2. 中国科学院大学, 北京 100049;
3. 冈山大学植物科学与资源研究所, 日本冈山 710-0046
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Institute of Plant Science and Resources, Okayama University, Okayama 710-0046, Japan
酸性土壤(pH < 5.5)约占世界耕地面积的40%、潜在可耕作土地的70%[1]。酸毒或氢离子毒害是酸性土壤中重要胁迫因子之一,严重限制土壤生产潜力的发挥。而且由于近几十年氨态氮肥的过量施用,不断加速土壤酸化速率,土壤酸化成为我国集约化农业体系中的主要问题之一[2]。特别需要注意的是,我国南方红壤区作为稻米的重要产区,也呈现明显酸化趋势。水稻作为约世界一半人口的主食[3],是我国南方居民的主要口粮,阐明酸毒的生理和分子机制,从而培育耐酸水稻品种以提高其在酸性土壤上的产量,是应对日益紧迫的粮食安全问题的必要措施。
低pH下,土壤中的氢离子浓度较高,常常引起土壤中金属离子如铝、铁和锰等的活化而间接造成植物伤害[4-6],因此,酸毒通常与金属离子胁迫共存,难以对其直接作用进行研究。关于酸性土壤中铝毒的研究已有大量报道[7-12],但关于酸毒或氢离子毒性本身的研究较少。酸毒严重抑制根系的生长发育,然而其重要性通常被低估[6,13-14]。与铝毒一样,酸毒可严重抑制根伸长[6],植物对酸毒最敏感的响应部位是伸长区和根毛[15]。低pH通过抑制根系的H+分泌[16],削弱细胞壁的完整性,导致根系生长受阻[17-19]。另外一个重要的酸毒机制是诱导产生活性氧(ROS)[20-22]。各种生物和非生物胁迫因素,如盐害、紫外线辐射、干旱、重金属、极端温度、营养缺乏和病虫害均会引起植物产生ROS而遭受氧化胁迫[23]。有研究报道,低pH削弱柑橘抗坏血酸代谢和谷胱甘肽代谢而诱导ROS积累[21],低pH会引起水稻1 318个基因发生差异表达,其中83%下调表达,这些基因最主要与氧化还原代谢和氧胁迫响应相关[24]。在分子机制方面,目前已在拟南芥中找到一些酸耐性基因,例如STOP1 [4,6],但在水稻中尚无相关报道。
一氧化氮(NO)是植物体中的一种重要气体信号分子,在植物生长发育和逆境响应中发挥重要作用[25-27],如促进种子萌发、参与诱导细胞程序性死亡、延缓叶片衰老、参与根系重力感应,以及参与乙烯释放和脱落酸信号转导等。植物中的NO合成可分为还原途径和氧化途径两种[28]。其中关于硝酸还原酶途径的研究最为透彻,主要发生在线粒体和细胞膜。目前NO在水稻酸毒中的角色尚不清楚。一般认为NO是植物次级抗氧化剂,可诱导激活抗氧化系统而清除ROS,亦可直接与ROS反应而清除ROS[29],在铝胁迫下NO可通过清除ROS而缓解铝毒[7-11],但它在植物酸毒中是否会发挥同样的作用值得探究。本研究选用前期筛选的酸耐性不同的两个水稻品种,分析了水稻的酸敏感性与ROS积累及氧化还原代谢相关酶的关系,并试图探讨酸毒下NO信号与ROS信号的调控关系,为阐明酸害的生理和分子机制奠定基础,从而为培育耐酸水稻品种提供理论指导。
1 材料与方法 1.1 植物材料与生长条件本研究选用酸耐性不同的两个籼稻品种Kasalath(酸耐性)和Jinguoyin(酸敏感)。水稻种子用10% H2O2消毒10 min,用去离子水洗净后在30℃恒温箱中避光浸泡催芽2 d。随后将刚露白的水稻种子转移至0.5 mmmol·L–1 CaCl2(pH 5.6)溶液的浮板上,26℃暗培养,CaCl2溶液每两天更换一次。
1.2 水稻酸耐性鉴定选择生长约5 d、整齐一致的水稻幼苗,分别在pH为4.2、4.5和4.8的含有0.5 mmmol·L–1 CaCl2水溶液中处理24 h,每个处理10株。用直尺测定处理前后根长,相减即为根伸长。
1.3 根系细胞膜完整性、丙二醛(MDA)、超氧阴离子和过氧化氢含量的测定根尖细胞膜的完整性采用伊文思蓝(Evans blue)吸收量的方法[30]测定。丙二醛的测定采用丙二醛(MDA)检测试剂盒(S0131S,碧云天,上海)。超氧阴离子的测定采用超氧阴离子检测试剂盒(BC1290,索莱宝,北京)。过氧化氢的测定采用过氧化氢检测试剂盒(BC3590,碧云天,上海)。蛋白浓度使用Bradford法[31]测定。
