2. 土壤与可持续农业国家重点实验室(中国科学院南京土壤研究所), 南京 210008
2. State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China
土壤团聚体协调土壤水、肥、气、热,影响土壤微生物栖居环境和微生物群落结构的变化,进而影响土壤物质能量循环;是胶体凝聚、胶结和黏结土壤原生颗粒,是生物和非生物因素共同作用的结果,其中微生物是形成土壤团聚体最活跃的生物因素之一[1-2]。
生物质炭是在无氧或缺氧条件下通过有机废弃物(例如废木材、有机肥、作物残余物、畜禽粪便等)热解产生的富炭多孔物质,具有很高的抗分解能力[3],故能在土壤中稳定地存在数千年[4]。已有研究表明,生物质炭不仅提高土壤碳储量和土壤养分含量,增加土壤pH、通气性和持水量,降低土壤容重;还有利于微生物的生长和繁殖,丰富微生物群落结构,促进土壤团聚体结构的形成和稳定[5-6]。Soinne等[7]指出,添加生物质炭改变土壤性质,减少土壤侵蚀,增加黏土团聚体稳定性,减少胶体物质的分离,进而减少农田中颗粒态磷的损失。Lehmann等[8]发现,生物质炭通过改善土壤容重、土壤持水量等土壤理化性质影响土壤微生物丰度,为微生物生长提供底物,改善微生物栖息地环境。何玉亭等[9]研究表明,烟秆炭和桑条炭均能促进红壤大团聚体(0.25~1 mm)的形成,提高红壤团聚体结构稳定性,增加土壤微生物群落丰度。但也有研究发现,施用生物质炭导致土壤团聚体稳定性降低[10]。这可能与生物质炭的制备材料、生产条件[11]、土壤类型、老化作用或其他环境因素有关[12]。
目前有关生物质炭改良土壤的研究主要来自短期试验,但是生物质炭中不稳定组分可以在短期内(数月)迅速降解[13],随后其表面形态、比表面积、孔隙度、元素组成、表面含氧官能团和pH等均会发生一系列的变化,进而显著改变土壤理化性质和微生物群落[8,12]。Duan等[14]研究发现,大田原位老化5年生物质炭纳米微孔结构被破坏,孔径、比表面积和铵吸附能力增大,同时对土壤pH的提升显著减弱。Zheng等[15]研究发现,添加生物质炭4年后对酸性稻田土壤理化性质和微生物群落组成均有影响。也有研究表明,生物质炭在田间原位老化过程中颗粒破碎,含氧官能团增加,与黏粒结合,增加土壤团聚和养分固持,改善土壤结构[16-17]。生物质炭的缓慢氧化特性决定了其对土壤团聚体的长期影响[18]。有必要关注老化生物质炭对土壤团聚体结构的影响机制[12]。因此,本研究针对不同地区稻麦轮作麦季土壤原位老化生物质炭,采用团聚体湿筛法和定量PCR技术,从非生物和生物角度探究生物质炭对土壤团聚体稳定性的长期影响,明确生物质炭在土壤结构改良上的可利用性和可持续性。
1 材料与方法 1.1 研究区概况选取2个独立进行的水稻-小麦轮作农田开展研究。试验地一[19]位于江苏省句容市茅山镇(31°92′N,119°28′E),土壤类型为潴育型水稻土,土壤质地为黏壤土,基本组成为黏粒26%、粉粒34%和砂粒40%。0~20 cm土壤pH 5.3、有机碳14.6 g·kg–1和全氮1.8 g·kg–1。试验地二[20]位于江苏省南京市秣陵镇(31°52'N,118°50'E),土壤类型为潜育型水稻土,土壤质地为黏壤土,基本组成为黏粒14%、粉粒80%和砂粒6%。0~20 cm土壤pH 5.6、有机碳14.6 g·kg–1和全氮1.3 g·kg–1。两试验地均位于长江中下游地区,属于北亚热带季风气候区,年均日照时间2 048 h,年均气温接近15.7℃,年均降水量约1 050 mm。
1.2 试验设计试验地一、二均设置两个处理:CK(施用NPK肥)和AB(施用NPK肥+生物质炭),每个处理均设置3个重复小区,采用随机区组设计。每个小区间均具有独立灌水排水系统。试验田周围设有2 m保护行。种植、灌溉、施肥以及病虫害防治等田间管理措施均遵循当地常规管理方式。
