary study on the isolation and identification of marine microalgae[D]. Qingdao, Shandong: Ocean University of China, 2011.[沈雄. 海洋微藻的分离及鉴定方法的初步研究[D]. 山东青岛: 中国海洋大学, 2011.]
(  0) 0) |
| [21] |
Wang Q, Xiang Y, Zhao M J, et al. Evaluation on the effect of 8 methods for rapid preparation of bacterial genomic DNA (In Chinese)[J]. Modern Preventive Medicine, 2017, 44(16): 3005-3009. [王群, 向瑶, 赵茂吉, 等. 8种快速制备细菌基因组DNA方法的效果评价[J]. 现代预防医学, 2017, 44(16): 3005-3009.]
(  0) 0) |
| [22] |
Hou M L, Ren Y F, Düsedau L, et al. Isolation and identification of protective and opportunistic pathogenic bacteria of invasive Gracilaria vermiculophylla (In Chinese)[J]. Periodical of Ocean University of China, 2024, 54(4): 78-85. [侯明磊, 任艺飞, Luisa Düsedau, 等. 入侵种真江蓠保护性细菌与条件致病菌的分离与鉴定[J]. 中国海洋大学学报(自然科学版), 2024, 54(4): 78-85.]
(  0) 0) |
| [23] |
Yan Q F, Liu B W, Liu G X. Chloroplast genome and description of Borodinellopsis insignis sp. nov. (chlamydomonadales, chlorophyta), a rare aerial Alga from China[J]. Plants, 2024, 13(22): 3199. DOI:10.3390/plants13223199
(  0) 0) |
| [24] |
Shetty P, Gitau M M, Maróti G. Salinity stress responses and adaptation mechanisms in eukaryotic green microalgae[J]. Cells, 2019, 8(12): 1657. DOI:10.3390/cells8121657
(  0) 0) |
| [25] |
Cui L Y, Xie X, Mao Q, et al. Response of soil microalgae to salt stress and its improvement effect on salinized soil (In Chinese)[J]. Earth Science, 2023, 48(11): 4270-4278. [崔丽洋, 谢茜, 毛青, 等. 土壤微藻对盐胁迫的响应及其对盐渍化土壤的改良作用[J]. 地球科学, 2023, 48(11): 4270-4278.]
(  0) 0) |
| [26] |
Gu W H, Wu S C, Liu X H, et al. Algal-bacterial consortium promotes carbon sink formation in saline environment[J]. Journal of Advanced Research, 2024, 60: 111-125. DOI:10.1016/j.jare.2023.08.004
(  0) 0) |
| [27] |
Zhang Z. Study on the parameter optimization and microbial interaction of microalgae-bacteria symbiotic system in livestock wastewater treatment[D]. Nanchang: Nanchang University, 2022.[张哲. 藻菌共生体系在畜禽养殖废水处理中的参数优化及微生物相互作用关系探究[D]. 南昌: 南昌大学, 2022.]
(  0) 0) |
| [28] |
Yang J, Cui F Z, Guo X Q, et al. Effects of different tillage methods and previous crops on winter wheat yield (In Chinese)[J]. Journal of Shanxi Agricultural Sciences, 2019, 47(3): 362-365. [杨佳, 崔福柱, 郭秀卿, 等. 不同耕作方式及前茬作物对冬小麦产量的影响[J]. 山西农业科学, 2019, 47(3): 362-365.]
(  0) 0) |
| [29] |
Wang T. Effects of tillage methods on physical and chemical properties and growth of multiple cropping soybean soil[D]. Alaer, Xinjiang: Tarim University, 2024.[王甜. 耕种方式对复播大豆土壤理化性质及生长发育的影响[D]. 新疆阿拉尔: 塔里木大学, 2024.]
(  0) 0) |
| [30] |
Tang M C, Li W J, Song T S, et al. Screening of a highly efficient phosphate-solubilizing bacterium and validation of its phosphate-solubilizing effect (In Chinese)[J]. Biotechnology Bulletin, 2020, 36(6): 102-109. [唐岷宸, 李文静, 宋天顺, 等. 一株高效解磷菌的筛选及其解磷效果验证[J]. 生物技术通报, 2020, 36(6): 102-109.]
