2. 中国科学院生态环境研究中心, 北京 100085
2. Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
气候变化是人类发展所面临的全球挑战之一[1]。氧化亚氮(N2O)是一种长寿命强效的温室气体,具有较二氧化碳高273倍的增温潜势,可在大气中存留约120年,也是臭氧层最主要的破坏物质[2-3]。全球N2O排放量自19世纪初的N 10~12 Tg⋅a–1增至目前约N 17 Tg⋅a–1。其中,自然源N2O排放一直稳定在约N 10 Tg⋅a–1,而人为源N2O排放自19世纪以来持续增至目前的N 6~7 Tg⋅a–1,人为源排放的增加是全球N2O排放增加的主要原因[4]。施用化肥及有机肥的农田土壤是N2O最大的人为排放源,排放量N 3~4 Tg⋅a–1,约占总人为排放的60%,且目前仍在以每十年N 0.27 Tg的速度增加[4]。农田土壤中,旱地是主要的N2O排放源。近十年旱地N2O排放因子约1.1%,而水田N2O排放因子在0.5%左右[5]。全球每年铵态或酰胺态氮肥使用量(120 Tg)占化肥氮总使用量(170 Tg)的70%左右[6–8]。铵态氮肥的大量使用,使氨氧化及其驱动的后续偶联过程对陆地N2O排放(直接和间接影响)具有重要贡献。但目前对土壤氨氧化特性及其对N2O排放的作用机制仍理解不清,是N2O减排和估算不确定性的主要来源之一[8]。
氨氧化是硝化过程的第一步和限速步骤,即在氨单加氧酶(AMO)和羟胺脱氢酶(HAO)的催化下,将氨(NH3)经羟胺(NH2OH)氧化至亚硝酸根(NO2–)的过程。氨氧化可通过NH2OH的不完全氧化和反应产物(NH2OH、一氧化氮NO、NO2–)的偶联反应直接产生N2O。自养氨氧化过程由三类氨氧化微生物进行,包括氨氧化细菌(Ammonia oxidizing bacteria,AOB)、氨氧化古菌(Ammonia oxidizing archaea,AOA)和全程硝化菌(Complete ammonia oxidizing bacteria,comammox)。三类氨氧化微生物偏好的生态位及N2O产率具有显著差异[8]。AOB偏好碱性高NH3环境,AOA偏好酸性低NH3环境[8]。comammox则与AOA更为相似,更适应于寡营养环境[9]。AOB可通过硝化反硝化作用和非生物反应产生N2O[10]。目前还未发现AOA和comammox可以进行硝化反硝化作用,因为缺少NO还原酶基因,但其可以通过中间产物的非生物反应产生N2O[9]。从量级上看,AOB的N2O产率显著高于AOA的N2O产率[11]。纯培养研究表明,与AOB和AOA产生的N2O相比,comammox虽是一个不可忽略的N2O产生源,但贡献很小[12]。异养微生物也可将还原态氮(包括有机氮)氧化为亚硝态氮和硝态氮,即异养硝化作用。异养硝化微生物对还原态氮的氧化能力远不如自养氨氧化微生物,因此异养硝化作用通常被认为可以忽略。但在酸性和有机碳较高的土壤中,其对硝化作用可能具有重要贡献[13]。因此,土壤中氨氧化微生物对氨氧化过程的相对贡献,决定着土壤整体的氨氧化能力及后续N2O的产生。尽管对特定氨氧化微生物类群的氨氧化和N2O排放已有一定认识,目前对不同土壤类型和农田管理措施下氨氧化微生物类群对N2O排放的相对贡献组成规律还缺乏系统的研究。
选择性抑制法被广泛用于研究不同种类氨氧化微生物的相对贡献。相较于稳定同位素,选择性抑制法操作简便、成本低,通过特异性抑制不同氨氧化微生物的活动,可以指示各类氨氧化微生物对氨氧化及N2O排放的相对贡献。研究表明,乙炔通过破坏AOB、AOA和comammox的AMO酶可抑制其氨氧化过程,是一种最为常用的自养硝化菌的特异性抑制剂,可用于区分自养硝化和异养硝化过程的N2O排放[14]。由于AOB对长链的炔烃更加敏感,辛炔常被作为AOB的特异性抑制剂,对AOA和comammox几乎没有抑制作用[15-16]。通过乙炔和辛炔的组合使用,可以区分AOB、AOA + comammox、异养硝化菌对土壤氨氧化过程及N2O排放的相对贡献。本研究通过采集我国不同pH和有机碳的典型农田耕层土壤(潮土、黑土、砖红壤、红壤),以及有机肥改良的砖红壤剖面土壤,采用选择性抑制法(乙炔或辛炔)区分AOB、AOA + comammox以及异养硝化菌对土壤硝化潜势、净硝化速率及N2O排放的相对贡献,以阐明氨氧化微生物对土壤氨氧化能力和N2O排放的相对贡献,以及与土壤性质和人为管理因素的关系,为制定与土壤氨氧化特性及土壤性质相匹配的农田管理措施、有效减少N2O排放提供新的科学依据。
1 材料与方法 1.1 土壤采集与基础信息根据pH差异选取潮土、黑土、砖红壤和红壤四种土壤类型,分别采自北京上庄、黑龙江哈尔滨、海南澄迈和江西鹰潭的典型传统管理农田耕层(0~20 cm)。潮土(SZ)采自北京上庄小麦–玉米轮作田块,黑土(HB)采自哈尔滨玉米–大豆轮作田块,砖红壤(XA)采自澄迈西岸村香蕉田块,红壤(YT)采自鹰潭花生–休闲田块。
在澄迈红山农场种植年限分别为1年(H1)和4年(H4)的两个菠萝田块,分别采集0~20 cm、20~40 cm及40~60 cm砖红壤剖面样品,探究人为管理(有机肥投入)导致土壤剖面性质改变后,对土壤氨氧化能力、氨氧化微生物活动及N2O排放的影响。
10个土壤样品均在各田块以五点法随机采集,混匀后剔除根系、植物残体及杂物,过2 mm筛,取1 kg用于理化性质测定及培养试验。土壤基础理化性质见表 1。
|
|
表 1 供试土壤基础理化性质 Table 1 Chemical and physical properties of soils |
