2. 中国科学院大学, 北京 100049;
3. 亚热带森林培育国家重点实验室, 浙江农林大学, 杭州 311300;
4. 自然资源部/广西壮族自治区岩溶动力学重点实验室, 中国地质科学院岩溶地质所, 广西桂林 541004
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. State Key Laboratory of Subtropical Forest Cultivation, Zhejiang Agricultural and Forestry University, Hangzhou 311300, China;
4. Key Laboratory of karst Dynamics, Ministry of Natural Resources/Guangxi, Institute of Karst Geology, Chinese Academy of Geological Sciences, Guilin, Guangxi 541004, China
氧化亚氮(Nitrous oxide,N2O)是大气中重要的温室气体,其单位质量的增温潜势是二氧化碳(CO2)的265倍[1],同时N2O还能在平流层中发生化学反应,引起臭氧层的破坏,导致地面紫外辐射增强,威胁人类的健康与生存[2]。N2O的来源有土壤、海洋和淡水湖泊,以及化石燃料燃烧等,而土壤是N2O主要排放源,约占总排放量的60%[3]。土壤N2O排放主要受其产生和还原双重作用的影响[4]。据估计,至2030年,随着农田面积的扩大和化肥使用量的增加,N2O排放量将增加35%~60%[3]。
土壤团聚体由原生颗粒(砂、粉、黏粒)、胶结物质和孔隙组成[5],是土壤结构的基本单元和微生物的重要栖息地[6]。不同粒径的土壤团聚体具有独特的物理化学特性,大团聚体孔隙连接度高、通气性好、周转率快、有机质丰富[7];微团聚体持水能力强、稳定性碳含量高,且受其保护的微生物不易被捕食[8]。因而,不同粒径团聚体形成了分化的微生物生态位[9],进而影响N2O产生和还原的功能微生物分布[10]。本文梳理了不同粒径团聚体N2O排放量的研究,整合分析其结果存在明显分歧;通过阐述土壤N2O产生和还原的微生物学过程和分析不同粒径土壤团聚体微生物的初始分布与环境扰动下的再分布过程,解释分歧存在的原因;进一步探讨氮循环功能基因在土壤团聚体中分布,评估不同粒径团聚体N2O产生和还原热区的可行性。
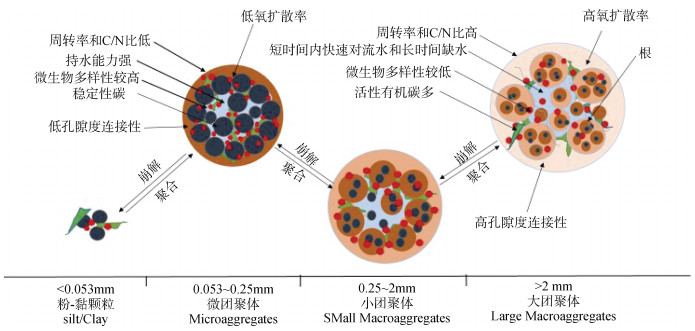
1 不同粒径土壤团聚体的N2O排放土壤团聚体是矿物颗粒在植物根系和土壤有机质、菌丝、土壤氧化物等有机和无机胶结物质作用下结合形成的二次颗粒。根据分级团聚理论,将土壤团聚体分成大团聚体(Large macroaggregates,> 2 mm)、小团聚体(Small macroaggregates,2~ 0.25 mm)、微团聚体(Microaggregates,0.25~ 0.053 mm)和粉-黏颗粒(Silt-plus Clay-size Particles,< 0.053 mm)[11]。不同粒径土壤团聚体具有不同的几何形状和孔隙结构,微团聚体的平均孔径较大团聚体小。这种差异影响通气条件、营养物质和溶解性有机碳的扩散。相对于微团聚体,大团聚体的通气条件较好,含有更多的不稳定性碳,其周转率和碳氮比(C/N)也较高[7],有利于增强好氧菌的活性。但是大团聚体内细菌更容易被捕食;特殊水分状态(如短时间内快速对流水和长时间缺水)会限制微生物运动和微生物酶运输,不利于细菌生长[12]。相反,微团聚体能够承受强大的机械和物理化学外力;能够在土壤中保持良好的稳定性;同时具有较低的养分有效性以及较低气体扩散率(图 1)[8]。因此,具有特殊独立化学条件的不同粒径土壤团聚体,可以被视为微生物产生和还原N2O的生物化学反应器[13-14]。目前在不同粒径土壤团聚体中N2O排放的结果存在较大差异(表 1),在不同的培养实验条件下,大团聚体、小团聚体和微团聚体的排放速率阈值分别为0.044~5 607 μg·kg-1·h-1、0.016~7 637 μg·kg-1·h-1、0.009~4.03 μg·kg-1·h-1。
|
图 1 土壤团聚体结构特征和周转过程概念图 Fig. 1 A conception map of structural characteristics and turnover processes of soil aggregates |
|
|
表 1 不同粒径土壤团聚体N2O排放速率 Table 1 N2O emission rate in different sizes of soil aggregates |
大量研究发现大团聚体较微团聚体具有更高的N2O排放能力[14]。Khalil等[15]发现较大团聚体中的N2O排放高于小团聚体,认为这是由于团聚体内的氧气浓度较低造成的。Diba等[8]研究发现火山灰土施用肥料时,大团聚体(4.5 mm)较小团聚体(< 2 mm)排放更多的N2O。Kimura等[16]采用培养实验研究两种土地利用类型下不同粒径团聚体中N2O排放,发现热带油棕和林地均以大团聚体N2O排放最多。Drury等[17]通过培养实验,发现N2O在大团聚体中排放速率(2~4 mm、4~8 mm)为7.0 mg·kg-1,而在小团聚体和微团聚体(0.25~0.5 mm、< 0.25 mm)中排放速率为0.04 mg·kg-1。同时,也有研究表明较小团聚体中的反硝化速率和N2O排放高于大团聚体[10]。Muñoz等[18]发现在施用肥料后,随着团聚体粒径的增大,N2O的排放量减少。Uchida[19]和Robinson[20]等发现,施用尿肥后,土壤中小团聚体较大团聚体N2O排放量更多。Sey [21]和周汉昌[10]等的研究结果也表明土壤团聚体最小粒径下N2O排放速率更高。由此可以看出,不同粒径土壤团聚体对N2O排放的贡献存在不一致性,而存在这种分歧的主要原因是产生和还原N2O的微生物在土壤中分布不同[21]。
2 土壤团聚体N2O排放的微生物学过程土壤团聚体N2O排放主要与N2O产生和还原有关。土壤N2O主要通过微生物的硝化作用(Nitrification)、反硝化作用(Denitrification)以及硝酸盐异化还原成铵(Dissimilatory nitrate reduction to ammonium,DNRA)、硝化-反硝化耦合作用(Nitrification-Coupled denitrification)、共反硝化作用(Co-denitrification)和非生物分解等过程产生[24]。而完全反硝化和非反硝化微生物携带的氧化亚氮还原酶(Nitrous oxide reductase,N2OR)是目前唯一明确的N2O微生物还原途径[25]。本文重点讨论微生物对N2O产生和还原的影响(图 2)。