试验地一生物质炭于2014年6月(15 t·hm–2)一次性施入句容试验地0~20 cm土层中,后续不再施用,为小麦秸秆在高温450℃限氧条件下制得,总碳含量513.0 g·kg–1、总氮11.3 g·kg–1、pH 10.5、表面积22.1 m2·g–1。自2014年6月开始,每个小区(3 m × 8 m)在移栽水稻当天施入化肥,施氮处理为尿素(以N计),每季用作基肥(120 kg·hm–2)、分蘖肥(120 kg·hm–2)和穗肥(60 kg·hm–2),分三次施入;每季过磷酸钙(以P2O5计)和氯化钾(以K2O计)均为125 kg·hm–2,作基肥一次性施入。
试验地二生物质炭于2012年6月(20 t·hm–2)一次性施入南京试验地0~20 cm土层中,后续不再施用,购自河南三利新能源有限公司,为小麦秸秆在高温400℃限氧条件下制得。总碳含量467.0 g·kg–1、总氮5.6 g·kg–1、pH 9.4、表面积8.9 m2·g–1、灰分20.8%。自2012年6月开始,每个小区(5 m × 4 m)在移栽水稻前一天施入化肥,施氮处理为尿素(以N计),每季用量为250 kg·hm–2,以4:3:3的比例分基肥和2次追肥施用;每季钙镁磷肥(以P2O5计)60 kg·hm–2和氯化钾(以K2O计)120 kg·hm–2用作基肥一次施入。
1.3 样品采集及测定方法两个试验地土壤样品均于2017年6月小麦收获后采集,每个处理3个重复小区,每个小区使用S型采样法采集5个0~20 cm混合土样。混合后分成三份,一份存储在4 ℃下用于土壤的基本理化特性和团聚体分析,一份土壤风干用于测定SOC、全氮和全磷含量,而另一份存储在-80 ℃下进行DNA提取和定量PCR测定。
土壤团聚体分级采用湿筛法[21]。干燥和重新润湿在稻麦轮作系统中非常普遍,故水稳性团聚体可以反映土壤结构和微生物群落的真实状态[16,22]。四种团聚体粒级,包括0.25~2 mm(大团聚体),0.053~0.25 mm(微团聚体),0.002~0.053 mm(粉砂粒),< 0.002 mm(黏粒),分别使用2、0.25和0.053 mm筛组和离心方法获得。将相当于100 g干土的新鲜土壤放置在2 mm筛上面,并在去离子水中浸泡10 min,通过手动上下晃动筛子3 cm,2min内重复50次实现团聚体分离,使所有土样通过2 mm筛子。筛子上残留的团聚体(> 2 mm)数量有限,使用镊子收集清洗 > 2 mm筛上面漂浮的植物残渣和石砾等。将通过2 mm筛子的土样倾倒通过0.25 mm筛子,从而得到大团聚体,将留在筛上的大团聚体转移到预先称重的烧杯中干燥。使用相同的方法将通过0.25 mm筛子的土样分多次通过0.053 mm筛,保留筛网上的微团聚体,同样将其转移到预先称重的烧杯中干燥。将通过0.053 mm筛子的土样转移到离心瓶中,通过逐步离心(600 r·min–1,15℃,4 min)分离出砂粒,并且将 < 0.002 mm粒级的上清液转移到其他离心瓶进行离心(4 200 r·min–1,15℃,36 min)得到黏粒。将不同粒级团聚体风干、称重,同时记录残渣和石砾质量,计算各粒级团聚体比例和回收率,同时用风干样品进一步测定不同粒级团聚体SOC、全氮和全磷含量。
土壤团聚体稳定性评价指标采用平均重量直径(MWD)、几何平均直径(GMD)、> 0.25 mm大团聚体比例(R> 0.25)衡量,分别按以下公式计算[23-24]:
| ${\rm{MWD}} = \frac{{\sum\nolimits_{i = 1}^n {\bar R \times {w_i}} }}{{\sum\nolimits_{i = 1}^n {{w_i}} }}$ |
| ${\rm{GMD}} = {\rm{Exp}}\left[ {\frac{{\sum\nolimits_{i = 1}^n {\ln {{\bar R}_i}} }}{{\sum\nolimits_{i = 1}^n {{m_i}} }}} \right]$ |
| ${R_{ > 0.25}} = \frac{{{M_{T > 0.25}}}}{{{M_T}}}$ |
式中,