选取所有供试土壤,在反应底物充足和完全有氧的条件下,结合选择性抑制剂测定土壤硝化潜势及不同氨氧化微生物的贡献[17](图 1),同时测定N2O排放。设计4个处理,分别为(1)空白(N0)、(2)施氮(N140)、(3)施氮加乙炔(N140+Acetylene,自养氨氧化菌抑制剂)及(4)施氮加辛炔(N140+ Octyne,AOB抑制剂),每个处理3个重复。取4 g风干土壤至120 mL血清瓶,重新湿润土壤至15%重量含水量,25℃下预培养一周。预培养后,施氮处理加入40 mL浓度为1 mmol⋅L–1的NH4Cl溶液,实现目标施氮量为N 140 mg⋅kg–1干土,空白处理加入40 mL去离子水,立即用硅胶塞和铝盖密封瓶口。乙炔和辛炔气体立即通过注射器注射进入瓶内顶空气体,添加浓度依据文献中的最适浓度确定,分别为1%v/v[18]和4 μmol⋅L–1 Caq[14]。25℃,175 r⋅min–1连续振荡培养72 h,期间分别于0、12、24、48、72 h将血清瓶从震荡机中取出,静置15 min,用注射器吸取1 mL上清液离心后进行无机氮的测定,以NO3–线性增长阶段的累积速率计算硝化潜势;于0、24、48、72 h抽取顶空气体16 mL用于测定培养期间N2O的累积排放量,气体抽取后补充16 mL氦气进入瓶中继续震荡。
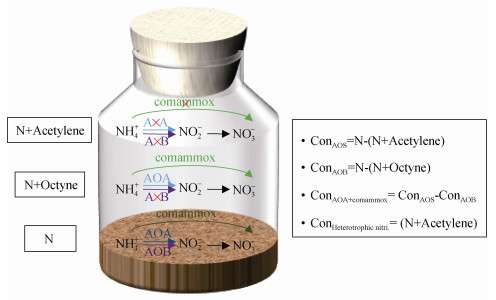
|
注:图中蓝色、紫色及绿色箭头分别表示AOA、AOB及comammox执行的氨氧化过程。右侧计算式中的Con表示不同氨氧化类群的相对贡献,AOS为自养氨氧化微生物、AOB为氨氧化细菌、AOA为氨氧化古菌、comammox为全程硝化细菌、Heterotrophic nitri.为异养硝化微生物。 Note: The blue, purple and green arrows indicate ammonia oxidation performed by AOA, AOB and comammox, respectively. The Con in the right box indicates the relative contribution of different ammonia-oxidizing microorganisms, AOS, AOB, AOA, comammox and Heterotrophic nitri. represent autotrophic ammonia-oxidizing microorganisms, ammonia-oxidizing bacteria, ammonia-oxidizing archaea, complete ammonia oxidizers and heterotrophic nitrifiers. 图 1 不同抑制剂确定氨氧化微生物相对贡献的原理示意图 Fig. 1 Schematic diagram of the mechanisms for calculating the relative contribution of different ammonia-oxidizing microorganisms using different inhibitors |
选取硝化潜势差异显著的3个耕层土样(SZ、HB、XA)及改良4年剖面的3个土层深度土样(H4-20、H4-40、H4-60),开展微宇宙静态培养实验,以研究土壤实际的净硝化速率、氨氧化微生物活动及N2O排放。实验设置3个处理,分别为(1)施氮(N100)、(2)施氮加乙炔(N100+Acetylene)、(3)施氮加辛炔(N100+Octyne),每个处理4个重复。称取100 g风干土壤样品于550 mL玻璃广口瓶中,调节土壤的重量含水量为15%,以parafilm封口膜封瓶口,在25℃恒温培养箱中避光预培养一周。预培养结束后均匀加入5 mL浓度143 mmol⋅L–1 NH4Cl溶液,实现目标施氮量N 100 mg⋅kg–1干土,施氮量依据田间传统施氮量设置。补充去离子水调节重量含水量至20%,用配有三通阀的橡胶塞密封培养瓶。乙炔和辛炔气体立即通过注射器注射进入瓶内顶空气体,添加浓度分别为1%v/v和4 μmol⋅L–1 Caq。在培养箱中以25℃条件连续培养14 d,0、1、3、7、14 d对土壤样品破坏取样,用于测定无机氮;1、2、3、5、7、10、14 d采集顶空气体,采气前将橡胶塞打开,使瓶内气体与空气充分流通混匀,分别于0、10、20、30 min采集培养瓶内顶空气体,采气时以注射器混匀瓶内气体后取20 mL至真空瓶内用于N2O气体分析。盖上瓶塞后再次补充气体抑制剂以继续培养和下次采气。
1.4 土壤及气体指标测定土壤无机氮测定通过1 mol⋅L–1 KCl溶液浸提,将浸提液加入96孔酶标板,采用水杨酸钠-硝普钠比色法在660 nm波长下测定NH4+浓度、磺胺重氮化反应比色法在540 nm波长下测定NO2–浓度、氯化钒还原比色法测定NO3–浓度[17],使用酶标仪(Infinite F50,Tecan Austria GmbH)读取数据。土壤pH以土水比1︰2.5浸提后用pH计测定(Seven Compact,Mettler Toledo),有机碳采用重铬酸钾氧化—分光光度法进行测定,全氮(TN)采用凯氏法进行测定,土壤质地采用比重计法进行测定[19]。
N2O浓度采用气相色谱仪(Agilent GC 8890)进行分析,检测器为电子捕获器ECD,检测温度350℃,柱温55℃。
1.5 数据处理与分析硝化潜势实验中气体累积排放量(E)的计算式为:
| $ E = \frac{{273}}{{273 + T}} \times {\text{M}}/22.4/m \times C({V_{\text{g}}} + {V_1} \times \alpha )/1000 $ | (1) |