1.4 酶活性测定抗氧化酶包括抗坏血酸过氧化物酶(APX,BC0220)、过氧化氢酶(CAT,BC0200)、过氧化物酶(POD,BC0090)和超氧化物歧化酶(SOD,BC0170)的活性均采用索莱宝的试剂盒进行测定。还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶活性的测定采用植物NADPH氧化酶(NADPH-OX)ELISA试剂盒(MB-10702A,酶标生物,江苏)。根尖蛋白质的提取根据Tian等[12]的方法略有修改。硝酸还原酶(NR)活性的测定采用植物硝酸还原酶活性检测试剂盒(索莱宝,北京)。一氧化氮合成酶(NOS)的活性采用一氧化氮合成酶检测试剂盒(碧云天,上海)测定。
1.5 ROS和NO含量测定根尖ROS的测定采用二氢乙啶(Dihydroethidium,DHE)荧光染料法。将处理24 h后的水稻根尖切下,置于1 mL 10 μmmol·L–1 DHE的水溶液中避光37℃孵育30 min,蒸馏水冲洗3次后,用荧光显微镜(Nikon,Minato,Tokyo,日本)拍照,使用的滤光片为EX 540/25、DM565、BA605/55。根系NO含量的测定采用3-氨基-4-甲氨基-2’-7’-二氟荧光素(DAF-FM DA)进行测定。将水稻根尖1 cm剪下,在20 mmmol·L–1的4-羟乙基哌嗪乙磺酸(HEPES)缓冲液(pH7.4)中清洗20 min,然后将根尖置于含10 μmmol·L–1荧光染料的1.5 mL离心管中避光染色30 min,然后在20 mmmol·L–1的HEPES缓冲液(pH7.4)避光清洗三次,每次15 min,制片,在荧光显微镜(Nikon,Minato,Tokyo,日本)下观察拍片,使用的滤光片为EX 465-495、DM 505和BA 512-558。
1.6 NIA1、NIA2和NIA3基因表达测定每个处理取处理后的15~20条根尖(0~1 cm),利用液氮研磨粉碎,重复4次。RNA的提取使用TGuide Cells/Tissue/Plant RNA Kit(天根,北京)。获得的RNA用Nanodrop 2000C(Thermo Fisher,美国)测定浓度和质量,并在1%琼脂糖上电泳检查完整性。用PrimeScript RT试剂盒(Takara,日本)反转录合成cDNA。使用SYBR Premix ExTaq(Takara,日本)在Roche LightCycler 480 II上进行实时荧光定量PCR。以水稻Histone3为内参基因,正向引物GGTCAACTTGTTGATTCCCCTCT,反向引物AACCGCAAAATCCAAAGAACG。NIA1(Os08g0468100)和NIA2(Os08g0468700)因其序列高度相似[32],用同一个定量引物,正向CGTGT CGTGCCAATTAGAAA,反向GGAATCAACCG CTAGCCTCT,NIA3(Os02g0770800)的定量引物是正向GAGTTCCGGTACATCGGAGT,反向TGCACAACCATCCATCAATC。基因的相对表达量采用Ct法计算。
1.7 统计分析及作图文中使用最小显著差异法(LSD)进行方差分析,SigmaPlot 14.0软件进行作图。
2 结果 2.1 水稻品种Kasalath和Jinguoyin的酸敏感性差异培养液pH从4.8降至4.2,酸敏感水稻品种Jinguoyin的根伸长显著降低,根尖出现卷曲,而酸耐性品种Kasalath的根伸长并未受到明显影响(图 1a,图 1b)。说明Jinguoyin是酸敏感水稻品种,而Kasalath为酸耐性品种。
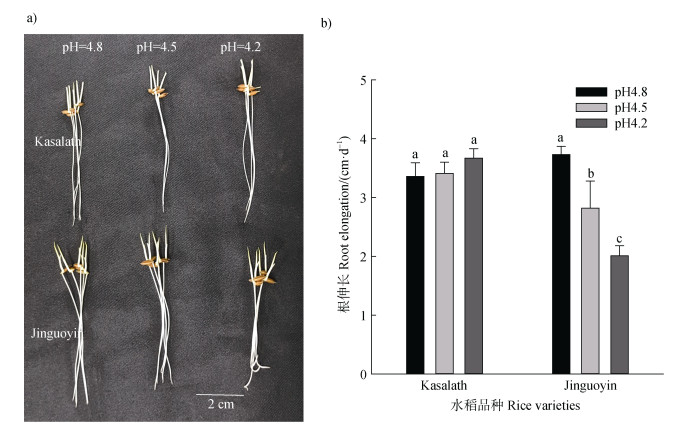
|
注:不同字母表示某个品种的根伸长在不同pH下的差异达显著水平(n = 10,P < 0.05)。 Note: Different letters indicate that the root elongation of a variety varies significantly at different pH values(n=10, P < 0.05). 图 1 pH对Kasalath和Jinguoyin根伸长的影响 Fig. 1 Effect of pH on the root elongation in Kasalath and Jinguoyin |