|
图 2 土壤N2O产生过程及其微生物机制 Fig. 2 Biotic processes of nitrous oxide(N2O)production in soil |
微生物硝化作用主要是将NH3氧化为
反硝化作用是微生物将
土壤N2O的产生还存在其他微生物过程,如:硝酸盐异化还原成铵、硝化-反硝化耦合作用和共反硝化等[24]。DNRA将
土壤N2O可以通过多个微生物途径产生,但N2O还原目前已知的过程仅为nosZ基因编码的氧化亚氮还原酶(Nitrous oxide reductase,N2OR)将N2O还原为N2 [42-43]。nosZ基因存在于细菌和古菌中,而真菌缺乏该酶,并且在放线菌或酸杆菌门基因组中也明显缺乏[25]。因此,真菌不能进行完全反硝化作用将N2O还原N2[44]。nosZ基因存在nosZI和nosZII两种基因类型[25]。相对于前者,后者编码的酶对N2O亲和力更高;同时,前者信号肽采用Sec分泌途径,酶的运输状态未折叠,后者则是Tat途径,酶的运输状态折叠[45]。通常情况下,土壤中nosZII较nosZI分布更广,并发现nosZII在非反硝化N2O还原过程中也能发挥作用[43]。nosZ基因与nirS基因出现频率明显高于nirK,nosZ和nirS有更大程度的共同进化史。这说明在良好的环境条件下,nirS型反硝化微生物更有可能发生完全反硝化作用,因此对N2O排放的贡献可能小于nirK型反硝化细菌[25]。
3 土壤团聚体对N2O排放的影响机制土壤团聚体形成与周转过程中,微生物可以通过代谢物黏结周围土壤颗粒,形成对其有利的生境[46]。同时,随着外界环境因子改变,微生物也面临栖息地再选择[47]。一种情形是,适应环境的微生物快速繁殖产生更多的衍生产物,而处于环境胁迫下的微生物会因为自我保护作用处于休眠状态,直到再次出现适合生存条件被激活或者极端环境导致死亡[48];另外一种情形是,土壤大团聚体发生崩解,微生物从团聚体内部重新释放到外部环境,选择适合生存的栖息地,或重新构造新的团聚体,进而导致微生物群落结构在不同微生境的演变[49]。上述过程均可能导致土壤N2O产生与还原的功能微生物群落在不同粒径团聚体中分异与演化,进而影响N2O排放。
3.1 土壤团聚体与微生物相互影响机制Tisdall和Oades[50]提出的团聚体形成模型为0.0002 mm→0.0002~0.002 mm→0.002~0.02 mm→0.02~0.250 mm→2 mm,微团聚体(< 0.25 mm)由有机分子胶结粉-黏颗粒和多价阳离子形成复合粒子,微团聚体再相互结合形成大团聚体(如图 1)。土壤微生物的活性在团聚体的形成和稳定过程中起着至关重要的作用[51-52]。细菌对大团聚体和微团聚体的形成具有较大的贡献;而真菌在大团聚体形成中发挥着重要作用,真菌释放的菌丝和糖蛋白能有效地黏着土壤颗粒[53-54]。硝化细菌(如Nitrospirae)由于硝化和反硝化过程减少土壤50%可利用的氮,减少大团聚体形成,而土壤腐生真菌粪壳菌目(Sordariales)则是大团聚体形成和稳定的关键微生物[55]。真菌的菌丝通过形成大团聚体可间接促进N2O的排放[56]。微生物通过分泌胞外多糖黏结土壤颗粒,增加颗粒间的凝聚力;同时由于胞外多糖的疏水性也有助于增加大团聚体的稳定性[54]。而微团聚体则可以通过有机键保持稳定[57]。当发生外界环境扰动(水淹、耕作、土壤动物活动和根系穿插等)时,土壤团聚体内部相对稳定的结构可能发生崩解[49];或者随着时间的推移,微生物直接消耗土壤团聚体中的胶结剂,导致大团聚体崩解并释放微团聚体和基本颗粒,进而为新团聚体的形成提供基础构件[6, 58]。
微生物群落和结构受土壤团聚体粒径的影响,细菌和真菌群落在不同粒径团聚体中存在明显结构差异。细菌和古菌更倾向存活于微团聚体,一方面能躲避土壤动物的捕食;另一方面作为原核生物的细菌和古菌较真核生物的真菌小,更易于在微小生境栖息[59]。Mummey等[60]发现α-变形杆菌纲(Alphaproteobacteria)、放线菌亚纲(Actinobacteria)、红色杆菌纲(Rubrobacteriaceae)和芽单孢菌纲(Gemmatimonadetes)等在微团聚体中具有较高的丰度,而酸杆菌门(Acidobacteria)在大团聚体中的丰度相对较高。Yang等[61]发现细菌在微团聚体中主要以芽胞杆菌科(Bacillaceae)和梭菌科(Clostridiaceaea)为主,而草酸杆菌科(Oxalobacteraceae)、鞘脂单胞菌科(Sphingomonadaceae)、噬纤维细菌科(Cytophagaceae)和芽单胞菌科(Gemmatimonadaceae)的相对丰度则在大团聚体中占主导地位。真菌主要分布在土壤大团聚体和团聚体表面[62],其中以毛球壳科(Lasiosphaeriaceae)和新科盾叶科(Rhizophlyctidaceae)为主[59-60]。Liao等[63]研究指出黑土中真菌子囊菌纲(Sordariomycetes)随团聚体粒径的减少而显著降低。子囊菌产生N2O占真菌的90%,其次为担子菌门(Basidiomycota)合子菌门(Zygomycota),分别占7%和3%[64]。还有研究证实土壤微团聚体(< 0.02 mm)微生物多样性和活性高,为微生物提供了良好的栖息地[65]。Bach等[66]通过田间实验也发现细菌和真菌在微团聚体中具有更丰富的多样性。
土壤氮素循环功能微生物在不同粒径团聚体的分布情况如表 1所示。研究发现固氮螺菌属(Azospirillum)在粉-黏团聚体(< 0.002 mm)中丰度最高[67]。硝化作用能在不同粒径的团聚体中发生,主要取决于活性硝化细菌的群落结构和丰度[68]。添加氮磷钾肥的情况下,AOA在大团聚体中的丰度显著高于微团聚体[69-70];而未添加肥料时,AOB和AOA丰度在微团聚体(< 0.25 mm)中高于其他粒径团聚体(0.25~2 mm、> 2 mm)[70]。在不同粒径团聚体上硝化细菌和反硝化细菌很少发生完全分离,但是氨氧化细菌在大团聚体(> 0.25 mm)中的丰度最高,反硝化细菌群落在微团聚体(< 0.25 mm)中占主导地位[21]。周汉昌等[10]研究发现在 < 1 mm的团聚体中,反硝化细菌群落数量显著高于粒径2~4 mm的团聚体。也有研究发现反硝化细菌在 < 0.002 mm的团聚体中生物量最大[71]。Kong等[72]开展了不同氮素管理条件对土壤硝化微生物、反硝化微生物和氮循环微生物的影响研究,发现微团聚体(0.053~0.25 mm)中硝化细菌、反硝化细菌以及总微生物群落明显大于大团聚体(> 0.25 mm)和粉-黏团聚体(< 0.053 mm)[72]。