式中,E为N2O累积量(N2O-N μg⋅kg–1),C为顶空气体中N2O的浓度(nL⋅L–1),Vg为顶空气体总体积(L),Vl为培养体系中的液体体积(L),α为N2O在25℃时的溶解系数(0.544)[20],T为培养温度(℃),M为N2O中N的摩尔质量,22.4为273 K温度下气体的摩尔体积(L⋅mol–1),m为瓶内烘干土重(kg)。
静态培养实验中气体排放通量(F)计算式为:
| $ F = \frac{{273}}{{273 + T}} \times {\text{M}} \times 60 \times 24 \times C \times {10^{ - 3}} \times V \times {\text{d}}c/{\text{d}}t/22.4/m $ | (2) |
式中,F为N2O排放通量(N2O-N μg⋅kg–1⋅d–1),T为培养温度(℃),M为N2O中N的摩尔质量,22.4为273 K温度下气体的摩尔体积(L⋅mol–1),m为瓶内烘干土重(kg),V为培养瓶顶空气体体积(L),C为气体浓度(nL⋅L–1),dc/dt为培养瓶内气体浓度的时间变化率(nL⋅L–1⋅min–1)。
上述数据处理采用Microsoft Excel 2019,各土样间硝化潜势、净硝化速率、N2O排放差异的显著性检验(P < 0.05)采用IBM SPSS Statistics 26进行,图表绘制采用Origin 2023。
2 结果 2.1 不同土壤类型和农田管理对土壤pH和有机碳的影响四个耕层土壤中,潮土(SZ)、黑土(HB)、砖红壤(XA)和红壤(YT)的酸碱度呈现北方土壤(SZ、HB)为碱性和中性,南方土壤(XA、YT)为酸性的趋势。黑土和砖红壤的有机碳显著高于潮土和红壤。
有机肥改良的剖面土壤中,改良1年的剖面样品(H1-20~H1-60)pH在土壤不同层次间还未显著变化(4.5~4.6),其有机碳由深层至表层呈现递增趋势,这主要是该剖面刚施入的有机肥还未矿化完全导致的有机碳短暂累积。改良4年的剖面样品(H4-20~H4-60)pH和有机碳均由深层至表层呈现递增的趋势。
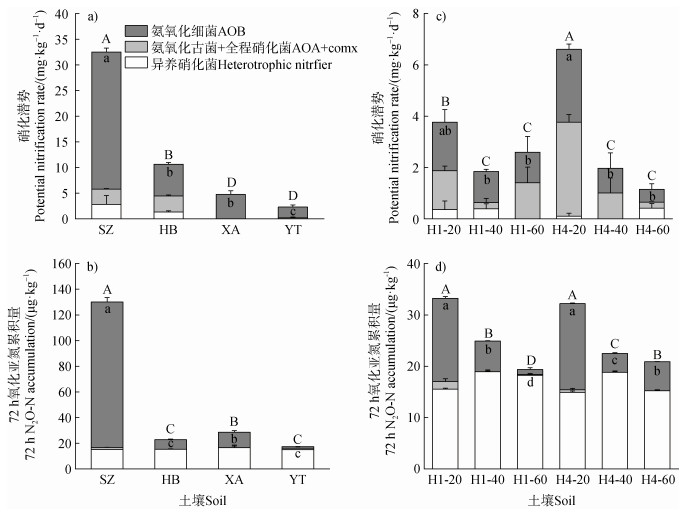
2.2 土壤硝化潜势、N2O排放及氨氧化微生物相对贡献四个耕层土壤中,潮土(SZ)、黑土(HB)、砖红壤(XA)和红壤(YT)的硝化潜势分别为N 32.5、6.6、4.8、2.3 mg⋅kg–1⋅d–1(图 2a)。它们在72 h内的N2O累积排放量分别为N 130.0、22.8、28.5、17.3 μg⋅kg–1(图 2b)。
|
注:图中误差线为标准误差SE(n = 3),不同大写字母表示硝化潜势或72 h N2O累积排放量在不同土壤间差异显著(P < 0.05);不同小写字母表示AOB对硝化潜势或72 h N2O累积排放量的贡献在不同土壤间差异显著(P < 0.05)。 Note: Error bars are standard errors(n = 3). Different capital letters indicate that the potential nitrification rate or 72 h N2O cumulative emissions differed significantly between soils(P < 0.05);different lowercase letters indicate that the contribution of AOB to potential nitrification rate or 72 h N2O cumulative emissions differed significantly between soils(P < 0.05). 图 2 不同土壤硝化潜势、72 h N2O累积排放量及氨氧化菌相对贡献 Fig. 2 Potential nitrification rate, cumulative 72 h N2O emissions and relative contribution of ammonia-oxidizers in different soils |
上述四个耕层土壤中,对于硝化潜势,AOB分别贡献82%、58%、100%和91%,AOA + comammox分别贡献9%、29%、0和9%,异养硝化菌分别贡献9%、13%、0和0。对于N2O排放,AOB分别贡献87%、33%、42%和6%,AOA + comammox分别贡献1%、0、0和7%,异养硝化菌分别贡献12%、67%、58%和87%。因此,AOB主导上述耕层土壤的硝化潜势。AOB主导潮土的N2O排放,而异养硝化菌主导黑土、砖红壤和红壤的N2O排放。AOA + comammox对所有供试土壤的N2O排放贡献均较小。
改良1年的砖红壤剖面中,土壤硝化潜势在40~60 cm(H1-60)、20~40 cm(H1-40)和0~20 cm(H1-20)分别为N 2.6、1.8和3.8 mg⋅kg–1⋅d–1(图 2c)。改良4年的砖红壤剖面中,土壤硝化潜势在40~60 cm(H4-60)、20~40 cm(H4-40)和0~20 cm(H4-20)分别为N 1.1、2.0和6.6 mg⋅kg–1⋅d–1,由深层至表层呈现显著增加(图 2c)。改良1年剖面中,H1-60、H1-40和H1-20的N2O排放分别为N 19.4、24.9和33.2 μg⋅kg–1(图 2d)。改良4年剖面中,H4-60、H4-40和H4-20的N2O排放分别为N 20.9、22.5和32.2 μg⋅kg–1。N2O排放由深层至表层均呈现显著的增加(图 2d)。在改良1年的剖面中,AOA + comammox和AOB对硝化潜势的贡献由深层至表层均在50%左右。在改良4年的剖面中,AOA + comammox对硝化潜势的贡献从21%(40~60 cm)显著增加至55%(0~20 cm)。以上结果说明,对于同一土壤,人为管理对土壤氨氧化能力及氨氧化微生物活动均有显著影响,且这一现象随种植年限增加(人为管理的影响增强)而加强。