土壤容重和田间持水量分别采用环刀法和环刀浸水法测定[25]。土壤pH和电导率按水土比5:1浸提后,分别用pH计(PHS-3C,上海)和电导率仪(FE30-K,上海)测定。土壤有机碳用外加热重铬酸钾氧化容量法测定;全氮用凯氏法测定;全磷用HClO4-H2SO4消煮、钼锑抗比色法测定[26]。
土壤样品DNA使用Fast DNA SPIN试剂盒(MP Biomedicals,美国)提取,取0.5 g土壤样品,用NanoDrop ND-1 000分光光度计(Nano Drop Technologies,美国)测定DNA浓度和质量,在0.8%琼脂糖电泳测定土壤DNA质量和大小。定量PCR扩增引物和反应条件如表 1所示。使用iCycler iQ5(美国Bio-Rad)进行实时定量PCR,扩增在20 μL反应混合物中进行,其中包括10 μL SYBR Green(日本TaKaRa),0.2 μL Rox DYEII,1 μL模板,0.4 μL前后引物(10 μmol–1)和8 μL无菌液。通过定量PCR估算土壤微生物丰度,描述为每克干土壤的基因拷贝数。
|
|
表 1 荧光实时定量PCR扩增引物和反应条件 Table 1 Amplification primers and reaction conditions of quantitative PCR |
统计分析前将土壤微生物拷贝数进行lg转换为归一化处理。采用IBM SPSS 22.0软件对土壤理化性质、土壤团聚体组成以及养分分布、微生物群落进行单因素方差分析(One-way ANOVA),Duncan法多重比较(α=0.05),结果以“平均值±标准差”形式表示,对平均重量直径与大团聚体比例、SOC、全磷、真菌、丛枝菌根真菌和古细菌的关系进行Pearson相关性分析。采用Origin Pro 2018软件绘图。
2 结果 2.1 原位老化生物质炭对土壤理化性质的影响如表 2所示,生物质炭原位老化后对土壤性质产生显著影响。句容和南京土壤AB处理pH较CK分别显著增加0.19和0.24个pH单位,大团聚体比例分别显著增加93.0%和61.5%,土壤田间持水量显著增加,平均重量直径和几何平均直径表现出增加趋势(P > 0.05)。句容土壤容重显著降低,南京土壤容重也呈降低趋势(P > 0.05)。综上所述,原位老化生物质炭改善土壤质量。
|
|
表 2 原位老化生物质炭对土壤理化性质的影响 Table 2 Effects of in situ aging biochar on soil physical and chemical properties |
如图 1a所示,各处理团聚体主要由0.002~0.053 mm粒级构成,该粒级约占团聚体总量70.0%。句容和南京土壤AB处理的大团聚体比例较CK分别显著增加93.0%和61.5%,0.002~0.053 mm和 < 0.002 mm粒级团聚体均呈减少趋势。由图 1b可知,与对照相比,句容和南京土壤AB处理全土SOC含量分别显著增加26.3%和26.9%,大团聚体中SOC含量分别显著增加72.4%和52.3%,微团聚体中SOC含量分别显著增加20.8%和30.0%。由图 1c可知,与对照相比,句容土壤全土全氮含量显著增加21.9%,大团聚体和微团聚体分别显著增加42.9%和18.2%。南京土壤全氮含量仅在全土中表现增加趋势(P > 0.05)。由图 1d可知,与CK处理相比,句容土壤全磷含量无显著变化,而南京土壤全土全磷含量显著增加25.4%。该结果表明,原位老化生物质炭有利于土壤大、微团聚体粒级形成和土壤碳、氮、磷养分增加。
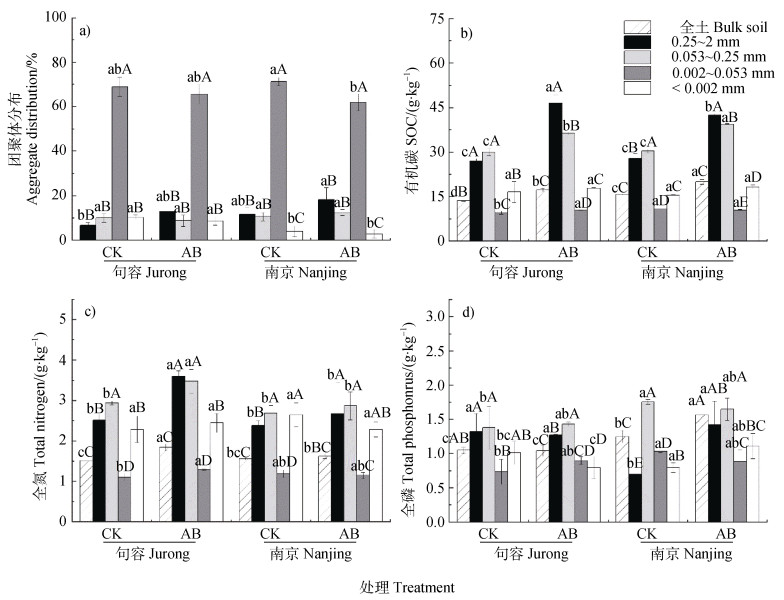
|