MDA是膜脂过氧化最重要的产物之一,pH从4.8下降至4.2,Jinguoyin根尖MDA浓度和伊文思蓝吸收量显著升高(图 2a,图 2b),表明Jinguoyin细胞膜完整性受到伤害。而Kasalath根尖MDA和伊文思蓝吸收量并未受到pH变化的显著影响。随着pH降低,Kasalath和Jinguoyin的根尖H2O2含量均未出现显著变化,而根尖O2·–含量均显著升高,且Jinguoyin中O2·–含量的增加幅度更大(图 2c,图 2d),因此,低pH引起的氧化胁迫可能是导致品种Jinguoyin酸毒的一个重要原因。
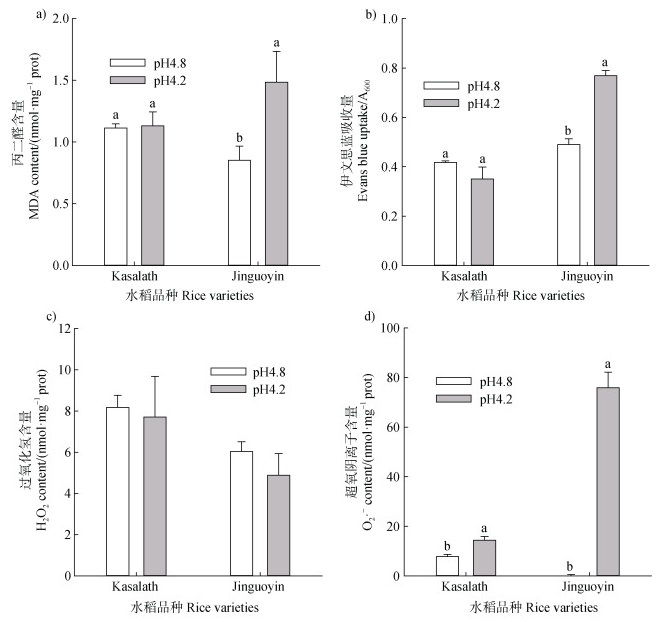
|
注:不同字母表示某个品种当前指标在不同pH下的差异达显著水平(n = 3,P < 0.05)。下同。 Note: Different letters indicate a significant difference in the current parameter of a variety at different pH values(n = 3, P < 0.05). 图 2 pH对Kasalath和Jinguoyin根尖细胞膜完整性和过氧化的影响 Fig. 2 Effect of pH on cell membrane integrity and peroxide in the root tips of Kasalath and Jinguoyin |
pH从4.8下降至4.2,Kasalath和Jinguoyin根尖SOD和APX活性均显著增加,且Jinguoyin的增加幅度更大(图 3a,图 3b)。抗氧化酶POD在Kasalath根尖未受到pH的显著影响,但在Jinguoyin中显著增加了106%(图 3c)。Kasalath和Jinguoyin根尖的CAT活性在低pH下均有增加的趋势,但是并不显著(图 3d)。NADPH氧化酶是根尖O2·–的主要来源之一,pH从4.8下降至4.2,Kasalath根尖的NADPH氧化酶未发生显著变化,而Jinguoyin根尖NADPH氧化酶活性显著增加了47.2%(图 3e)。
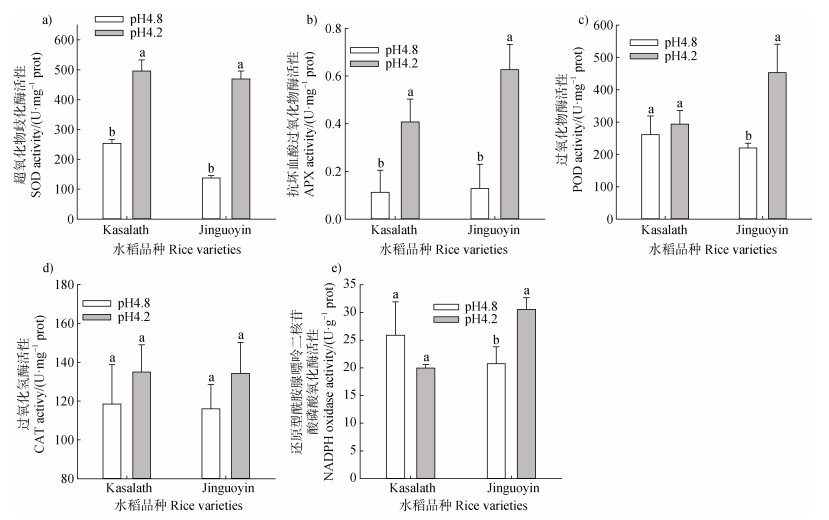
|
图 3 pH对Kasalath和Jinguoyin根尖抗氧化酶系统和还原型烟酰胺腺嘌呤二核苷酸磷酸氧化酶活性的影响 Fig. 3 Effect of pH on the activity of antioxidant enzymes and NADPH oxidase in the root tips of Kasalath and Jinguoyin |
培养液pH从4.8下降至4.2,酸敏感品种Jinguoyin根尖NO和ROS信号均明显增强,而酸耐性品种Kasalath根尖NO和ROS信号并未明显变化(图 4),表明NO和ROS可能参与酸毒害反应。Jinguoyin受酸毒后,NO信号主要集中在根冠和伸长区,而ROS信号在整个根尖均有分布。

|
注Note:pH 4.8(a,c,e,g);pH 4.2(b,d,f,h);Kasalath(a,b,e,f);Jinguoyin(c,d,g,h);NO(a,b,c,d);ROS(e,f,g,h). 图 4 pH对Kasalath和Jinguoyin根尖NO和ROS信号的影响 Fig. 4 Effect of pH on NO and ROS in the root tips of Kasalath and Jinguoyin |
为了探明酸毒诱导的NO和ROS信号的上下游调控关系,通过清除根尖NO来确定NO对ROS和根伸长的影响。添加NO清除剂2-(4-羧基苯基)- 4, 4, 5, 5-四甲基咪唑啉-1-氧基-3-氧化物钾盐(cPTIO)可以显著提高Jinguoyin在低pH下的根生长(图 5a,图 5b),同时显著降低低pH下根尖的NO和ROS信号(图 5c,图 5d)。

|