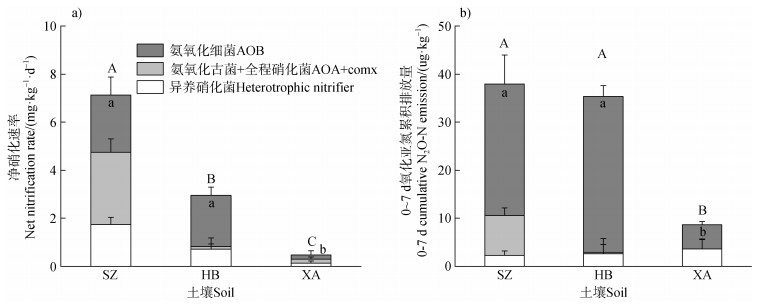
2.3 土壤净硝化速率、N2O排放及氨氧化微生物相对贡献在微宇宙静态培养实验选取的3个耕层土样中,潮土(SZ)、黑土(HB)和砖红壤(XA)的净硝化速率分别为N 7.1、3.0和0.5 mg⋅kg–1⋅d–1(图 3a)。它们在7 d内的N2O累积排放量分别为N 38.0、35.4和8.7 μg⋅kg–1(图 3b)。
|
注:图中误差线为标准误差SE(n = 4)。不同大写字母表示净硝化速率或N2O累积排放量(0~7 d)在不同土壤间差异显著(P < 0.05)。不同小写字母表示AOB对净硝化速率或N2O累积排放量(0~7 d)的贡献在不同土壤间差异显著(P < 0.05)。下同。 Note: Error lines are standard errors (n = 4). Different capital letters indicate that the net nitrification rate or cumulative N2O emissions (0–7 d) differed significantly (P < 0.05) among soils; different lowercase letters indicate that the contribution of AOB to the net nitrification rate or cumulative N2O emissions (0–7 d) differed significantly (P < 0.05) among soils. The same below. 图 3 不同类型土壤净硝化速率、N2O累积排放量(0~7 d)及氨氧化菌相对贡献 Fig. 3 Net nitrification rate, cumulative N2O emissions (0–7 d) and relative contribution of ammonia-oxidizers in different soils |
上述三个耕层土壤中,对于净硝化速率,AOB分别贡献33%、72%和36%,AOA + comammox分别贡献42%、4%和36%,异养硝化菌分别贡献24%、24%和28%。对于N2O排放,AOB分别贡献72%、92%和58%,AOA + comammox分别贡献22%、1%和0,异养硝化菌分别贡献6%、7%和42%。AOB和AOA + comammox对潮土和砖红壤的净硝化贡献相当,而黑土的净硝化由AOB主导。此外,上述三个土壤中的N2O排放均由AOB主导。
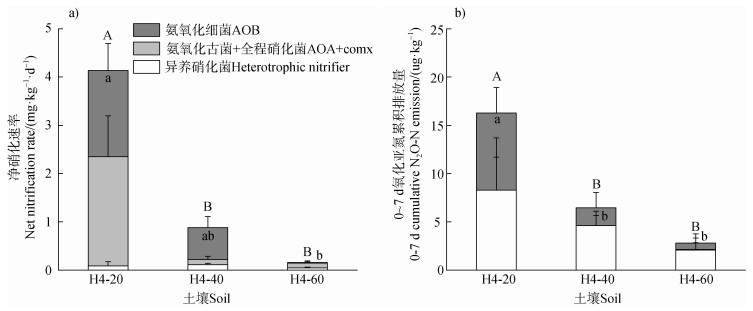
改良4年砖红壤的微宇宙静态培养实验结果显示,土壤净硝化速率在40~60、20~40 cm和0~20 cm分别为N 0.2、0.9和4.1mg⋅kg–1⋅d–1,由深层至表层呈现显著增加(图 4a)。N2O排放由深层至表层同样呈现显著增加(图 4b),H4-60、H4-40和H4-20的N2O排放分别为N 2.8、6.5和16.3 μg⋅kg–1。AOA + comammox对净硝化速率的贡献由深层至表层保持在60%左右,但AOB的贡献从8%(40~60 cm)显著增至43%(0~20 cm)。综上,人为管理对土壤硝化、氨氧化微生物活动及N2O排放潜势的影响,均会反映在其实际反应速率上。
|
图 4 剖面土壤净硝化速率、N2O累积排放量(0~7 d)及氨氧化菌相对贡献 Fig. 4 Net nitrification rate, cumulative N2O emissions (0–7 d) and relative contribution of ammonia-oxidizers in different profile soils |
比较土壤硝化潜势与净硝化速率对pH的响应表明,硝化潜势与净硝化速率均与pH呈正相关关系(图 5a)。对于硝化速率较高的土壤(SZ和HB),其硝化潜势(N 12~30 mg⋅kg–1⋅d–1)是净硝化速率(N 4~10 mg⋅kg–1⋅d–1)的3倍。而对于硝化速率较低的土壤(XA),其硝化潜势与净硝化速率在同一量级(图 5a)。在硝化潜势和净硝化速率测定条件下N2O的排放对pH的响应规律并不完全一致,主要表现为黑土在两种测定条件下N2O排放量的显著差异(图 5b)。
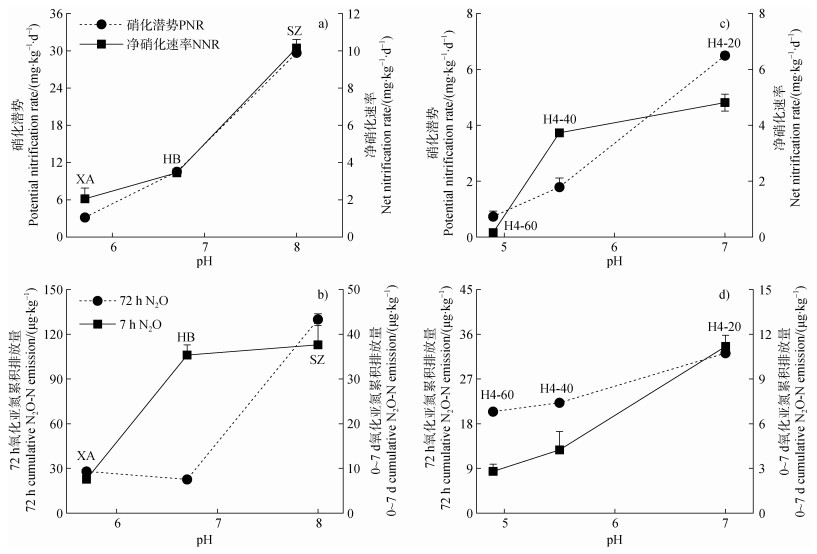
|
注:图中误差线为标准误差SE(PNR n = 3,NNR n = 4)。 Note: PNR and NNR represent the potential nitrification rate and net nitrification rate, respectively. The error bars are standard errors (PNR n = 3, NNR n = 4). 图 5 硝化潜势与净硝化速率及N2O排放对pH的响应 Fig. 5 Potential nitrification rate versus net nitrification rate and the response of N2O production to pH during the period |