注:不同小写字母表示同一粒级团聚体不同处理之间差异显著(P < 0.05),不同大写字母表示同一处理不同粒级团聚体之间差异显著(P < 0.05)。下同。 Note: Different small letters indicate significant differences between different treatments the same in size fraction of aggregates (P < 0.05). Different capital letters indicate significant differences between different size fractions of aggregates in the same treatment (P < 0.05). The same as below. 图 1 原位老化生物质炭对土壤团聚体及养分分布的影响 Fig. 1 Effects of in situ aging biochar on soil aggregates and nutrient distribution |
原位老化生物质炭对土壤微生物丰度的影响如表 3所示。与对照相比,句容和南京土壤真菌丰度均显著增加,放线菌丰度表现出增加趋势;同时,南京土壤丛枝菌根真菌和古细菌丰度也显著增加(P < 0.05)。综上说明,原位老化生物质炭增加部分土壤微生物丰度。
|
|
表 3 原位老化生物质炭对土壤微生物丰度(lg copies·g-1)的影响 Table 3 Effects of in situ aging biochar on soil microbial abundance(lg copies·g-1) |
土壤平均重量直径与大团聚体比例、SOC、全磷、真菌、丛枝菌根真菌和古细菌的相关性分析如图 2所示。平均重量直径与大团聚体比例、SOC含量、真菌和丛枝菌根真菌丰度极显著正相关(P < 0.01);与全磷和古细菌丰度显著正相关(P < 0.05)。该结果表明,土壤团聚体稳定性与土壤碳、磷养分和真菌、丛枝菌根真菌和古细菌丰度显著正相关。
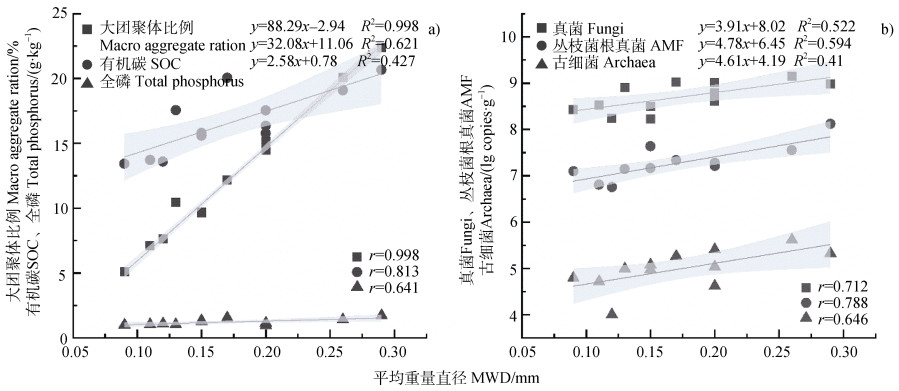
|
注:阴影部分表示95%置信区间。 Note: The shaded bands with different colors show 95% point-wise confidence intervals. 图 2 团聚体平均重量直径与土壤养分(a)和微生物丰度(b)的相关关系 Fig. 2 Correlations of mean weight diameter(MWD)with soil nutrients(a)and microbial abundance(b) |
土壤团聚体平均重量直径、几何平均直径和大团聚体比例是表示土壤团聚体稳定性的关键指标,其值越高,代表土壤对侵蚀和耕作的抵抗力越好[16]。本研究表明,句容和南京两地原位老化生物质炭仍可提高稻麦轮作土壤pH、大团聚体比例和平均重量直径(表 2);大、微团聚体比例显著增加,0.002~0.053 mm和 < 0.002 mm粒级团聚体比例均呈减少趋势(图 1),改善土壤结构。生物质炭随着老化过程其表面部分芳香碳结构被(含氧)烷基碳取代,羧基、羰基等酸性官能团增多,与阳离子结合形成羧酸盐和酚盐,释放出H+,导致生物质炭老化后pH降低,对土壤的碱性效应下降[12],土壤pH增加减少。生物质炭通过与矿物颗粒结合增加其内部黏结力,提高土壤团聚体的抗碎裂性,还刺激土壤微生物分泌物及胶结物质,从而有利于大团聚体的形成并增强团聚体稳定性[33]。在相对温和的模拟老化过程中,生物质炭比表面积较新鲜生物质炭增大一倍以上[34],对土壤稀释作用增强;Liang等[35]发现,添加生物质炭后,小麦在返青期生长急剧增加,根系分泌物增多,土壤微生物和动物呼吸作用增强,使土壤疏松,从而改善土壤结构。这可能还与老化生物质炭刺激土壤微生物活性,通过菌丝缠绕或分泌胶结物质等方式促进小粒级团聚体向大粒级团聚体转化有关[9]。
本研究也表明,土壤团聚体平均重量直径与大团聚体、SOC含量和全磷含量呈显著正相关(图 2a)。生物质炭可与土壤团聚体结合,减少其与外界接触面积,降低土壤有机质矿化速率,促进土壤固碳[36]。从污泥、猪粪和麦草制取的生物质炭,也可通过一定的物理吸附效应和化学作用抑制土壤CO2排放[37],提高土壤固碳潜力[12]。或者通过增强土壤团聚体胶结作用,使微团聚体黏结在一起,再通过菌丝体缠绕等形成大团聚体[38]。生物质炭还可以吸附磷酸盐[39],利于微生物和植物根系活动,促进土壤有机质合成,增强土壤抗侵蚀能力,提高团聚体稳定性[40],从而减轻农田颗粒态磷的损失,增强对磷的吸收并且降低溶解态磷地表径流损失风险[7]。