3.2 环境因素对团聚体中功能微生物丰度以及N2O排放的影响环境变化影响土壤团聚体功能微生物群落重新分布,进而影响N2O排放[73]。底物浓度、土壤含水量、通气状况和土地利用方式等诸多环境因素与土壤N2O排放通量密切相关。(1)土壤底物浓度(如碳、氮含量等)影响N2O排放。土壤大团聚体中有机碳和总氮的浓度显著高于微团聚体,且易于分解[74-75]。研究表明,土壤有机碳和总氮含量与细菌和古菌的amoA基因丰度以及硝化速率密切相关[70];有机碳和
氮循环功能基因可作为一项指标来衡量N2O排放。目前已有研究表明,土壤N2O产生潜力能用amoA、narG基因表征,N2O还原强度可通过nosZ基因丰度反映[93-94]。Rasche等[95]发现古细菌amoA、nirS和nosZ基因丰度与土壤N2O排放呈正相关。反硝化功能基因napA、narG、nirK、nirS、nosZ基因能评估N2O排放潜力;产生N2O功能基因(nirS/nirK)及其还原功能基因(nosZ)之间的差异也可预测N2O排放[96]。因此,通过提高nosZ反硝化基因丰度,降低土壤(nirK+nirS)/nosZ比值,可使N2O还原大于产生,从而减少N2O排放[76, 97]。
不同粒径土壤团聚体中微生物群落分布不同,携带的功能基因也存在一定差异(表 2),导致其N2O产生和还原热区也不一致。Xin等[98]在长期耕种的水稻生态系统中发现AOA和AOB的amoA基因主要分布在小团聚体内(0.25~2 mm);Blaud等[9]研究发现,林地和草地中nifH、amoA bacteria、narG、nirS和nosZ在小团聚体中(0.25~0.5 mm)分布最多,而这些功能基因在农田土壤中主要分布于 < 0.25 mm的微团聚体。Kong等[72]研究表明amoA和nosZ基因在微团聚体(0.053~0.25 mm)中分布最多,同时在 > 0.25 mm的大团聚体中,amoA基因丰度较nosZ低。周汉昌等[10]采用室内培养试验研究了氮循环关键功能基因在不同粒径土壤团聚体中的分布,发现 < 1 mm的土壤团聚体中narG和nosZ基因丰度均高于4~8 mm团聚体,且随着团聚体粒径增加而减少,narG和nosZ基因丰度与N2O排放呈正相关关系。大量研究结果表明,氮循环关键功能基因分布热区在微团聚体(0.053~0.25 mm)和小团聚体(0.25~2.0 mm),且N2O产生和还原的功能基因nirS和nosZ分布趋势一致。由此可以看出,微团聚体和小团聚体可能是N2O产生和还原的热区,而N2O产生和还原的相对贡献目前无法量化,因而尚不能通过nirS和nosZ丰度准确预测不同粒径团聚体N2O排放速率。微团聚体功能基因丰度高,可能是因为微团聚体相比大团聚体更加稳定,利于硝化/反硝化微生物的栖息与被保护。值得注意的是,如果微团聚体中养分亏缺、竞争激烈,微生物可能会选择更大的团聚体作为栖息地。不同土地利用方式、土壤含水量以及底物浓度也会影响N2O产生和还原的功能基因在土壤团聚体中的分布。
|
|
表 2 不同粒径团聚体N2O排放速率及功能基因分布 Table 2 Distribution of functional genes and N2O emission rate in different sizes of soil aggregates |
但是Liu等[99]研究表明:在长期的重复火灾作用下,环境因素而非反硝化基因丰度控制N2O排放。可能由于上述工作仅分析了反硝化相关功能基因丰度,并未涉及其他N2O产生排放过程[100]。因此,通过功能基因丰度直接量化N2O排放趋势还存在一定的不确定性,需充分考虑土壤基本性质和环境因素。
4 总结与展望土壤团聚体是微生物进行硝化/反硝化作用的生化反应器。不同粒径土壤团聚体中孔隙特征、通气条件、底物浓度以及水分含量等性状分异能改变微生物群落结构和活性,从而导致不同粒径团聚体中N2O排放差异较大。目前功能微生物对N2O排放的研究重心逐渐从全土尺度转移到团聚体尺度,聚焦于微生物群落结构和多样性以及功能微生物与团聚体的生物物理关联机制下N2O排放研究。
今后的研究主要加强以下几个方面:
1)土壤团聚体N2O产生和还原热区探究。土壤团聚体内部和周围孔隙是微生物存活的生境,有机质、氧气浓度、水分条件和底物成分等差异可能是导致微生物群落差异的主要驱动因素。土壤大团聚体通气条件好,可利用有机碳含量高,但是容易被外界环境因素扰动,而微团聚体内部可能是微生物相对稳定和隐蔽的栖息地。微团聚体具有长期稳定的碳库,能够持续为硝化/反硝化微生物提供生长环境,并且可能为微生物提供物理保护。近年来大量研究表明微团聚体内存在较高的微生物丰度,氮循环功能基因也在微团聚体中大量分布。但是目前对土壤团聚体中氮循环功能基因分布特征研究较少,尚不能明确功能微生物对土壤微团聚体粒径的偏好。今后还需要结合室内控制试验和环境因子梯度,明确N2O产生和还原的潜在热区,进一步探究微团聚体和大团聚体对N2O排放的贡献。
2)环境因子阈值范围确定。大团聚体和微团聚体均可能为硝化/反硝化微生物提供有利的生境,不同粒径土壤团聚体N2O排放潜势存在差异,可能是由底物浓度、通气条件以及水分含量等异质性引起。一旦外界环境压力超出阈值范围,则对参与硝化/反硝化过程的微生物生存产生胁迫,从而改变硝化/反硝化过程中N2O排放。通过研究确定环境因子的临界范围,可进一步明晰不同粒径土壤团聚体N2O产生和还原所占的比例,可初步判断N2O排放情况,有利于更好地分析硝化/反硝化对N2O的贡献率以及微生物驱动机制。
3)对系列功能基因(酶)开展整体性研究,而不仅是单个主要基因(酶)。硝化/反硝化过程能够在微生物体内发生,并排放N2O,是一系列功能基因和酶的运转,而非某一个功能基因和酶发挥作用。例如:目前用nosZ/(nirS + nirK)或者(nirS+nirK)/nosZ来衡量N2O排放,仅关注了反硝化在N2O排放中的关键作用,而忽略了其他作用的功能基因对N2O排放的贡献。虽然针对个别主要的功能基因能够突出研究重点,但是也可能造成氮循环功能基因认识的盲区,甚至是误区。因此,结合其他过程的功能基因(酶)展开研究,探究不同途径(如硝化作用、硝酸盐异化还原成铵、共反硝化作用等)对N2O排放的相对贡献。发掘这些功能基因是否能够和N2O排放建立相互关系以及这些基因(酶)之间互相的调控机制。功能基因和酶的活性相结合可能会成为衡量N2O排放潜势的新途径。功能基因不能够完全用来量化N2O排放潜势,N2O产生不仅由微生物活性决定,硝化/反硝化过程中酶的活性也是N2O产生的决定性因素。为此,将功能基因和酶结合研究,可能是量化N2O排放潜势的有效途径。