本研究中耕层土壤的硝化潜势随着土壤pH由酸性到碱性而呈现显著的增加趋势(图 2a)。土壤硝化潜势的提升主要是由于AOB的贡献增强,这与先前在农田潮棕壤、钙积土和灰化土中的研究结果[21-26]一致。有研究表明,土壤pH的升高显著改变了AOB的群落结构和数量[27],从而提升了AOB对氨氧化的贡献。此外,本研究设置相当于田间传统施氮量的氮底物添加量,反映了田间氨氧化过程发生的主要时期(即施肥后高氨浓度阶段)中AOB的贡献。然而,在不施肥氨浓度较低时,AOB的贡献会有所下降[28]。
在改良的砖红壤剖面土壤中,随土壤pH与肥力由深层至表层逐渐提升,AOB和AOA + comammox对硝化潜势的贡献均显著增加。这一结果与Ouyang[29]、王萍萍[30]等的结果一致。一般而言,随着有机肥管理年限的增加,土壤pH的改良效果逐渐显著,土壤中的有效态氮(NH3)浓度呈指数级提高[31],偏好高pH富营养环境的AOB是主要的“受益者”[32]。此外,基于DNA-SIP标记、选择性抑制剂以及功能基因测序的研究结果一致表明,在农田土壤中,comammox对氨氧化有不可忽略的贡献,且高氨浓度和高pH促进其分布和活动[33-37]。有机肥的投入还可以为AOA和comammox提供偏好氮源,改变土壤理化性质[38],促进其生长。
本研究在黑土(HB)中检测到了异养硝化菌对硝化的贡献,可能是由于其较高的C/N。有研究表明,较高C/N的土壤可以为异养硝化菌的生长提供更多可利用碳源,增强异养硝化的贡献[19,39-40]。
3.2 土壤氨氧化相对贡献对N2O排放的影响氨氧化通过消耗土壤O2并提供NO2–和NO3–底物引起硝化反硝化或异养反硝化是N2O产生的主要途径,且该过程随AOB主导作用的加强而增强[41]。本研究中,由AOB主导氨氧化的潮土(SZ)在培养48 h出现NO2–的累积峰,与N2O的排放高峰耦合,可能的机制一是为减轻高浓度NO2–的毒害作用,AOB发生了硝化反硝化作用;二是由于AOB迅速的氨氧化过程,消耗土壤O2,抑制了NOB的活动,引起了NO2–累积,进而导致硝化–反硝化耦合作用的发生。
尽管有研究表明,在未施肥的酸性土壤中,AOA + comammox是氨氧化的主导微生物[42],本研究却发现在施氮后的酸性红壤和砖红壤中,AOB对硝化潜势有较显著的贡献,且AOB和异养硝化菌对N2O的贡献量接近90%。这可能是因为AOB通过其硝化反硝化作用或驱动反硝化产生的N2O远高于AOA和comammox的N2O产生量。对全球农田土壤的研究结果也表明,comammox Nitrospira对N2O排放的贡献非常有限,N2O排放贡献由AOB主导[43]。异养硝化菌的N2O产生量也较AOA和comammox高[44-45]。
氨氧化相关的非生物学过程也会产生N2O。一方面是氨氧化过程中产生的NH2OH底物,可通过AOA和comammox与NH2OH氧化相关的非生物学过程产生N2O[46]。另一方面,氨氧化产物NH2OH、NO和NO2–的化学分解也可以产生N2O,此过程在三类氨氧化菌中均可发生[47]。因此,本研究中氨氧化微生物对N2O的贡献中包括了中间产物非生物过程的贡献。
3.3 氨氧化微生物相对贡献研究方法的差异乙炔和辛炔分别是常用的自养氨氧化和AOB氨氧化的特异性炔烃抑制剂,被广泛应用于室内培养实验中。二者的结合使用可以比较准确地定量各类氨氧化微生物对氨氧化过程的相对贡献。然而,基于其炔烃的性质,使用过程中可能作为异养硝化菌的碳源[48],进而刺激异养硝化的发生,从而高估培养体系中的异养硝化作用。此外,不同土壤质地会对乙炔和辛炔的扩散效果产生影响,导致对体系内氨氧化微生物相对贡献的估计误差。值得注意的是,乙炔和辛炔的化学结构特性可能将反应体系中的NO氧化为NO2[49-51],减少N2O的化学排放量,从而低估AOA + comammox的N2O排放量。但上述偏差,不会影响本研究的基本结论。总体而言,本研究使用炔烃抑制剂区分不同氨氧化能力的土壤中主导的氨氧化微生物类群,结果与前人利用DNA稳定同位素探测(SIP)技术获得的结果一致[52]。未来可以将抑制剂法与同位素或微生物基因表达分析结合,在限制特定氨氧化微生物活性的基础上,更加清楚地明确N2O的排放源[35,53]。由于同一类氨氧化微生物内部也存在生态位的分化,例如嗜酸AOB TAO100[54]、comammox clade A和comammox clade B的差异[34],因此继续开发针对氨氧化微生物,尤其是对AOA或comammox的特异性抑制剂是非常必要的,这将有助于拓展对硝化微生物的认知边界。
4 结论潮土、黑土、砖红壤及红壤旱地农田耕层土壤的硝化潜势随土壤由酸性到碱性而显著增加,AOB主导四种耕层土壤的硝化潜势。在潮土、黑土及砖红壤中,净硝化速率和N2O排放均随pH的升高而显著提升,与硝化潜势的规律一致。AOB主导黑土的净硝化速率,在潮土和砖红壤中AOB与AOA + comammox对净硝化速率的贡献相当。潮土、黑土及砖红壤的N2O排放均由AOB主导。在有机肥改良的砖红壤剖面土壤中,pH、硝化潜势、净硝化速率和N2O排放均由深层至表层而显著增加,硝化潜势和净硝化速率的提升由AOA + comammox作用的显著增加主导,N2O排放的增加由AOB作用的增加主导,且这一现象随有机肥改良年限的增加而加强。
| [1] |
IPCC. Summary for policymakers[M]//Global warming of 1.5℃. Cambridge: Cambridge University Press, 2018. https://www.ipcc.ch/site/assets/uploads/sites/2/2022/06/SPM_version_report_LR.pdf.
(  0) 0) |
| [2] |