3.2 土壤微生物丰度对原位老化生物质炭的响应及其与土壤团聚体稳定性的关系生物质炭通过改变土壤理化性质直接影响微生物活动,进而改变土壤微生物群落丰度[6]。本研究结果显示,老化生物质炭能显著增加土壤真菌丰度,而放线菌丰度无显著变化(表 3)。有研究发现,生物质炭有利于增加蛋白水解酶(L-亮氨酸氨基肽酶)活性以及氨基酸和胺的利用,从而增加微生物代谢活性[41]。Zhu等[42]研究也表明,生物质炭通过其表面官能团(羧酸基等含氧基团)吸附并提供土壤微生物生长所需要的养分。Yao等[43]利用定量PCR发现,生物质炭的高孔隙度和大表面积可以改善土壤通气和持水能力,促进真菌菌丝生长,为土壤真菌提供良好的栖息地。此外,真菌能降解生物质炭中的顽固性碳[8]。Watzinger等[44]研究表明,放线菌丰度在酸性黏磐土比在钙质黑土中增加更显著,因为生物质炭对酸性黏磐土pH影响更大,而放线菌对酸性土壤比较敏感。Zheng等[15]则发现,酸性稻田中添加生物质炭4年后,放线菌丰度显著减少。这可能受土壤质地影响。
土壤团聚体和微生物不可分割,前者是后者生存的场所,后者是前者形成的主要因素之一[2]。本试验条件下土壤平均重量直径与真菌丰度极显著相关,相关系数为0.712(图 2b)。李景等[45]研究发现,耕作土壤细菌和古菌香农指数与平均重量直径显著相关,而真菌香农指数与平均重量直径相关性不显著。相反,何玉亭等[9]研究发现平均重量直径与真菌相关系数为0.890,支持本研究结果。同时,丛枝菌根真菌对植物与土壤理化性质变化反应灵敏,能够与80%以上陆生植物形成共生体,能够利用植物光合产物在土壤中形成根外菌丝,该菌丝体可为解磷细菌提供营养元素,促进解磷菌的生长,从而提高植物对磷的吸收[46]。而且丛枝菌根真菌在土壤碳、氮固存中起着关键作用,其菌丝的缠绕及其分泌的糖蛋白(球囊霉素)和多糖物质有利于土壤团聚体形成和结构稳定[47]。因此,老化生物质炭可能通过增加真菌和丛枝菌根真菌丰度,提高团聚体稳定性,促进植物对土壤碳、氮、磷养分的吸收。
4 结论老化生物质炭改善土壤pH和田间持水量等理化性质,增加0.25~2 mm大团聚体比例以及SOC和全磷含量,提高土壤团聚体稳定性。定量PCR结果表明,句容、南京两地稻田土壤微生物丰度均有不同程度的增加。老化生物质炭有利于土壤微生物的生长,增加真菌和丛枝菌根真菌丰度,促进土壤团聚体形成,间接提高土壤团聚体稳定性。综上表明,老化生物质炭能提高稻麦轮作麦季土壤团聚体稳定性,增加土壤微生物丰度。
| [1] |
Hemkemeyer M, Christensen B T, Martens R, et al. Soil particle size fractions harbour distinct microbial communities and differ in potential for microbial mineralisation of organic pollutants[J]. Soil Biology & Biochemistry, 2015, 90: 255-265.
(  0) 0) |
| [2] |
Li N, Han X Z, You M Y, et al. Research review on soil aggregates and microbes (In Chinese)[J]. Ecology and Environmnet, 2013, 22(9): 1625-1632. DOI:10.3969/j.issn.1674-5906.2013.09.027 [李娜, 韩晓增, 尤孟阳, 等. 土壤团聚体与微生物相互作用研究[J]. 生态环境学报, 2013, 22(9): 1625-1632.]