(  0) 0) |
| [31] |
Li X Y, Zhang F, Wang Z. Present situation and development trend in building remote sensing monitoring models of soil salinization (In Chinese)[J]. Remote Sensing for Natural Resources, 2022, 34(4): 11-21. [李星佑, 张飞, 王筝. 土壤盐渍化遥感监测模型构建方法现状与发展趋势[J]. 自然资源遥感, 2022, 34(4): 11-21.]
(  0) 0) |
| [32] |
Jiang L J, Yang L Y, Xiao L, et al. Quantitative studies on phosphorus transference occurring between Microcystis aeruginosa and its attached bacterium(Pseudomonas sp. )[M]//Eutrophication of Shallow Lakes with Special Reference to Lake Taihu, China. Dordrecht: Springer Netherlands, 2007: 161—165.
(  0) 0) |
| [33] |
Wei H Y, Li Y, Peng S Y, et al. Promoting crop growth under stress conditions by exopolysaccharides: re-view and perspective (In Chinese)[J]. Jiangsu Journal of Agricultural Sciences, 2022, 38(4): 1123-1134. [魏宏宇, 李怡, 彭帅英, 等. 胞外多糖促进胁迫条件下农作物生长的研究与展望[J]. 江苏农业学报, 2022, 38(4): 1123-1134.]
(  0) 0) |
| [34] |
Mishra A, Jha B. Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress[J]. Bioresource Technology, 2009, 100(13): 3382-3386. DOI:10.1016/j.biortech.2009.02.006
(  0) 0) |
| [35] |
Chang H X, Li M Y, Mairiyangu Y S, et al. Screening and growth-promoting characteristics of multifunctional exopolysaccharides-producing bacteria (In Chinese)[J]. Biotechnology Bulletin, 2024, 40(3): 273-285. [常海霞, 李明源, 麦日艳古·亚生, 等. 产胞外多糖多功能促生菌的筛选鉴定及促生评价[J]. 生物技术通报, 2024, 40(3): 273-285.]
(  0) 0) |
| [36] |
Li X Y, Li B, Wang C Q, et al. Effects of long-term straw returning on organic carbon and extracellular enzymes in paddy soil aggregates (In Chinese)[J]. Acta Pedologica Sinica, 2024, 61(1): 235-246. DOI:10.11766/trxb202206170325 [李新悦, 李冰, 王昌全, 等. 长期秸秆还田对水稻土团聚体有机碳及胞外酶的影响[J]. 土壤学报, 2024, 61(1): 235-246.]
(  0) 0) |
| [37] |
Wei S C, Xie W J, Xia J B, et al. Research progress on soil aggregates and associated organic carbon in salinized soils (In Chinese)[J]. Chinese Journal of Applied Ecology, 2021, 32(1): 369-376. [魏守才, 谢文军, 夏江宝, 等. 盐渍化条件下土壤团聚体及其有机碳研究进展[J]. 应用生态学报, 2021, 32(1): 369-376.]
(  0) 0) |
| [38] |
Wu Z Y, Zhou G F, Chen H M, et al. Isolation of Clostridium strain from rice paddy soil and its synergistic nitrogen fixation and hydrogen production ability (In Chinese)[J]. Research of Environmental Sciences, 2022, 35(11): 2588-2595. [吴志宇, 周革非, 陈慧敏, 等. 一株稻田土壤梭状芽孢杆菌的分离及其固氮产氢能力研究[J]. 环境科学研究, 2022, 35(11): 2588-2595.]
(  0) 0) |
| [39] |
Hu Y P, Lin D M, Hu H Z, et al. Isolation, identification and functional characteristics of associative nitrogen-fixing bacteria in upland rice (In Chinese)[J]. Journal of Fujian Agriculture and Forestry University(Natural Science Edition), 2024, 53(6): 797-807. [胡应平, 林冬梅, 胡弘正, 等. 旱稻联合固氮菌的分离、鉴定及功能特性[J]. 福建农林大学学报(自然科学版), 2024, 53(6): 797-807.]
(  0) 0) |
 2026, Vol. 63
2026, Vol. 63