Ravishankara A R, Daniel J S, Portmann R W. Nitrous oxide(N2O): The dominant ozone-depleting substance emitted in the 21st century[J]. Science, 2009, 326(5949): 123-125. DOI:10.1126/science.1176985
(  0) 0) |
| [3] |
Smith K A. Changing views of nitrous oxide emissions from agricultural soil: Key controlling processes and assessment at different spatial scales[J]. European Journal of Soil Science, 2017, 68(2): 137-155. DOI:10.1111/ejss.12409
(  0) 0) |
| [4] |
Tian H Q, Xu R T, Canadell J G, et al. A comprehensive quantification of global nitrous oxide sources and sinks[J]. Nature, 2020, 586(7828): 248-256. DOI:10.1038/s41586-020-2780-0
(  0) 0) |
| [5] |
Wang Q H, Zhou F, Shang Z Y, et al. Data-driven estimates of global nitrous oxide emissions from croplands[J]. National Science Review, 2020, 7(2): 441-452. DOI:10.1093/nsr/nwz087
(  0) 0) |
| [6] |
FAO. World fertilizer trends and outlook to 2020[R]. Rome, Italy: Food and Agriculture Organization of the United Nations, 2017. https://www.fao.org/3/i6895e/i6895e.pdf.
(  0) 0) |
| [7] |
Lehtovirta-Morley L E. Ammonia oxidation: Ecology, physiology, biochemistry and why they must all come together[J]. FEMS Microbiology Letters, 2018, 365(9): fny058.
(  0) 0) |
| [8] |
Prosser J I, Hink L, Gubry-Rangin C, et al. Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies[J]. Global Change Biology, 2020, 26(1): 103-118. DOI:10.1111/gcb.14877
(  0) 0) |
| [9] |
Kits K D, Sedlacek C J, Lebedeva E V, et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle[J]. Nature, 2017, 549(7671): 269-272. DOI:10.1038/nature23679
(  0) 0) |
| [10] |
Kozlowski J A, Kits K D, Stein L Y. Comparison of nitrogen oxide metabolism among diverse ammonia-oxidizing bacteria[J]. Frontiers in Microbiology, 2016, 7: 1090.
(  0) 0) |
| [11] |
Hink L, Gubry-Rangin C, Nicol G W, et al. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions[J]. The ISME Journal, 2018, 12(4): 1084-1093. DOI:10.1038/s41396-017-0025-5
(  0) 0) |
| [12] |
Han P, Wu D M, Sun D Y, et al. N2O and NOy production by the comammox bacterium Nitrospira inopinata in comparison with canonical ammonia oxidizers[J]. Water Research, 2021, 190: 116728. DOI:10.1016/j.watres.2020.116728
(  0) 0) |
| [13] |
Chen Z M, Ding W X, Xu Y H, et al. Importance of heterotrophic nitrification and dissimilatory nitrate reduction to ammonium in a cropland soil: Evidences from a 15N tracing study to literature synthesis[J]. Soil Biology & Biochemistry, 2015, 91: 65-75.