(  0) 0) |
| [3] |
Leng L J, Huang H J, Li H, et al. Biochar stability assessment methods: A review[J]. Science of the Total Environment, 2019, 647: 210-222. DOI:10.1016/j.scitotenv.2018.07.402
(  0) 0) |
| [4] |
Sun X, Han X G, Ping F, et al. Effect of rice-straw biochar on nitrous oxide emissions from paddy soils under elevated CO2 and temperature[J]. Science of the Total Environment, 2018, 628: 1009-1016.
(  0) 0) |
| [5] |
Gomez J D, Denef K, Stewart C E, et al. Biochar addition rate influences soil microbial abundance and activity in temperate soils[J]. European Journal of Soil Science, 2014, 65(1): 28-39. DOI:10.1111/ejss.12097
(  0) 0) |
| [6] |
Palansooriya K N, Wong J T F, Hashimoto Y, et al. Response of microbial communities to biochar-amended soils: A critical review[J]. Biochar, 2019, 1(1): 3-22. DOI:10.1007/s42773-019-00009-2
(  0) 0) |
| [7] |
Soinne H, Hovi J, Tammeorg P, et al. Effect of biochar on phosphorus sorption and clay soil aggregate stability[J]. Geoderma, 2014, 219/220: 162-167. DOI:10.1016/j.geoderma.2013.12.022
(  0) 0) |
| [8] |
Lehmann J, Rillig M C, Thies J, et al. Biochar effects on soil biota-A review[J]. Soil Biology & Biochemistry, 2011, 43(9): 1812-1836.
(  0) 0) |
| [9] |
He Y T, Wang C Q, Shen J, et al. Effects of two biochars on red soil aggregate stability and microbial community (In Chinese)[J]. Scientia Agricultura Sinica, 2016, 49(12): 2333-2342. DOI:10.3864/j.issn.0578-1752.2016.12.009 [何玉亭, 王昌全, 沈杰, 等. 两种生物质炭对红壤团聚体结构稳定性和微生物群落的影响[J]. 中国农业科学, 2016, 49(12): 2333-2342.]
(  0) 0) |
| [10] |
Ye L L, Wang C H, Zhou H, et al. Effects of rice straw-derived biochar addition on soil structure stability of an ultisol (In Chinese)[J]. Soils, 2012, 44(1): 62-66. DOI:10.3969/j.issn.0253-9829.2012.01.010 [叶丽丽, 王翠红, 周虎, 等. 添加生物质黑炭对红壤结构稳定性的影响[J]. 土壤, 2012, 44(1): 62-66.]
(  0) 0) |
| [11] |
Gundale M J, DeLuca T H. Temperature and source material influence ecological attributes of ponderosa pine and Douglas-fir charcoal[J]. Forest Ecology and Management, 2006, 231(1/2/3): 86-93.
(  0) 0) |
| [12] |
Yuan H J, Deng G S, Zhou S G, et al. Biochar ageing and its effects on greenhouse gases emissions: A review (In Chinese)[J]. Ecology and Environmental Sciences, 2019, 28(9): 1907-1914. [袁海静, 邓桂森, 周顺桂, 等. 生物炭的老化及其对温室气体排放影响的研究进展[J]. 生态环境学报, 2019, 28(9): 1907-1914.]
(  0) 0) |
| [13] |
Kuzyakov Y, Subbotina I, Chen H Q, et al. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling[J]. Soil Biology & Biochemistry, 2009, 41(2): 210-219.
(  0) 0) |
| [14] |
Duan P P, Zhang X, Zhang Q Q, et al. Field-aged biochar stimulated N2O production from greenhouse vegetable production soils by nitrification and denitrification[J]. Science of the Total Environment, 2018, 642: 1303-1310. DOI:10.1016/j.scitotenv.2018.06.166
(  0) 0) |
| [15] |
Zheng J F, Chen J H, Pan G X, et al. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China[J]. Science of the Total Environment, 2016, 571: 206-217. DOI:10.1016/j.scitotenv.2016.07.135
(  0) 0) |
| [16] |
Zhang Q Q, Song Y F, Wu Z, et al. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation[J]. Journal of Cleaner Production, 2020, 242: 118435. DOI:10.1016/j.jclepro.2019.118435
(  0) 0) |
| [17] |
Luo S S, Wang S J, Tian L, et al. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland[J]. Applied Soil Ecology, 2017, 117/118: 10-15. DOI:10.1016/j.apsoil.2017.04.024
(  0) 0) |
| [18] |
Verheijen F G A, Jeffery S, Bastos A, et al. Biochar application to soils: A critical scientific review of effects on soil properties, processes and functions . Luxembourg: European Commission Publication Office, 2010.