| [1] |
Pachauri R K, Meyer L A, Barros V R, et al. Climate change 2014: Synthesis report[M]//Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: Intergovernmental Panel on Climate Change(IPCC), 2014.
(  0) 0) |
| [2] |
Ravishankara A R, Daniel J S, Portmann R W. Nitrous oxide(N2O): The dominant ozone-depleting substance emitted in the 21st century[J]. Science, 2009, 326(5949): 123-125. DOI:10.1126/science.1176985
(  0) 0) |
| [3] |
Solomon S, Qin D, Manning M, et al. Climate change 2007: The physical science basis[M]//Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Summary for policymakers. Geneva: Intergovernmental Panel on Climate Change(IPCC), 2007.
(  0) 0) |
| [4] |
Stein L Y. The long-term relationship between microbial metabolism and greenhouse gases[J]. Trends in Microbiology, 2020, 28(6): 500-511. DOI:10.1016/j.tim.2020.01.006
(  0) 0) |
| [5] |
Bosilj P, Gould I, Duckett T, et al. Estimating soil aggregate size distribution from images using pattern spectra[J]. Biosystems Engineering, 2020, 198: 63-77. DOI:10.1016/j.biosystemseng.2020.07.012
(  0) 0) |
| [6] |
Li F Q, Xue C, Qiu P F, et al. Soil aggregate size mediates the responses of microbial communities to crop rotation[J]. European Journal of Soil Biology, 2018, 88: 48-56. DOI:10.1016/j.ejsobi.2018.06.004
(  0) 0) |
| [7] |
Six J, Bossuyt H, Degryze S, et al. A history of research on the link between(micro)aggregates, soil biota, and soil organic matter dynamics[J]. Soil & Tillage Research, 2004, 79(1): 7-31.
(  0) 0) |
| [8] |
Diba F, Shimizu M, Hatano R. Effects of soil aggregate size, moisture content and fertilizer management on nitrous oxide production in a volcanic ash soil[J]. Soil Science and Plant Nutrition, 2011, 57(5): 733-747. DOI:10.1080/00380768.2011.604767
(  0) 0) |
| [9] |
Blaud A, van der Zaan B, Menon M, et al. The abundance of nitrogen cycle genes and potential greenhouse gas fluxes depends on land use type and little on soil aggregate size[J]. Applied Soil Ecology, 2018, 125: 1-11. DOI:10.1016/j.apsoil.2017.11.026
(  0) 0) |
| [10] |
Zhou H C, Zhang W Z, Liu Y, et al. Relationships of N2O emission with abundance and composition of denitrifying microorganisms in soil aggregates (In Chinese)[J]. Acta Pedologica Sinica, 2015, 52(5): 1144-1152. [周汉昌, 张文钊, 刘毅, 等. 土壤团聚体N2O释放与反硝化微生物丰度和组成的关系[J]. 土壤学报, 2015, 52(5): 1144-1152.]
(  0) 0) |
| [11] |
Cambardella C A, Elliott E T. Carbon and nitrogen distribution in aggregates from cultivated and native grassland soils[J]. Soil Science Society of America Journal, 1993, 57(4): 1071-1076. DOI:10.2136/sssaj1993.03615995005700040032x
(  0) 0) |
| [12] |
Ananyeva K, Wang W, Smucker A J M, et al. Can intra-aggregate pore structures affect the aggregate's effectiveness in protecting carbon?[J]. Soil Biology & Biochemistry, 2013, 57: 868-875.