注:不同字母表示当前指标在不同处理下的差异达显著水平(n = 10,P < 0.05)。cPTIO为2-(4-羧基苯基)-4, 4, 5, 5-四甲基咪唑啉-1-氧基-3-氧化物钾盐。 Note: Different letters indicate a significant difference on the current parameter of a variety at different pH values(n = 10, P < 0.05). cPTIO is 2-(4-Carboxyphenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt. 图 5 NO清除剂cPTIO对Jinguoyin根生长(a,b)、根尖NO信号(c)和ROS信号(d)的影响 Fig. 5 Effect of NO scavenger cPTIO on root growth(a, b), NO(c)and ROS(d)in the root tips of Jinguoyin |
植物中的NO主要由硝酸还原酶和一氧化氮合酶合成,为了确定Jinguoyin中酸毒诱导的NO主要是哪个酶在发挥作用,观察外源添加这两个酶的抑制剂Gln(硝酸还原酶反馈抑制剂)和L-NAME(一氧化氮合成酶抑制剂)对根伸长和NO信号的影响。硝酸还原酶反馈抑制剂Gln可显著提高根系在低pH下的生长,而一氧化氮合酶抑制剂L-NAME未表现出显著的缓解作用(图 6a,图 6b)。同时,Gln明显降低Jinguoyin在低pH下的根尖NO信号,而L-NAME对根尖NO信号无影响(图 6c)。

|
注:不同字母表示当前指标在不同处理下的差异达显著水平(n = 10,P < 0.05)。Mock为空白对照,Gln为谷氨酰胺,L-NAME为N'-硝基-L-精氨酸甲酯盐酸盐。 Note: Different letters indicate a significant difference in the current parameter of a variety at different pH values(n = 10, P < 0.05). Mock is the control, Gln is glutamine, and L-NAME is NG-Nitro-L-arginine Methyl Ester, Hydrochloride. 图 6 酶抑制剂Gln和L-NAME对Jinguoyin根生长(a,b)和根尖NO信号(c)的影响 Fig. 6 Effect of enzyme inhibitor Gln and L-NAME on root growth(a, b)and NO(c)in the root tips of Jinguoyin |
低pH显著提高Kasalath和Jinguoyin根尖硝酸还原酶活性,而Jinguoyin中的增加幅度显著高于Kasalath(210.3% vs 47.5%)(图 7a)。低pH诱导Jinguoyin的一氧化氮合成酶活性增加17.3%,而对Kasalath无显著影响(图 7b)。可见,硝酸还原酶是Jinguoyin在酸毒胁迫下NO合成的主要来源。
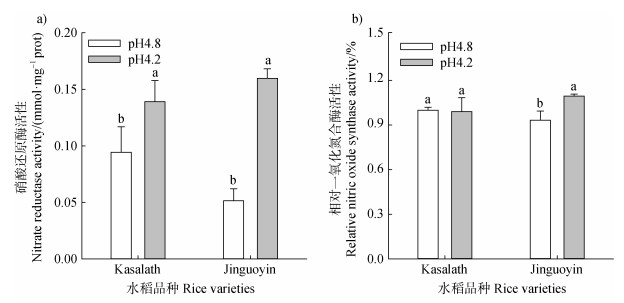
|
注:不同字母表示某个品种当前指标在不同pH下的差异达显著水平(n = 3,P < 0.05)。下同。 Note: Different letters indicate a significant difference in the current parameter of a variety at different pH values(n = 3, P < 0.05). The same below. 图 7 pH对Kasalath和Jinguoyin根尖硝酸还原酶活性(a)和一氧化氮合成酶活性(b)的影响 Fig. 7 Effect of pH on the activity of nitrate reductase(a)and nitric oxide synthase(b)in the root tips of Kasalath and Jinguoyin |
水稻基因组含有3个编码硝酸还原酶的基因,NIA1(Os08g0468100)、NIA2(Os08g0468700)和NIA3(Os02g0770800)。低pH显著提高了Jinguoyin中NIA1和NIA2的表达,其表达量提高了1.29倍,但对Kasalath中NIA1和NIA2的表达无显著影响(图 8a)。Kasalath和Jinguoyin中NIA3的表达均受到低pH的诱导,但Jinguoyin中的变化幅度更大(图 8b)。