(  0) 0) |
| [19] |
He T H, Liu D Y, Yuan J J, et al. A two years study on the combined effects of biochar and inhibitors on ammonia volatilization in an intensively managed rice field[J]. Agriculture, Ecosystems & Environment, 2018, 264: 44-53.
(  0) 0) |
| [20] |
Wu Z, Zhang X, Dong Y B, et al. Biochar amendment reduced greenhouse gas intensities in the rice-wheat rotation system: Six-year field observation and meta-analysis[J]. Agricultural and Forest Meteorology, 2019, 278: 107625. DOI:10.1016/j.agrformet.2019.107625
(  0) 0) |
| [21] |
Six J, Elliott E, Paustian K, et al. Aggregation and soil organic matter accumulation in cultivated and native grassland soils[J]. Soil Science Society of America Journal, 1998, 62(5): 1367-1377. DOI:10.2136/sssaj1998.03615995006200050032x
(  0) 0) |
| [22] |
Bach E M, Hofmockel K S. Soil aggregate isolation method affects measures of intra-aggregate extracellular enzyme activity[J]. Soil Biology & Biochemistry, 2014, 69: 54-62.
(  0) 0) |
| [23] |
Hou X N, Li H, Zhu L B, et al. Effects of biochar and straw additions on lime concretion black soil aggregate composition and organic carbon distribution (In Chinese)[J]. Scientia Agricultura Sinica, 2015, 48(4): 705-712. [侯晓娜, 李慧, 朱刘兵, 等. 生物炭与秸秆添加对砂姜黑土团聚体组成和有机碳分布的影响[J]. 中国农业科学, 2015, 48(4): 705-712.]
(  0) 0) |
| [24] |
Shang J, Geng Z C, Zhao J, et al. Effects of biochar on water thermal properties and aggregate stability of Lou soil (In Chinese)[J]. Chinese Journal of Applied Ecology, 2015, 26(7): 1969-1976. [尚杰, 耿增超, 赵军, 等. 生物炭对塿土水热特性及团聚体稳定性的影响[J]. 应用生态学报, 2015, 26(7): 1969-1976.]
(  0) 0) |
| [25] |
Wang Y L. Analysis of experimental results of measuring soil field water holding capacity by ring knife method (In Chinese)[J]. Ground Water, 2016, 38(3): 55-57. DOI:10.3969/j.issn.1004-1184.2016.03.020 [王艳丽. 环刀法测定土壤田间持水量实验结果分析[J]. 地下水, 2016, 38(3): 55-57.]
(  0) 0) |
| [26] |
Lu R K. Analytical methods for soil and agro- chemistry (In Chinese). Beijing: China Agricultural Science and Technology Press, 2000. [鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000.]
(  0) 0) |
| [27] |
Muyzer G, de Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA[J]. Applied and Environmental Microbiology, 1993, 59(3): 695-700. DOI:10.1128/aem.59.3.695-700.1993
(  0) 0) |
| [28] |
May L A, Smiley B, Schmidt M G. Comparative denaturing gradient gel electrophoresis analysis of fungal communities associated with whole plant corn silage[J]. Canadian Journal of Microbiology, 2001, 47(9): 829-841. DOI:10.1139/w01-086
(  0) 0) |
| [29] |
Płaza G A, Upchurch R, Brigmon R, et al. Rapid DNA extraction for screening soil filamentous fungi using PCR amplification[J]. Polish Journal of Environmental Studies, 2004, 13(3): 315-318.
(  0) 0) |
| [30] |
Lumini E, Orgiazzi A, Borriello R, et al. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach[J]. Environmental Microbiology, 2010, 12(8): 2165-2179.