(  0) 0) |
| [14] |
Taylor A E, Vajrala N, Giguere A T, et al. Use of aliphatic n-alkynes to discriminate soil nitrification activities of ammonia-oxidizing thaumarchaea and bacteria[J]. Applied and Environmental Microbiology, 2013, 79(21): 6544-6551. DOI:10.1128/AEM.01928-13
(  0) 0) |
| [15] |
Hink L, Nicol G W, Prosser J I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil[J]. Environmental Microbiology, 2017, 19(12): 4829-4837. DOI:10.1111/1462-2920.13282
(  0) 0) |
| [16] |
Taylor A E, Giguere A T, Zoebelein C M, et al. Modeling of soil nitrification responses to temperature reveals thermodynamic differences between ammonia-oxidizing activity of archaea and bacteria[J]. The ISME Journal, 2017, 11(4): 896-908. DOI:10.1038/ismej.2016.179
(  0) 0) |
| [17] |
Nadeem S, Bakken L R, Frostegård Å, et al. Contingent effects of Liming on N2O-emissions driven by autotrophic nitrification[J]. Frontiers in Environmental Science, 2020, 8: 598513. DOI:10.3389/fenvs.2020.598513
(  0) 0) |
| [18] |
Zhang J B, Sun W J, Zhong W H, et al. The substrate is an important factor in controlling the significance of heterotrophic nitrification in acidic forest soils[J]. Soil Biology & Biochemistry, 2014, 76: 143-148.
(  0) 0) |
| [19] |
Lu R K. Analytical methods for soil and agro-chemistry (In Chinese). Beijing: China Agricultural Science and Technology Press, 2000. [鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000.]
(  0) 0) |
| [20] |
Giguere A T, Taylor A E, Suwa Y, et al. Uncoupling of ammonia oxidation from nitrite oxidation: Impact upon nitrous oxide production in non-cropped Oregon soils[J]. Soil Biology & Biochemistry, 2017, 104: 30-38.
(  0) 0) |
| [21] |
Zhao J, Meng Y Y, Drewer J, et al. Differential ecosystem function stability of ammonia-oxidizing archaea and bacteria following short-term environmental perturbation[J]. mSystems, 2020, 5(3): e00309-20.
(  0) 0) |
| [22] |
Tao R, Wakelin S A, Liang Y C, et al. Response of ammonia-oxidizing archaea and bacteria in calcareous soil to mineral and organic fertilizer application and their relative contribution to nitrification[J]. Soil Biology & Biochemistry, 2017, 114: 20-30.
(  0) 0) |
| [23] |
Stienstra A W, Klein Gunnewiek P, Laanbroek H J. Repression of nitrification in soils under a climax grassland vegetation[J]. FEMS Microbiology Ecology, 1994, 14(1): 45-52. DOI:10.1111/j.1574-6941.1994.tb00089.x
(  0) 0) |
| [24] |
Sun R B, Guo X S, Wang D Z, et al. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle[J]. Applied Soil Ecology, 2015, 95: 171-178. DOI:10.1016/j.apsoil.2015.06.010
(  0) 0) |
| [25] |
Tang Y Q, Yu G R, Zhang X Y, et al. Environmental variables better explain changes in potential nitrification and denitrification activities than microbial properties in fertilized forest soils[J]. Science of the Total Environment, 2019, 647: 653-662. DOI:10.1016/j.scitotenv.2018.07.437
(  0) 0) |
| [26] |
Yu W T, Xu Y G, Bi M L, et al. Activity and composition of ammonia-oxidizing bacteria in an aquic brown soil as influenced by land use and fertilization[J]. Pedosphere, 2010, 20(6): 789-798. DOI:10.1016/S1002-0160(10)60069-0
(  0) 0) |
| [27] |
Che J, Zhao X Q, Zhou X, et al. High pH-enhanced soil nitrification was associated with ammonia-oxidizing bacteria rather than Archaea in acidic soils[J]. Applied Soil Ecology, 2015, 85: 21-29. DOI:10.1016/j.apsoil.2014.09.003
(  0) 0) |
| [28] |
Ouyang Y, Norton J M, Stark J M. Ammonium availability and temperature control contributions of ammonia oxidizing bacteria and archaea to nitrification in an agricultural soil[J]. Soil Biology & Biochemistry, 2017, 113: 161-172.
(  0) 0) |
| [29] |
Ouyang Y, Evans S E, Friesen M L, et al. Effect of nitrogen fertilization on the abundance of nitrogen cycling genes in agricultural soils: A meta-analysis of field studies[J]. Soil Biology & Biochemistry, 2018, 127: 71-78.
(  0) 0) |
| [30] |
Wang P P, Duan Y H, Xu M G, et al. Nitrification potential in fluvo-aquic soils different in fertility and its influencing factors (In Chinese)[J]. Acta Pedologica Sinica, 2019, 56(1): 124-134. [王萍萍, 段英华, 徐明岗, 等. 不同肥力潮土硝化潜势及其影响因素[J]. 土壤学报, 2019, 56(1): 124-134.]
(  0) 0) |
| [31] |
Kemmitt S J, Wright D, Goulding K W T, et al. pH regulation of carbon and nitrogen dynamics in two agricultural soils[J]. Soil Biology & Biochemistry, 2006, 38(5): 898-911.
(  0) 0) |
| [32] |
Zhong W H, Bian B Y, Gao N, et al. Nitrogen fertilization induced changes in ammonia oxidation are attributable mostly to bacteria rather than archaea in greenhouse-based high N input vegetable soil[J]. Soil Biology & Biochemistry, 2016, 93: 150-159.