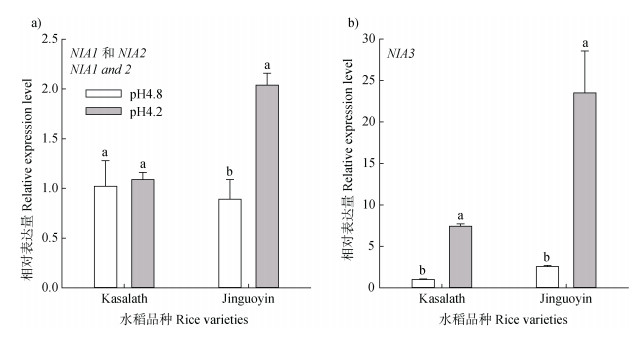
|
注:不同字母表示某个品种当前指标在不同pH下的差异达显著水平(n = 3,P < 0.05)。 Note: Different letters indicate a significant difference in the current parameter of a variety at different pH values(n = 3, P < 0.05). 图 8 pH对Kasalath和Jinguoyin根尖NIA1和NIA2(a)及NIA3(b)的影响 Fig. 8 Effect of pH on the expression of NIA1 and 2(a)and NIA3(b)in the root tips of Kasalath and Jinguoyin |
低pH降低根系的H+分泌[16],削弱细胞壁的完整性[17-19]以及诱导ROS产生[20-22],从而抑制根系生长。本研究中,低pH通过诱导以O2·–为主的ROS积累,对水稻品种Jinguoyin产生酸毒(图 2h,图 3c,图 3d)。NADPH氧化酶是植物遭遇生物和非生物逆境胁迫时产生O2·–的主要来源[33-34],本研究中低pH显著提高了Jinguoyin中NADPH氧化酶的活性(图 3e),因此推测Jinguoyin中升高的O2·–含量是由提高的NADPH氧化酶活性产生的。SOD能够催化O2·–歧化生成氧和过氧化氢,Jinguoyin中SOD活性虽然也显著提高(图 3a),但却未发挥应有的作用。可能原因是Jinguoyin中NADPH氧化酶催化产生O2·–速率要高于SOD消耗O2·–的速率,或者Jinguoyin中提高的SOD活性与NADPH氧化酶催化产生的O2·–所在位置并不一致,或者兼而有之。NADPH氧化酶催化产生O2·–主要位于质外体[35-36],在此部位负责清除O2·–的是铜锌-SOD(以铜或锌离子为辅基的SOD),但一般质外体的ROS清除机制并不高效[37],因此导致Jinguoyin积累O2·–而产生酸毒。
3.2 NO诱导根尖ROS积累在植物体内,NO作为气体信号分子通过激活抗氧化系统而间接清除ROS,亦可直接与ROS反应而清除ROS,因此它被称为次级抗氧化剂[29]。例如,水稻一氧化氮合酶介导的早期NO爆发,通过增强根系抗氧化能力,缓解水分胁迫诱导的氧化损伤[38]。大豆硝酸还原酶合成的NO通过调节根系抗坏血酸-谷胱甘肽循环,可以消除铝诱导的氧化胁迫[7]。NO也可提高红芸豆、大豆和小麦等植物的铝耐性[8-11]。本研究中,添加NO清除剂cPTIO同时清除酸敏感品种Jinguoyin根尖的NO和ROS(图 5b,图 5c),显著提高Jinguoyin在低pH下的根生长(图 5a),这表明低pH对酸敏感品种Jinguoyin的毒害作用与低pH诱导产生的NO有关。而且酸毒诱导的NO信号处于ROS信号的上游,可通过降低酸敏感品种Jinguoyin中NO信号而降低氧化胁迫来缓解酸毒。但是,NO在酸毒中诱导酸敏感品种Jinguoyin ROS积累的机制尚不清楚。有研究发现,在大豆铝胁迫中,NO通过促进细胞质葡萄糖-6-磷酸脱氢酶活性,进而提高NADPH氧化酶的活性,从而导致ROS积累,以产生氧化胁迫[39]。本研究中,酸敏感品种Jinguoyin根尖的NADPH氧化酶活性在低pH下大幅提高(图 3e),且NO含量也有显著提高(图 5c),但是否通过NO促进葡萄糖-6-磷酸脱氢酶活性而引起NADPH氧化酶活性升高,从而导致ROS积累,尚需进一步的验证。
3.3 硝酸还原酶合成NO目前,已知植物NO的生物合成途径至少有硝酸还原酶途径和一氧化氮合酶途径等7种[27]。本研究中,低pH导致Jinguoyin的硝酸还原酶活性的增加幅度远高于一氧化氮合酶(图 7),且外源添加硝酸还原酶反馈抑制剂Gln明显抑制低pH下Jinguoyin根尖NO信号的产生,而外源添加一氧化氮合酶抑制剂L-NAME对低pH下Jinguoyin根尖NO信号并无影响(图 6b),因此,低pH下Jinguoyin中NO的合成是通过硝酸还原酶途径。水稻品种Jinguoyin根尖硝酸还原酶活性的提高,一方面是由于低pH可直接激活硝酸还原酶[40],另一方面低pH会抑制亚硝酸还原酶活性,导致底物亚硝酸浓度提高,从而进一步促进硝酸还原酶合成NO[41]。