(  0) 0) |
| [13] |
Rillig M C, Muller L A, Lehmann A. Soil aggregates as massively concurrent evolutionary incubators[J]. The ISME Journal, 2017, 11(9): 1943-1948. DOI:10.1038/ismej.2017.56
(  0) 0) |
| [14] |
Wang B, Brewer P E, Shugart H H, et al. Soil aggregates as biogeochemical reactors and implications for soil-atmosphere exchange of greenhouse gases—A concept[J]. Global Change Biology, 2019, 25(2): 373-385. DOI:10.1111/gcb.14515
(  0) 0) |
| [15] |
Khalil K, Renault P, Mary B. Effects of transient anaerobic conditions in the presence of acetylene on subsequent aerobic respiration and N2O emission by soil aggregates[J]. Soil Biology & Biochemistry, 2005, 37(7): 1333-1342.
(  0) 0) |
| [16] |
Kimura S D, Melling L, Goh K J. Influence of soil aggregate size on greenhouse gas emission and uptake rate from tropical peat soil in forest and different oil palm development years[J]. Geoderma, 2012, 185/186: 1-5. DOI:10.1016/j.geoderma.2012.03.026
(  0) 0) |
| [17] |
Drury C F, Yang X M, Reynolds W D, et al. Influence of crop rotation and aggregate size on carbon dioxide production and denitrification[J]. Soil & Tillage Research, 2004, 79(1): 87-100.
(  0) 0) |
| [18] |
Muñoz C, Torres P, Alvear M, et al. Physical protection of C and greenhouse gas emissions provided by soil macroaggregates from a Chilean cultivated volcanic soil[J]. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science, 2012, 62(8): 739-748.
(  0) 0) |
| [19] |
Uchida Y, Clough T, Kelliher F, et al. Effects of aggregate size, soil compaction, and bovine urine on N2O emissions from a pasture soil[J]. Soil Biology & Biochemistry, 2008, 40(4): 924-931.
(  0) 0) |
| [20] |
Robinson A, Di H J, Cameron K C, et al. Effect of soil aggregate size and dicyandiamide on N2O emissions and ammonia oxidizer abundance in a grazed pasture soil[J]. Soil Use and Management, 2014, 30(2): 231-240.
(  0) 0) |
| [21] |
Sey B K, Manceur A M, Whalen J K, et al. Small-scale heterogeneity in carbon dioxide, nitrous oxide and methane production from aggregates of a cultivated sandy-loam soil[J]. Soil Biology & Biochemistry, 2008, 40(9): 2468-2473.
(  0) 0) |
| [22] |
Bandyopadhyay K K, Lal R. Effect of land use management on greenhouse gas emissions from water stable aggregates[J]. Geoderma, 2014, 232/233/234: 363-372.
(  0) 0) |
| [23] |
Mangalassery S, Sjögersten S, Sparkes D L, et al. The effect of soil aggregate size on pore structure and its consequence on emission of greenhouse gases[J]. Soil & Tillage Research, 2013, 132: 39-46.
(  0) 0) |
| [24] |
Butterbach-Bahl K, Baggs E M, Dannenmann M, et al. Nitrous oxide emissions from soils: How well do we understand the processes and their controls?[J]. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 2013, 368(1621): 20130122. DOI:10.1098/rstb.2013.0122
(  0) 0) |
| [25] |
Hallin S, Philippot L, Löffler F E, et al. Genomics and ecology of novel N2O-reducing microorganisms[J]. Trends in Microbiology, 2018, 26(1): 43-55. DOI:10.1016/j.tim.2017.07.003
(  0) 0) |
| [26] |
Kuypers M M M, Marchant H K, Kartal B. The microbial nitrogen-cycling network[J]. Nature Reviews Microbiology, 2018, 16(5): 263-276. DOI:10.1038/nrmicro.2018.9
(  0) 0) |
| [27] |
Prosser J I, Hink L, Gubry-Rangin C, et al. Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies[J]. Global Change Biology, 2020, 26(1): 103-118. DOI:10.1111/gcb.14877
(  0) 0) |
| [28] |
Cao W C, Song H, Wang Y J, et al. Key production processes and influencing factors of nitrous oxide emissions from agricultural soils (In Chinese)[J]. Journal of Plant Nutrition and Fertilizers, 2019, 25(10): 1781-1798. DOI:10.11674/zwyf.18441 [曹文超, 宋贺, 王娅静, 等. 农田土壤N2O排放的关键过程及影响因素[J]. 植物营养与肥料学报, 2019, 25(10): 1781-1798.]
(  0) 0) |
| [29] |
Liu N, Yan Z S, Fan Y J, et al. Effect of different nitrogen application levels on the content of soluble protein and key enzyme activities in nitrogen metabolism of sugar beet (In Chinese)[J]. Chinese Agricultural Science Bulletin, 2015, 31(30): 149-154. DOI:10.11924/j.issn.1000-6850.casb15040052 [刘娜, 闫志山, 范有君, 等. 不同氮素水平对甜菜氮代谢酶和可溶性蛋白含量的影响[J]. 中国农学通报, 2015, 31(30): 149-154.]
(  0) 0) |
| [30] |
Daims H, Lebedeva E V, Pjevac P, et al. Complete nitrification by Nitrospira bacteria[J]. Nature, 2015, 528(7583): 504-509. DOI:10.1038/nature16461
(  0) 0) |
| [31] |
Kits K D, Jung M Y, Vierheilig J, et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata[J]. Nature Communications, 2019, 10: 1836. DOI:10.1038/s41467-019-09790-x
(  0) 0) |
| [32] |
Opperman D J, Murgida D H, Dalosto S D, et al. A three-domain copper-nitrite reductase with a unique sensing loop[J]. IUCrJ, 2019, 6(2): 248-258. DOI:10.1107/S2052252519000241
(  0) 0) |
| [33] |
Priemé A, Braker G, Tiedje J M. Diversity of nitrite reductase(nirK and nirS)gene fragments in forested upland and wetland soils[J]. Applied and Environmental Microbiology, 2002, 68(4): 1893-1900. DOI:10.1128/AEM.68.4.1893-1900.2002
(  0) 0) |
| [34] |
Zumft W G. Nitric oxide reductases of prokaryotes with emphasis on the respiratory, heme-copper oxidase type[J]. Journal of Inorganic Biochemistry, 2005, 99(1): 194-215. DOI:10.1016/j.jinorgbio.2004.09.024
(  0) 0) |
| [35] |
Blomberg M R A, Ädelroth P. Mechanisms for enzymatic reduction of nitric oxide to nitrous oxide-A comparison between nitric oxide reductase and cytochrome c oxidase[J]. Biochimica et Biophysica Acta-Bioenergetics, 2018, 1859(11): 1223-1234. DOI:10.1016/j.bbabio.2018.09.368
(  0) 0) |
| [36] |
Suharti, Strampraad M J F, Schröder I, et al. A novel copper a containing menaquinol NO reductase from Bacillus azotoformans[J]. Biochemistry, 2001, 40(8): 2632-2639. DOI:10.1021/bi0020067
(  0) 0) |
| [37] |
Putz M, Schleusner P, Rütting T, et al. Relative abundance of denitrifying and DNRA bacteria and their activity determine nitrogen retention or loss in agricultural soil[J]. Soil Biology & Biochemistry, 2018, 123: 97-104.