(  0) 0) |
| [33] |
Li C Y, Hu H W, Chen Q L, et al. Comammox Nitrospira play an active role in nitrification of agricultural soils amended with nitrogen fertilizers[J]. Soil Biology & Biochemistry, 2019, 138: 107609.
(  0) 0) |
| [34] |
Li C Y, Hu H W, Chen Q L, et al. Niche differentiation of clade A comammox Nitrospira and canonical ammonia oxidizers in selected forest soils[J]. Soil Biology & Biochemistry, 2020, 149: 107925.
(  0) 0) |
| [35] |
Li C Y, Hu H W, Chen Q L, et al. Niche specialization of comammox Nitrospira clade A in terrestrial ecosystems[J]. Soil Biology & Biochemistry, 2021, 156: 108231.
(  0) 0) |
| [36] |
Li C Y, He Z Y, Hu H W, et al. Niche specialization of comammox Nitrospira in terrestrial ecosystems: Oligotrophic or copiotrophic?[J]. Critical Reviews in Environmental Science and Technology, 2023, 53(2): 161-176. DOI:10.1080/10643389.2022.2049578
(  0) 0) |
| [37] |
Zhu G B, Wang X M, Wang S Y, et al. Towards a more labor-saving way in microbial ammonium oxidation: A review on complete ammonia oxidization(comammox)[J]. Science of the Total Environment, 2022, 829: 154590. DOI:10.1016/j.scitotenv.2022.154590
(  0) 0) |
| [38] |
Li X, Han S, Wan W J, et al. Manure fertilizes alter the nitrite oxidizer and comammox community composition and increase nitrification rates[J]. Soil and Tillage Research, 2020, 204: 104701. DOI:10.1016/j.still.2020.104701
(  0) 0) |
| [39] |
Lan T, Liu R, Suter H, et al. Stimulation of heterotrophic nitrification and N2O production, inhibition of autotrophic nitrification in soil by adding readily degradable carbon[J]. Journal of Soils and Sediments, 2020, 20(1): 81-90. DOI:10.1007/s11368-019-02417-0
(  0) 0) |
| [40] |
Zhang N, Miao S J, Qiao Y F, et al. N2O emissions from black soils in northeast China (In Chinese)[J]. Acta Pedologica Sinica, 2022, 59(4): 899-909. [张楠, 苗淑杰, 乔云发, 等. 东北农田黑土N2O排放研究进展[J]. 土壤学报, 2022, 59(4): 899-909.]
(  0) 0) |
| [41] |
Huang T, Gao B, Hu X K, et al. Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous Fluvo-aquic soil[J]. Scientific Reports, 2014, 4(1): 3950. DOI:10.1038/srep03950
(  0) 0) |
| [42] |
Bai X, Hu X J, Liu J J, et al. Ammonia oxidizing bacteria dominate soil nitrification under different fertilization regimes in black soils of northeast China[J]. European Journal of Soil Biology, 2022, 111: 103410. DOI:10.1016/j.ejsobi.2022.103410
(  0) 0) |
| [43] |
Jiang L P, Yu J, Wang S Y, et al. Complete ammonia oxidization in agricultural soils: High ammonia fertilizer loss but low N2O production[J]. Global Change Biology, 2023, 29(7): 1984-1997. DOI:10.1111/gcb.16586
(  0) 0) |
| [44] |
Zhang J B, Müller C, Cai Z C. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils[J]. Soil Biology & Biochemistry, 2015, 84: 199-209.
(  0) 0) |
| [45] |
Zhu T B, Zhang J B, Cai Z C. The contribution of nitrogen transformation processes to total N2O emissions from soils used for intensive vegetable cultivation[J]. Plant and Soil, 2011, 343(1): 313-327.
(  0) 0) |
| [46] |
Kits K D, Jung M Y, Vierheilig J, et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata[J]. Nature Communications, 2019, 10(1): 1836. DOI:10.1038/s41467-019-09790-x
(  0) 0) |
| [47] |
Heil J, Vereecken H, Brüggemann N. A review of chemical reactions of nitrification intermediates and their role in nitrogen cycling and nitrogen trace gas formation in soil[J]. European Journal of Soil Science, 2016, 67(1): 23-39. DOI:10.1111/ejss.12306
(  0) 0) |
| [48] |
Nielsen T H, Nielsen L P, Revsbech N P. Nitrification and coupled nitrification-denitrification associated with a soil-manure interface[J]. Soil Science Society of America Journal, 1996, 60(6): 1829-1840. DOI:10.2136/sssaj1996.03615995006000060031x
(  0) 0) |
| [49] |
Bollmann A, Conrad R. Acetylene blockage technique leads to underestimation of denitrification rates in oxic soils due to scavenging of intermediate nitric oxide[J]. Soil Biology & Biochemistry, 1997, 29(7): 1067-1077.
(  0) 0) |
| [50] |
Bollmann A, Conrad R. Enhancement by acetylene of the decomposition of nitric oxide in soil[J]. Soil Biology & Biochemistry, 1997, 29(7): 1057-1066.
(  0) 0) |
| [51] |
Caranto J D, Lancaster K M. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(31): 8217-8222.
(  0) 0) |
| [52] |
Shen J P, Zhang L M, Di H J, et al. A review of ammonia-oxidizing bacteria and archaea in Chinese soils[J]. Frontiers in Microbiology, 2012, 3: 296.
(  0) 0) |
| [53] |
Decock C, Six J. How reliable is the intramolecular distribution of 15N in N2O to source partition N2O emitted from soil?[J]. Soil Biology & Biochemistry, 2013, 65: 114-127.
(  0) 0) |
| [54] |
Hayatsu M, Tago K, Uchiyama I, et al. An acid-tolerant ammonia-oxidizing γ-proteobacterium from soil[J]. The ISME Journal, 2017, 11(5): 1130-1141.
(  0) 0) |
 2024, Vol. 61
2024, Vol. 61