此外,通过转录合成新的硝酸还原酶也可促进Jinguoyin根尖硝酸还原酶活性的提高。水稻中有两种硝酸还原酶,一种是NADH依赖的硝酸还原酶,由NIA1和NIA2编码;一种是NADPH依赖的硝酸还原酶,由NIA3编码。一般水稻硝酸还原酶的活性以NADH依赖的硝酸还原酶为主[32]。在酸敏感水稻Jinguoyin中,低pH使NIA1、NIA2和NIA3的表达均大幅升高(图 8),引起硝酸还原酶的活性升高,促进根尖NO信号的富集(图 5c)。而在酸耐性水稻Kasalath中NIA3的表达受到低pH诱导(图 8b),但NIA3编码的NADPH依赖的硝酸还原酶对硝酸还原酶活性的贡献并不高[32],它的上调引起硝酸还原酶活性升高但增加程度不高(图 7a),并未引起根尖NO信号发生明显变化。
4 结论本研究利用耐酸性不同的两个籼稻品种Kasalath和Jinguoyin,发现在酸敏感水稻Jinguoyin中低pH引起的酸毒与NO介导的ROS富集相关,酸毒引起的NO信号主要与硝酸还原酶活性的提高有关,而硝酸还原酶活性的提高与硝酸还原酶基因NIA1和NIA2的表达上调有关。
| [1] |
von Uexküll H R, Mutert E. Global extent, development and economic impact of acid soils[J]. Plant and Soil, 1995, 171(1): 1-15. DOI:10.1007/BF00009558
(  0) 0) |
| [2] |
Guo J H, Liu X J, Zhang Y, et al. Significant acidification in major Chinese croplands[J]. Science, 2010, 327(5968): 1008-1010. DOI:10.1126/science.1182570
(  0) 0) |
| [3] |
Food and Agriculture Organization (FAO). World agriculture : Towards 2015/2030 summary report[R]. Rome, 2002.
(  0) 0) |
| [4] |
Iuchi S, Koyama H, Iuchi A, et al. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(23): 9900-9905. DOI:10.1073/pnas.0700117104
(  0) 0) |
| [5] |
Kochian L V, Piñeros M A, Liu J P, et al. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance[J]. Annual Review of Plant Biology, 2015, 66: 571-598. DOI:10.1146/annurev-arplant-043014-114822
(  0) 0) |
| [6] |
Kobayashi Y, Kobayashi Y, Watanabe T, et al. Molecular and physiological analysis of Al3+ and H+ rhizotoxicities at moderately acidic conditions[J]. Plant Physiology, 2013, 163(1): 180-192. DOI:10.1104/pp.113.222893
(  0) 0) |
| [7] |
Wang H H, Li Y, Hou J J, et al. Nitrate reductasemediated nitric oxide production alleviates Al-induced inhibition of root elongation by regulating the ascorbate-glutathione cycle in soybean roots[J]. Plant and Soil, 2017, 410(1/2): 453-465.