(  0) 0) |
| [38] |
Simon J. Enzymology and bioenergetics of respiratory nitrite ammonification[J]. FEMS Microbiology Reviews, 2002, 26(3): 285-309. DOI:10.1111/j.1574-6976.2002.tb00616.x
(  0) 0) |
| [39] |
He J Z, Zhang L M. Key processes and microbial mechanisms of soil nitrogen transformation (In Chinese)[J]. Microbiology China, 2013, 40(1): 98-108. [贺纪正, 张丽梅. 土壤氮素转化的关键微生物过程及机制[J]. 微生物学通报, 2013, 40(1): 98-108.]
(  0) 0) |
| [40] |
Rütting T, Boeckx P, Müller C, et al. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle[J]. Biogeosciences, 2011, 8(7): 1779-1791. DOI:10.5194/bg-8-1779-2011
(  0) 0) |
| [41] |
Tanimoto T, Hatano K I, Kim D H, et al. Co-denitrification by the denitrifying system of the fungus Fusarium oxysporum[J]. FEMS Microbiology Letters, 1992, 93(2): 177-180. DOI:10.1111/j.1574-6968.1992.tb05086.x
(  0) 0) |
| [42] |
Graf D R H, Jones C M, Hallin S. Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions[J]. PLoS One, 2014, 9(12): e114118. DOI:10.1371/journal.pone.0114118
(  0) 0) |
| [43] |
Sanford R A, Wagner D D, Wu Q Z, et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(48): 19709-19714. DOI:10.1073/pnas.1211238109
(  0) 0) |
| [44] |
Baggs E M. Soil microbial sources of nitrous oxide: Recent advances in knowledge, emerging challenges and future direction[J]. Current Opinion in Environmental Sustainability, 2011, 3(5): 321-327. DOI:10.1016/j.cosust.2011.08.011
(  0) 0) |
| [45] |
Pauleta S R, Carepo M S P, Moura I. Source and reduction of nitrous oxide[J]. Coordination Chemistry Reviews, 2019, 387: 436-449. DOI:10.1016/j.ccr.2019.02.005
(  0) 0) |
| [46] |
Li F Q, Qiu P F, Shen B, et al. Soil aggregate size modifies the impacts of fertilization on microbial communities[J]. Geoderma, 2019, 343: 205-214. DOI:10.1016/j.geoderma.2019.02.039
(  0) 0) |
| [47] |
Dini-Andreote F, Stegen J C, van Elsas J D, et al. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(11): E1326-E1332. DOI:10.1073/pnas.1414261112
(  0) 0) |
| [48] |
Rittershaus E S C, Baek S H, Sassetti C M. The normalcy of dormancy: Common themes in microbial quiescence[J]. Cell Host & Microbe, 2013, 13(6): 643-651.
(  0) 0) |
| [49] |
Rillig M C, Lehmann A, Aguilar-Trigueros C A, et al. Soil microbes and community coalescence[J]. Pedobiologia, 2016, 59(1/2): 37-40.
(  0) 0) |
| [50] |
Tisdall J M, Oades J M. Organic matter and water-stable aggregates in soils[J]. Journal of Soil Science, 1982, 33(2): 141-163. DOI:10.1111/j.1365-2389.1982.tb01755.x
(  0) 0) |
| [51] |
Blagodatsky S, Smith P. Soil physics meets soil biology: Towards better mechanistic prediction of greenhouse gas emissions from soil[J]. Soil Biology & Biochemistry, 2012, 47: 78-92.
(  0) 0) |
| [52] |
Xiao J J, Xing D, Mao M M, et al. Mechanism of arbuscular mycorrhizal fungal affecting soil aggregates in rhizosphere of mulberry(Morus alba) (In Chinese)[J]. Acta Pedologica Sinica, 2020, 57(3): 773-782. [肖玖军, 邢丹, 毛明明, 等. AM真菌对桑树根围土壤团聚体的影响机制[J]. 土壤学报, 2020, 57(3): 773-782.]
(  0) 0) |
| [53] |
Lehmann A, Zheng W S, Rillig M C. Soil biota contributions to soil aggregation[J]. Nature Ecology & Evolution, 2017, 1(12): 1828-1835.
(  0) 0) |
| [54] |
Li N, Han X Z, You M Y, et al. Research review on soil aggregates and microbes (In Chinese)[J]. Ecology and Environment, 2013, 22(9): 1625-1632. DOI:10.3969/j.issn.1674-5906.2013.09.027 [李娜, 韩晓增, 尤孟阳, 等. 土壤团聚体与微生物相互作用研究[J]. 生态环境学报, 2013, 22(9): 1625-1632.]
(  0) 0) |
| [55] |
Tian X L, Wang C B, Bao X G, et al. Crop diversity facilitates soil aggregation in relation to soil microbial community composition driven by intercropping[J]. Plant and Soil, 2019, 436(1/2): 173-192. DOI:10.1007/s11104-018-03924-8
(  0) 0) |
| [56] |
Okiobe S T, Augustin J, Mansour I, et al. Disentangling direct and indirect effects of mycorrhiza on nitrous oxide activity and denitrification[J]. Soil Biology & Biochemistry, 2019, 134: 142-151.