(  0) 0) |
| [31] |
Vetriani C, Jannasch H W, MacGregor B J, et al. Population structure and phylogenetic characterization of marine benthic archaea in deep-sea sediments[J]. Applied and Environmental Microbiology, 1999, 65(10): 4375-4384. DOI:10.1128/AEM.65.10.4375-4384.1999
(  0) 0) |
| [32] |
Heuer H, Krsek M, Baker P, et al. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients[J]. Applied and Environmental Microbiology, 1997, 63(8): 3233-3241. DOI:10.1128/aem.63.8.3233-3241.1997
(  0) 0) |
| [33] |
Sun F F, Lu S G. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil[J]. Journal of Plant Nutrition and Soil Science, 2014, 177(1): 26-33. DOI:10.1002/jpln.201200639
(  0) 0) |
| [34] |
Liu Y Y, Sohi S P, Jing F Q, et al. Oxidative ageing induces change in the functionality of biochar and hydrochar: Mechanistic insights from sorption of atrazine[J]. Environmental Pollution, 2019, 249: 1002-1010. DOI:10.1016/j.envpol.2019.03.035
(  0) 0) |
| [35] |
Liang B Q, Lehmann J, Sohi S P, et al. Black carbon affects the cycling of non-black carbon in soil[J]. Organic Geochemistry, 2010, 41(2): 206-213. DOI:10.1016/j.orggeochem.2009.09.007
(  0) 0) |
| [36] |
Li J Z, Dai K, Zhang L M, et al. Effects of biochar application on soil organic carbon distribution and soil aggregate composition of red soils in Yunnan tobacco planting area (In Chinese)[J]. Acta Scientiae Circumstantiae, 2016, 36(6): 2114-2120. [李江舟, 代快, 张立猛, 等. 施用生物炭对云南烟区红壤团聚体组成及有机碳分布的影响[J]. 环境科学学报, 2016, 36(6): 2114-2120.]
(  0) 0) |
| [37] |
Xu X Y, Kan Y, Zhao L, et al. Chemical transformation of CO2 during its capture by waste biomass derived biochars[J]. Environmental Pollution, 2016, 213: 533-540. DOI:10.1016/j.envpol.2016.03.013
(  0) 0) |
| [38] |
Xiao J J, Xing D, Mao M M, et al. Mechanism of arbuscular mycorrhizal fungal affecting soil aggregates in rhizosphere of mulberry (Morus alba) (In Chinese)[J]. Acta Pedologica Sinica, 2020, 57(3): 773-782. [肖玖军, 邢丹, 毛明明, 等. AM真菌对桑树根围土壤团聚体的影响机制[J]. 土壤学报, 2020, 57(3): 773-782.]
(  0) 0) |
| [39] |
Lehmann J. Bio-energy in the black[J]. Frontiers in Ecology & the Environment, 2007, 5(7): 381-387.
(  0) 0) |
| [40] |
Zhu Q L, Wang C, Yan J H, et al. Effects of straw and waste application on soil aggregates and soil carbon, nitrogen and phosphorus in the jasmine garden (In Chinese)[J]. Journal of Soil and Water Conservation, 2017, 31(4): 191-197. [朱秋丽, 王纯, 严锦华, 等. 施加秸秆与废弃物对茉莉园土壤团聚体及碳氮磷含量的影响[J]. 水土保持学报, 2017, 31(4): 191-197.]
(  0) 0) |
| [41] |
Tian J, Wang J Y, Dippold M, et al. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil[J]. Science of the Total Environment, 2016, 556: 89-97.
(  0) 0) |
| [42] |
Zhu X M, Chen B L, Zhu L Z, et al. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review[J]. Environmental Pollution, 2017, 227: 98-115. DOI:10.1016/j.envpol.2017.04.032
(  0) 0) |
| [43] |
Yao Q, Liu J J, Yu Z H, et al. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China[J]. Soil Biology & Biochemistry, 2017, 110: 56-67.
(  0) 0) |
| [44] |
Watzinger A, Feichtmair S, Kitzler B, et al. Soil microbial communities responded to biochar application in temperate soils and slowly metabolized 13C-labelled biochar as revealed by 13C PLFA analyses: Results from a short-term incubation and pot experiment[J]. European Journal of Soil Science, 2014, 65(1): 40-51.
(  0) 0) |
| [45] |
Li J, Wu H J, Wu X P, et al. Effects of long-term tillage measurements on soil aggregate characteristic and microbial diversity (In Chinese)[J]. Chinese Journal of Applied Ecology, 2014, 25(8): 2341-2348. [李景, 吴会军, 武雪萍, 等. 长期不同耕作措施对土壤团聚体特征及微生物多样性的影响[J]. 应用生态学报, 2014, 25(8): 2341-2348.]
(  0) 0) |
| [46] |
Yue F X, Li J W, Wang Y F, et al. Mechanism of the improvement effect by biochar and AM fungi on the availability of soil nutrients in coal mining area (In Chinese)[J]. Journal of Plant Nutrition and Fertilizer, 2019, 25(8): 1325-1334. [悦飞雪, 李继伟, 王艳芳, 等. 生物炭和AM真菌提高矿区土壤养分有效性的机理[J]. 植物营养与肥料学报, 2019, 25(8): 1325-1334.]
(  0) 0) |
| [47] |
Peng S L, Guo T, Liu G C. The effects of arbuscular mycorrhizal hyphal networks on soil aggregations of purple soil in southwest China[J]. Soil Biology & Biochemistry, 2013, 57: 411-417.
(  0) 0) |
 2021, Vol. 58
2021, Vol. 58