(  0) 0) |
| [8] |
Cai M Z, Zhang S N, Wang F M, et al. Protective effect of exogenously applied nitric oxide on aluminum-induced oxidative stress in soybean plants[J]. Russian Journal of Plant Physiology, 2011, 58(5): 791-798. DOI:10.1134/S1021443711050037
(  0) 0) |
| [9] |
Sun C L, Lu L L, Liu L J, et al. Nitrate reductasemediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum)[J]. New Phytologist, 2014, 201(4): 1240-1250. DOI:10.1111/nph.12597
(  0) 0) |
| [10] |
Wang H, Huang J, Bi Y. Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots[J]. Plant Science, 2010, 179(3): 281-288. DOI:10.1016/j.plantsci.2010.05.014
(  0) 0) |
| [11] |
Zhang H, Li Y H, Hu L Y, et al. Effects of exogenous nitric oxide donor on antioxidant metabolism in wheat leaves under aluminum stress[J]. Russian Journal of Plant Physiology, 2008, 55(4): 469-474. DOI:10.1134/S1021443708040067
(  0) 0) |
| [12] |
Tian Q Y, Sun D H, Zhao M G, et al. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos[J]. New Phytologist, 2010, 174(2): 322-331.
(  0) 0) |
| [13] |
Bose J, Babourina O, Shabala S, et al. Aluminumdependent dynamics of ion transport in Arabidopsis: Specificity of low pH and aluminum responses[J]. Physiologia Plantarum, 2010, 139(4): 401-412.
(  0) 0) |
| [14] |
Lager I, Andréasson O, Dunbar T L, et al. Changes in external pH rapidly alter plant gene expression and modulate auxin and elicitor responses[J]. Plant, Cell & Environment, 2010, 33(9): 1513-1528.
(  0) 0) |
| [15] |
Tsang D L, Edmond C, Harrington J L, et al. Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway[J]. Plant Physiology, 2011, 156(2): 596-604. DOI:10.1104/pp.111.175372
(  0) 0) |
| [16] |
Yan F, Schubert S, Mengel K. Effect of Low Root Medium pH on Net Proton Release, Root Respiration, and Root Growth of Corn(Zea mays L.) and Broad Bean (Vicia faba L.)[J]. Plant Physiology, 1992, 99(2): 415-421. DOI:10.1104/pp.99.2.415
(  0) 0) |
| [17] |
Monshausen G B, Bibikova T N, Messerli M A, et al. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(52): 20996-21001. DOI:10.1073/pnas.0708586104
(  0) 0) |
| [18] |
Tanimoto E, Fujii S, Yamamoto R, et al. Measurement of viscoelastic properties of root cell walls affected by low pH in lateral roots of Pisum sativum L[J]. Plant and Soil, 2000, 226(1): 21-28. DOI:10.1023/A:1026460308158
(  0) 0) |
| [19] |
Koyama H, Toda T, Hara T. Brief exposure to low-pH stress causes irreversible damage to the growing root in Arabidopsis thaliana: Pectin-Ca interaction may play an important role in proton rhizotoxicity[J]. Journal of Experimental Botany, 2001, 52(355): 361-368.
(  0) 0) |
| [20] |
Chen H F, Zhang Q, Zhang Z H. Comparative transcriptome combined with metabolomic and physiological analyses revealed ROS-mediated redox signaling affecting rice growth and cellular iron homeostasis under varying pH conditions[J]. Plant and Soil, 2019, 434(1/2): 343-361.
(  0) 0) |
| [21] |
Long A, Huang W L, Qi Y P, et al. Low pH effects on reactive oxygen species and methylglyoxal metabolisms in Citrus roots and leaves[J]. BMC Plant Biology, 2019, 19(1): 477. DOI:10.1186/s12870-019-2103-5
(  0) 0) |
| [22] |
Yang T Y, Huang W T, Zhang J, et al. Raised pH conferred the ability to maintain a balance between production and detoxification of reactive oxygen species and methylglyoxal in aluminum-toxic Citrus sinensis leaves and roots[J]. Environmental Pollution, 2021, 268: 115676. DOI:10.1016/j.envpol.2020.115676
(  0) 0) |
| [23] |
Gill S S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiology and Biochemistry, 2010, 48(12): 909-930. DOI:10.1016/j.plaphy.2010.08.016
(  0) 0) |
| [24] |
Chen H F, Zhang Q, Zhang Z H. Comparative transcriptome combined with metabolomic and physiological analyses revealed ROS-mediated redox signaling affecting rice growth and cellular iron homeostasis under varying pH conditions[J]. Plant and Soil, 2019, 434(1/2): 343-361.