(  0) 0) |
| [57] |
Ran Y G, Ma M H, Liu Y, et al. Physicochemical determinants in stabilizing soil aggregates along a hydrological stress gradient on reservoir riparian habitats: Implications to soil restoration[J]. Ecological Engineering, 2020, 143: 105664. DOI:10.1016/j.ecoleng.2019.105664
(  0) 0) |
| [58] |
Six J, Paustian K, Elliott E T, et al. Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon[J]. Soil Science Society of America Journal, 2000, 64(2): 681-689. DOI:10.2136/sssaj2000.642681x
(  0) 0) |
| [59] |
Wright D A, Killham K, Glover L A, et al. Role of pore size location in determining bacterial activity during predation by protozoa in soil[J]. Applied and Environmental Microbiology, 1995, 61(10): 3537-3543. DOI:10.1128/aem.61.10.3537-3543.1995
(  0) 0) |
| [60] |
Mummey D, Holben W, Six J, et al. Spatial stratification of soil bacterial populations in aggregates of diverse soils[J]. Microbial Ecology, 2006, 51(3): 404-411. DOI:10.1007/s00248-006-9020-5
(  0) 0) |
| [61] |
Yang C, Liu N, Zhang Y J. Soil aggregates regulate the impact of soil bacterial and fungal communities on soil respiration[J]. Geoderma, 2019, 337: 444-452. DOI:10.1016/j.geoderma.2018.10.002
(  0) 0) |
| [62] |
Frey S D. Aggregation-microbial aspects[M]//Hillel D. Encyclopedia of soils in the environment. New York: Academic Press, 2005: 22-28.
(  0) 0) |
| [63] |
Liao H, Zhang Y C, Zuo Q Y, et al. Contrasting responses of bacterial and fungal communities to aggregate-size fractions and long-term fertilizations in soils of northeastern China[J]. Science of the Total Environment, 2018, 635: 784-792. DOI:10.1016/j.scitotenv.2018.04.168
(  0) 0) |
| [64] |
Mothapo N, Chen H H, Cubeta M A, et al. Phylogenetic, taxonomic and functional diversity of fungal denitrifiers and associated N2O production efficacy[J]. Soil Biology & Biochemistry, 2015, 83: 160-175.
(  0) 0) |
| [65] |
Poll C, Thiede A, Wermbter N, et al. Micro-scale distribution of microorganisms and microbial enzyme activities in a soil with long-term organic amendment[J]. European Journal of Soil Science, 2003, 54(4): 715-724. DOI:10.1046/j.1351-0754.2003.0569.x
(  0) 0) |
| [66] |
Bach E M, Williams R J, Hargreaves S K, et al. Greatest soil microbial diversity found in micro-habitats[J]. Soil Biology & Biochemistry, 2018, 118: 217-226.
(  0) 0) |
| [67] |
Chotte J L, Schwartzmann A, Bally R, et al. Changes in bacterial communities and Azospirillum diversity in soil fractions of a tropical soil under 3 or 19 years of natural fallow[J]. Soil Biology & Biochemistry, 2002, 34(8): 1083-1092.
(  0) 0) |
| [68] |
Nahidan S, Nourbakhsh F, Henneberger R, et al. Aggregate size distribution of ammonia-oxidizing bacteria and archaea at different landscape positions[J]. Geomicrobiology Journal, 2017, 34(10): 895-902. DOI:10.1080/01490451.2017.1297511
(  0) 0) |
| [69] |
Jiang Y J, Jin C, Sun B. Soil aggregate stratification of nematodes and ammonia oxidizers affects nitrification in an acid soil[J]. Environmental Microbiology, 2014, 16(10): 3083-3094. DOI:10.1111/1462-2920.12339
(  0) 0) |
| [70] |
Li P P, Han Y L, He J Z, et al. Soil aggregate size and long-term fertilization effects on the function and community of ammonia oxidizers[J]. Geoderma, 2019, 338: 107-117. DOI:10.1016/j.geoderma.2018.11.033
(  0) 0) |
| [71] |
Lensi R, Clays-Josserand A, Jocteur Monrozier L. Denitrifiers and denitrifying activity in size fractions of a mollisol under permanent pasture and continuous cultivation[J]. Soil Biology & Biochemistry, 1995, 27(1): 61-69.
(  0) 0) |
| [72] |
Kong A Y Y, Hristova K, Scow K M, et al. Impacts of different N management regimes on nitrifier and denitrifier communities and N cycling in soil microenvironments[J]. Soil Biology & Biochemistry, 2010, 42(9): 1523-1533.
(  0) 0) |
| [73] |
Zhou J, Ning D. Stochastic community assembly: Does it matter in microbial ecology?[J]. Microbiology and Molecular Biology Reviews, 2017, 81(4): e00002-00017.
(  0) 0) |
| [74] |
Rabbi S M F, Daniel H, Lockwood P V, et al. Physical soil architectural traits are functionally linked to carbon decomposition and bacterial diversity[J]. Scientific Reports, 2016, 6: 33012. DOI:10.1038/srep33012
(  0) 0) |
| [75] |
Xiao S S, Zhang W, Ye Y Y, et al. Soil aggregate mediates the impacts of land uses on organic carbon, total nitrogen, and microbial activity in a Karst ecosystem[J]. Scientific Reports, 2017, 7: 41402. DOI:10.1038/srep41402
(  0) 0) |
| [76] |
Aamer M, Shaaban M, Hassan M U, et al. Biochar mitigates the N2O emissions from acidic soil by increasing the nosZ and nirK gene abundance and soil pH[J]. Journal of Environmental Management, 2020, 255: 109891. DOI:10.1016/j.jenvman.2019.109891
(  0) 0) |
| [77] |
Huang R, Wang Y Y, Liu J, et al. Variation in N2O emission and N2O related microbial functional genes in straw- and biochar-amended and non-amended soils[J]. Applied Soil Ecology, 2019, 137: 57-68. DOI:10.1016/j.apsoil.2019.01.010
(  0) 0) |
| [78] |
Mathieu O, Lévêque J, Hénault C, et al. Emissions and spatial variability of N2O, N2 and nitrous oxide mole fraction at the field scale, revealed with 15N isotopic techniques[J]. Soil Biology & Biochemistry, 2006, 38(5): 941-951.
(  0) 0) |
| [79] |
Weier K, Doran J W, Power J F, et al. Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate[J]. Soil Science Society of America Journal, 1993, 57(1): 66-72. DOI:10.2136/sssaj1993.03615995005700010013x
(  0) 0) |
| [80] |
Ying J Y, Li X X, Wang N N, et al. Contrasting effects of nitrogen forms and soil pH on ammonia oxidizing microorganisms and their responses to long-term nitrogen fertilization in a typical steppe ecosystem[J]. Soil Biology & Biochemistry, 2017, 107: 10-18.