(  0) 0) |
| [25] |
Zhang Y Y, Zhang W H, Xue L, et al. Advance on NO function in plant growth, development and abiotic stress tolerance (In Chinese)[J]. Acta Botanica Boreali-Occidentalia Sinica, 2012, 32(4): 835-842. DOI:10.3969/j.issn.1000-4025.2012.04.032 [张艳艳, 章文华, 薛丽, 等. 一氧化氮在植物生长发育和抗逆过程中的作用研究进展[J]. 西北植物学报, 2012, 32(4): 835-842.]
(  0) 0) |
| [26] |
Xia H W, Shi G X, Huang M, et al. Advances on effects of nitric oxide on resistances of plants to heavy metal stress (In Chinese)[J]. Acta Ecologica Sinica, 2015, 35(10): 3139-3147. [夏海威, 施国新, 黄敏, 等. 一氧化氮对植物重金属胁迫抗性的影响研究进展[J]. 生态学报, 2015, 35(10): 3139-3147.]
(  0) 0) |
| [27] |
Xiong J, Fu G F, Yang Y J, et al. Roles of nitric oxide in growth of plant root (In Chinese)[J]. Journal of Huazhong Agricultural University, 2011, 30(3): 375-383. DOI:10.13300/j.cnki.hnlkxb.2011.03.009 [熊杰, 符冠富, 杨永杰, 等. 一氧化氮在植物根系生长发育过程中的作用研究进展[J]. 华中农业大学学报, 2011, 30(3): 375-383.]
(  0) 0) |
| [28] |
Gupta K J, Fernie A R, Kaiser W M, et al. On the origins of nitric oxide[J]. Trends in Plant Science, 2011, 16(3): 160-168. DOI:10.1016/j.tplants.2010.11.007
(  0) 0) |
| [29] |
Sahay S, Gupta M. An update on nitric oxide and its benign role in plant responses under metal stress[J]. Nitric Oxide, 2017, 67: 39-52. DOI:10.1016/j.niox.2017.04.011
(  0) 0) |
| [30] |
Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid Peroxidation Is an Early Symptom Triggered by Aluminum, But Not the Primary Cause of Elongation Inhibition in Pea Roots[J]. Plant Physiology, 2001, 125(1): 199-208. DOI:10.1104/pp.125.1.199
(  0) 0) |
| [31] |
Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Analytical Biochemistry, 1976, 72(1/2): 248-254.
(  0) 0) |
| [32] |
Araki R, Kousaka K, Namba K, et al. 2'‐ Deoxymugineic acid promotes growth of rice(Oryza sativa L.) by orchestrating iron and nitrate uptake processes under high pH conditions[J]. Plant Journal, 2015, 81(2): 233-246. DOI:10.1111/tpj.12722
(  0) 0) |
| [33] |
Hao F, Wang X, Chen J. Involvement of plasmamembrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings[J]. Plant Science, 2006, 170(1): 151-158. DOI:10.1016/j.plantsci.2005.08.014
(  0) 0) |
| [34] |
Scharte J, Schön H, Tjaden Z, et al. Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(19): 8061-8066. DOI:10.1073/pnas.0812902106
(  0) 0) |
| [35] |
Gilroy S, Suzuki N, Miller G, et al. A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling[J]. Trends in Plant Science, 2014, 19(10): 623-630. DOI:10.1016/j.tplants.2014.06.013
(  0) 0) |
| [36] |
Gilroy S, Białasek M, Suzuki N, et al. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants[J]. Plant Physiology, 2016, 171(3): 1606-1615. DOI:10.1104/pp.16.00434
(  0) 0) |
| [37] |
Choudhury F K, Rivero R M, Blumwald E, et al. Reactive oxygen species, abiotic stress and stress combination[J]. Plant Journal, 2017, 90(5): 856-867. DOI:10.1111/tpj.13299
(  0) 0) |
| [38] |
Cao X C, Zhu C Q, Zhong C, et al. Nitric oxide synthase-mediated early nitric oxide burst alleviates water stress-induced oxidative damage in ammoniumsupplied rice roots[J]. BMC Plant Biology, 2019, 19(1): 108. DOI:10.1186/s12870-019-1721-2
(  0) 0) |
| [39] |
Wang H H, Hou J J, Li Y, et al. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration[J]. Plant and Soil, 2017, 416(1/2): 39-52.
(  0) 0) |
| [40] |
Kaiser W M, Brendle-Behnisch E. Acid-base-modulation of nitrate reductase in leaf tissues[J]. Planta, 1995, 196(1): 1-6. DOI:10.3760/cma.j.issn.0254-1785.1995.01.001
(  0) 0) |
| [41] |
Ferrari T E, Varner J E. Intact tissue assay for nitrite reductase in barley aleurone layers[J]. Plant Physiology, 1971, 47(6): 790-794. DOI:10.1104/pp.47.6.790
(  0) 0) |
 2023, Vol. 60
2023, Vol. 60