(  0) 0) |
| [81] |
Senbayram M, Budai A, Bol R, et al. Soil NO3- level and O2 availability are key factors in controlling N2O reduction to N2 following long-term liming of an acidic sandy soil[J]. Soil Biology & Biochemistry, 2019, 132: 165-173.
(  0) 0) |
| [82] |
Trivedi P, Rochester I J, Trivedi C, et al. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities[J]. Soil Biology & Biochemistry, 2015, 91: 169-181.
(  0) 0) |
| [83] |
Bateman E J, Baggs E. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space[J]. Biology and Fertility of Soils, 2005, 41(6): 379-388. DOI:10.1007/s00374-005-0858-3
(  0) 0) |
| [84] |
Ruser R, Flessa H, Russow R, et al. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting[J]. Soil Biology & Biochemistry, 2006, 38(2): 263-274.
(  0) 0) |
| [85] |
Jia W L, Liang S, Zhang J, et al. Nitrous oxide emission in low-oxygen simultaneous nitrification and denitrification process: Sources and mechanisms[J]. Bioresource Technology, 2013, 136: 444-451. DOI:10.1016/j.biortech.2013.02.117
(  0) 0) |
| [86] |
Zhu X, Burger M, Doane T A, et al. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(16): 6328-6333. DOI:10.1073/pnas.1219993110
(  0) 0) |
| [87] |
An S S, Mentler A, Mayer H, et al. Soil aggregation, aggregate stability, organic carbon and nitrogen in different soil aggregate fractions under forest and shrub vegetation on the Loess Plateau, China[J]. Catena, 2010, 81(3): 226-233. DOI:10.1016/j.catena.2010.04.002
(  0) 0) |
| [88] |
Zhu K, Ma M H, Ran Y G, et al. In mitigating CO2 emission in the reservoir riparian: The influences of land use and the dam-triggered flooding on soil respiration[J]. Soil & Tillage Research, 2020, 197: 104522.
(  0) 0) |
| [89] |
Vilain G, Garnier J, Tallec G, et al. Effect of slope position and land use on nitrous oxide(N2O)emissions(Seine Basin, France)[J]. Agricultural and Forest Meteorology, 2010, 150(9): 1192-1202. DOI:10.1016/j.agrformet.2010.05.004
(  0) 0) |
| [90] |
Shi H A, Li L J, You M Y, et al. Impact of soil temperature and moisture on soil N2O emission from mollisols under different land-use types (In Chinese)[J]. Journal of Agro-Environment Science, 2013, 32(11): 2286-2292. DOI:10.11654/jaes.2013.11.026 [石洪艾, 李禄军, 尤孟阳, 等. 不同土地利用方式下土壤温度与土壤水分对黑土N2O排放的影响[J]. 农业环境科学学报, 2013, 32(11): 2286-2292.]
(  0) 0) |
| [91] |
Li M, Qing J, Hong Y, et al. Effects of nitrogen addition on ecological stoichiometric characteristics of carbon, nitrogen and phosphorus in Stipa baicalensis grassland soil aggregates (In Chinese)[J]. Acta Prataculture Sinica, 2019, 28(12): 29-40. DOI:10.11686/cyxb2019297 [李明, 秦洁, 红雨, 等. 氮素添加对贝加尔针茅草原土壤团聚体碳、氮和磷生态化学计量学特征的影响[J]. 草业学报, 2019, 28(12): 29-40.]
(  0) 0) |
| [92] |
Wei W, Isobe K, Shiratori Y, et al. N2O emission from cropland field soil through fungal denitrification after surface applications of organic fertilizer[J]. Soil Biology & Biochemistry, 2014, 69: 157-167.
(  0) 0) |
| [93] |
Zhong L, Du R, Ding K, et al. Effects of grazing on N2O production potential and abundance of nitrifying and denitrifying microbial communities in meadow-steppe grassland in Northern China[J]. Soil Biology & Biochemistry, 2014, 69: 1-10.
(  0) 0) |
| [94] |
Wang L, Li K, Song YQ, et al. The N2O consumption ability in the surface paddy soil layer and its coupling relationship to N2O reducing microorganisms (In Chinese)[J]. Acta Ecologica Sinica, 2019, 39(20): 7602-7610. [王玲, 李昆, 宋雅琦, 等. 浅表层水稻土N2O消耗能力及其与N2O还原微生物的耦合关系[J]. 生态学报, 2019, 39(20): 7602-7610.]
(  0) 0) |
| [95] |
Rasche F, Knapp D, Kaiser C, et al. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest[J]. The ISME Journal, 2011, 5(3): 389-402. DOI:10.1038/ismej.2010.138
(  0) 0) |
| [96] |
Levy-Booth D J, Prescott C E, Grayston S J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems[J]. Soil Biology & Biochemistry, 2014, 75: 11-25.
(  0) 0) |
| [97] |
Li S S, Chen C, Duan P P, et al. Effects of biochar application on N2O emissions and abundance of nitrogen related functional genes in an acidic vegetable soil (In Chinese)[J]. Journal of Plant Nutrition and Fertilizers, 2018, 24(2): 414-423. [李双双, 陈晨, 段鹏鹏, 等. 生物质炭对酸性菜地土壤N2O排放及相关功能基因丰度的影响[J]. 植物营养与肥料学报, 2018, 24(2): 414-423.]
(  0) 0) |
| [98] |
Xin X, Liu Q, Liu W, et al. Distribution of nitrifiers and nitrification associated with different sizes of aggregates along a 2000 year chronosequence of rice cultivation[J]. Catena, 2014, 119: 71-77. DOI:10.1016/j.catena.2014.03.012
(  0) 0) |
| [99] |
Liu X, Chen C R, Wang W J, et al. Soil environmental factors rather than denitrification gene abundance control N2O fluxes in a wet sclerophyll forest with different burning frequency[J]. Soil Biology & Biochemistry, 2013, 57: 292-300.
(  0) 0) |
| [100] |
Wallenstein M D, Myrold D D, Firestone M, et al. Environmental controls on denitrifying communities and denitrification rates: Insights from molecular methods[J]. Ecological Applications, 2006, 16(6): 2143-2152. DOI:10.1890/1051-0761(2006)016[2143:ECODCA]2.0.CO;2
(  0) 0) |
 2021, Vol. 58
2021, Vol. 58


